Back to Journals » Therapeutics and Clinical Risk Management » Volume 11
Docetaxel-related interstitial pneumonitis
Authors Wang C, Chang H, Chang C
Received 14 June 2015
Accepted for publication 7 August 2015
Published 9 December 2015 Volume 2015:11 Pages 1813—1816
DOI https://doi.org/10.2147/TCRM.S90488
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Garry Walsh
Chung-Jen Wang, Hou-Tai Chang, Cheng-Yu Chang
Division of Chest Medicine, Department of Internal Medicine, Far Eastern Memorial Hospital, New Taipei City, Taiwan
Abstract: Docetaxel (Taxotere®) is an agent that is indicated for the treatment of patients with locally advanced or metastatic non-small-cell lung cancer. In recent years, docetaxel-related interstitial lung disease (ILD) has been reported in several case series studies. The onset of ILD occurred ~10–20 days (median time: 18 days) after docetaxel administration. Here, we reported the case of a patient who had pulmonary toxicity of ILD within 3 days after using a relatively low-dose docetaxel administration. Although some articles have described patients who progressed to respiratory failure and needed intubation, this patient responded well to steroid treatment and discontinued docetaxel administration.
Keywords: docetaxel, interstitial lung disease
Case report
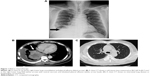
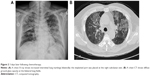
A 47-year-old male smoker presented with chronic cough and dyspnea for 2 months. The chest X-ray (Figure 1A) and chest computed tomography (CT) showed right lower lung tumor and lobulated effusion (Figure 1B), and the lung window showed no interstitial lesion (Figure 1C). Pathological immunostaining was positive for p63 and CK7 and negative for CK20 and TTF-1, which indicated squamous cell carcinoma. After a series of studies, including brain CT and whole body bone scan, the cancer was classified as cT4 (9.87 cm) N3M0, stage IIIb, due to multiple enlarged lymph nodes in the retrocaval, prevascular, bilateral lower paratracheal, subcarinal, and right interlobar regions. He received chemotherapy with docetaxel (30 mg/m2, day 1) after premedication with intravenous dexamethasone (10 mg). He developed severe cough and progressive dyspnea 3 days after administration. He had no systemic inflammatory or infection symptoms at the time. In room air, the arterial blood gas analysis revealed: pH, 7.362; partial pressure of carbon dioxide, 40.3 mmHg; partial pressure of oxygen, 44.6 mmHg; and bicarbonate (HCO3) level, 23.4 mmol/L. The serum examination showed no leukocytosis or neutropenia. The chest X-ray revealed diffused interstitial infiltrates (Figure 2A), and chest CT showed diffuse ground glass opacities at bilateral lung fields and nonsegmental predominance of lung opacity without prominent hilum engorgement (Figure 2B). The patient had no heart disease history and fair urine output. The cardiac echography showed good left ventricular ejection fraction (74%). The fluid overload or cardiogenic edema was less likely.
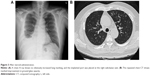
Under the impression of interstitial lung disease (ILD), steroid (intravenous methylprednisolone, 2 mg/kg) and broad spectrum antibiotics were prescribed. We tapered the steroid gradually within 14 days of treatment. The clinical symptoms, chest X-ray (Figure 3A), and chest CT (Figure 3B) showed significant improvement after treatment. He also received serological tests, sputum culture, blood culture, Legionella pneumophila, Mycoplasma pneumoniae, Chlamydophila pneumonia, influenza virus A and B, and autoimmune profile examinations, and all tests showed negative findings. Written informed consent was obtained by the patient. Permission of case report publication from the Ethics Review Committee of the Far Eastern Memorial Hospital was also sought.
Discussion
Among previously published cases of docetaxel-related interstitial pneumonitis, this is the first to describe a patient who developed acute pulmonary toxicity within 3 days after administration of low-dose docetaxel (30 mg/m2). Taxanes are known to cause proliferation of cytotoxic T-cells, leading to a hypersensitivity type of lung damage, or might cause direct pulmonary damage through reactive oxygen metabolites. According to our report, low-dose docetaxel treatment was less toxic but not an effective prevention method of lung damage. The physicians should be aware of the possibility of interstitial pneumonitis by the patient’s symptoms and daily physical examination after administration of taxanes.
The acute pulmonary toxicity of ILD after docetaxel administration is an uncommon adverse event in patients with cancer.1 Approximately 4.6% of patients experience pulmonary toxicity induced by triweekly conventional-dose docetaxel monotherapy (75 mg/m2).2,3 Pneumonitis in most cases occurred after two cycles with a conventional dose.3 The incidence of pulmonary toxicity is more related to the docetaxel delivery schedule than the dose, that is, a weekly schedule leads to lower myelosuppression but more pneumonitis than triweekly administration.4 Moreover, the largest published series of docetaxel-related interstitial pneumonitis reported 18 different cases of a total of 392 patients with metastatic non-small-cell lung cancer treated with docetaxel. The time from the last docetaxel administration to the onset of toxicity findings on the chest radiograph was approximately 10–20 days (median time: 18 days).5
Although docetaxel-related side effects are rare, the mortality rate is high. The overall mortality rate due to drug-associated interstitial fibrosis, estimated based on published case reports, appears to be ~40%, with 12 deaths among 30 cases in which mortality data were available.2 This result was also seen in another literature review, which summarizes a total of 31 cases of taxane-induced pneumonitis, in which 35% needed ventilator support, and the mortality rate was 42% in the whole group; those in need of intubation had a mortality rate of 82%.6
The treatment of choice is administration of systemic glucocorticoid therapy in selected patients in whom an infectious etiology was excluded with appropriate cultures. The patient is usually treated with prednisolone 40–60 mg/day for 2–3 weeks; 60–240 mg/day may be used in patients who have impending respiratory failure, along with a slow and careful tapering-off period.7
Although the occurrence of docetaxel-related ILD is rare, it will lead to respiratory failure if treatment is delayed. ILD responds well to steroid treatment and discontinuation of docetaxel administration. Our case indicates the acute pulmonary toxicity of ILD when administering docetaxel.
Disclosure
The authors report no conflicts of interest in this work.
References
Eivind S, Gunhild H, Karsten G, et al. Lethal pneumonitis after docetaxel chemotherapy: case report and review of the literature. Acta Oncol. 2013;52:1034–1038. | ||
Read WL, Mortimer JE, Picus J. Severe interstitial pneumonitis associated with docetaxel administration. Cancer. 2002;94:847–853. | ||
Wang GS, Yang KY, Perng RP. Life-threatening hypersensitivity pneumonitis induced by docetacel (taxotere). Br J Cancer. 2001;85:1247–1250. | ||
Chen YM, Shih JF, Perng RP, Tsai CM, Whang-Peng J. A randomized trial of different docetaxel schedules in non-small cell lung cancer patients who failed previous platinum-based chemotherapy. Chest. 2006;129:1031–1038. | ||
Tamiya A, Naito T, Miura S, et al. Interstitial lung disease associated with docetaxel in patients with advanced non-small cell lung cancer. Anticancer Res. 2012;32:1103–1106. | ||
Nagata S, Ueda N, Yoshida Y, Matsuda H, Maehara Y. Severe interstitial pneumonitis associated with the administration of taxanes. J Infect Chemother. 2010;16:340–344. | ||
Khan A, McNally D, Tutschka PJ, Bilgrami S. Paclitaxel-induced acute bilateral pneumonitis. Ann Pharmacother. 1997;31:1471. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.