Back to Journals » Clinical Ophthalmology » Volume 15
Do Corneal Tissue Providers Inform Their Community That They Export Corneas? An Audit of Publicly Available Sector Websites
Authors Machin H , Philippy B, Pollock GA
Received 21 July 2021
Accepted for publication 27 September 2021
Published 5 October 2021 Volume 2021:15 Pages 4029—4034
DOI https://doi.org/10.2147/OPTH.S330468
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Heather Machin,1,2 Brian Philippy,3 Graeme A Pollock1,2
1Lions Eye Donation Service, Centre for Eye Research Australia, Royal Victorian Eye and Ear Hospital, Melbourne, Victoria, Australia; 2Department of Surgery, Ophthalmology, University of Melbourne, Melbourne, Victoria, Australia; 3Distribution Department, Lions Medical Eye Bank and Research Center of East Virginia, Norfolk, VA, USA
Correspondence: Heather Machin Email [email protected]
Aim: The exportation of corneas from one nation to another, for transplantation services, is responsible for 23% of all global transplants. Global allocation is possible because of the end-of-life donations from citizens and residents of export nations. To date, there is no information indicating if export nation donors are aware that their corneas may be exported, nor if organizations that export provide information regarding their export engagement to their community. To ascertain if and how exporters inform their community, we audited known export organization public websites.
Materials and Methods: We designed and conducted a double-blind audit of known exporting eye banks, eye tissue sharing and distributor organization websites.
Results: We audited 79 websites, from 9 nations. Of the 79, 46 (58.2%) did not mention corneal tissue exportation, 17 (21.5%) implied exportation, and 16 (10.2%) explicitly mentioned it. Of the 16 that mentioned they exported, 75% (12/16) provided information regarding their export license, and 12.5% (2/16) indicated partnership with a third party. We could not locate information explaining how organizations decided on how and to whom they export.
Discussion: Organizations that export corneal tissue across national borders do not share sufficient information regarding their export activities on their website. The general public and donors within export nations may not be aware that this practice occurs or could occur with their donation. Export organizations and the eye tissue sector must evaluate their communication strategies and collaborate, preferably nationally, to develop publicly appropriate information regarding corneal tissue exportation.
Keywords: corneal tissue, distributors and brokers, export, eye banks, transparency, website
Introduction
Since 1961, eye banks and eye tissue distributors have moved donated human corneas across national borders.1 The practice has provided transplant options to recipients located in other nations that are without their own eye bank or where their eye bank is unable to supply sufficient quantities of corneal tissue to meet their nation’s waiting corneal transplant recipient needs. It has also provided an allocation option and outlet for export nations that may not be able to place the corneal tissue domestically at the time of the donation, or where exportation is used as a routine allocation practice simultaneous to domestic allocation.2–5
The practice has been described as exportation and importation, allocation, sharing, provision, movement, transfer or collectively as transnational activity.6,7 There is no known annual global record indicating the degree of corneal tissue transnational activity, however a 20168 paper that examined global corneal transplant numbers, indicated that 23% of all global transplants used imported corneas (being n=42,251 of n=184,576 corneal transplants provided between their 12-month reporting period). Their study indicated there were n=9 export nations, with the USA (85%), Sri Lanka (9%) and Italy (3%) the largest participants. They identified lesser export nations as Australia, Columbia, the Czech Republic, France, The Netherlands, and The Philippines. Additionally, the Eye Bank Association of America indicated in their 2019 Annual Data Report that n=3 of their n=11 international member eye banks exported corneas. This included at least one Canadian eye bank export member.9
There is little information to describe the practice of corneal transnational activity10 and there is no indication of general public or donor awareness within export nations, regarding exportation of their corneas on donation. Nor is there any indication at the national level that donors are uniformly informed or consented-for-export at the point of their donation.11,12 There has been no research published to indicate if and how exporting eye banks and distributors currently provide information to their donors or the public about their organization’s export participation.10 Finally, there is no understanding of the current degree of transparency by export organizations about their activities nor the gaps in existing publicly available information that requires address.
To commence examination in this field, we conducted a review to determine how export organizations publicly inform their community about their export participation. This provided an overview of current practice and a baseline for future comparison and development. We excluded examination of the point-of-donation information and consent, and the public’s comprehension of existing available information, though we acknowledge these also require evaluation.
To conduct our research, we selected to examine the public websites of known participating corneal tissue export organizations. This placed all organizations on a level playing field, in terms of equal opportunity to upload information (for example, open text, pamphlets, diagrams, video vignette and links) relatively quickly and easily on the same public delivery platform. We selected websites over social media, as websites formally represent an organization, as its virtual front-door. It indicated how the organization wanted to be perceived because in general, website content requires organizational sign-off on design and content prior to live uploads. This provided a degree of conscious decision-making and control by the organization regarding what information they want e-visitors to access.
Materials and Methods
We developed a question set (available in Supplementary 1) for use in a double-blind audit. The questions reflected the recommendations outlined in the key guiding document of the eye banking field, being The Barcelona Principles (Principle numbers 1 and 7,13 which provide a recommendation on how to conduct consent and good transnational practice). We uploaded the questions to our audit-tool - RedCap (powered by Vanderbilt, USA). Two Investigators (BP and HM) audited independently between September-December 2019 with a subsequent separate independent examination of the results conducted (by GP) between February-March 2020 (Figure 1). As the websites were publicly available, the research was exempt from human ethics approval.
 |
Figure 1 The audit process. |
We confirmed exporting organizations through empirical inquiry, where we searched for organizations in nations mentioned in: the 2016 paper we have previously described;8 other peer published papers;10 conference presentations;14 national/regional professional association publications or websites; and for some, through private email correspondence with our Investigators. We focused on the exporting organizations (defined as: eye banks, tissue sharing cooperatives and third-party brokers/distributors), rather than importing organizations, as our focus was on determining the level of available information to the export nation’s public. We included websites that were accessible to our reviewers in the USA (BP) and Australia (HM, GP), via the public search engine Google®.
We excluded organizations: without websites; without functioning websites; websites unavailable in English (as English was the common language of our Investigators); and those not listed in peer publications or peer associations as an exporter. We excluded organizations in publications that simply stated the nation, without further evidence to support our researchers to determine which organizations in that nation exported.
Audit duration per website ranged from 10–30 minutes, depending on the structure and content of the website and the ease to collect available information or confirm information was not present. While we collected a wealth of information from each website, this paper presents the outcomes from five key questions (Questions 15–19 within Supplementary 1), being: 1. Stated that they export, 2. Export accredited for their stated purpose [meaning export license present], 3. Outlined their export allocation decision-making process, 4. Stated engagement of distributors/brokers or mention of allocation-agency affiliation, and 5. Stated that permission to export is part of their consent process.
Results
We identified n=87 export provider websites, from n=12 nations. After our exclusion criteria was applied, n=79 websites, from n=9 nations remained. As some nations had only one or two exporting organizations, we intentionally de-identify our country level and organization level results, and present the results as one global collective.
Of the n=79 websites, n=46 (58%) did not mention corneal tissue exportation, n=17 (21.5%) implied exportation, and n=16 (10.5%) explicitly mentioned it. Of the n=16 that explicitly stated they exported, 75% (n=12/16, (being 12/79,15%) provided information regarding their export license and 12.5% (n=2/16)(being 2/79, 2.5%) indicated partnership with a third-party, indicating an international collaboration or connection. We could not locate information explaining how the organization’s decision-making was framed on any of the n=16 website (for example, how they selected one import nation or recipient/surgeon over another). Nor could we locate information regarding their inclusion or exclusion of a consent-for-export process or if they informed donors at the point of donation that their donation may be exported. (Table 1).
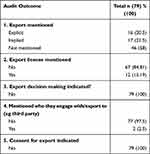 |
Table 1 Audit Questions and Results |
The language used on the websites, of those who implied exportation (n=17/79, 21.5%), indicated involvement in or support towards international/global eye banking efforts. For example, they mention partnering with or supporting transplant facilities, allocation agencies, other non-state actors, or surgeons and hospitals around the world. The websites did not state that they exported corneas to the mentioned partners but alluded to an established international connection. Finally, the largest group in our cohort were those organizations that did not mention on their website their exportation engagement (n=46/79, 58%). There was no indication on their website to allude to possible international affiliations either.
Discussion
Organizations that export corneal tissue across national borders, provide an inadequate amount of information to inform the public, about their export activities on their own organization’s website. As such, the general public in export nations, may not be aware that this practice occurs or could occur with their donation. While some organizations may inform verbally, at the point of donation, to the next-of-kin, there is no general information readily available for the public to consider and scrutinize, prior to their death. Additionally, there is no universal method available to the public, to allow them to determine one way or another, which eye banks or affiliated organizations export, and which ones do not, and which distributors are involved. This may compromise consent in some jurisdictions,11,15 and may subsequently erode public and donor trust in the system. Erosion of trust may result in a withdrawal of donation if export populations or a donor’s next-of-kin discovered that exportation occurred without their knowledge or consent.
Having audited the websites of exporters in this field, we found that exporters were not routinely informing the public about their activity on their website. Without doing so, we were left wondering why those organizations were withholding information. This seemed illogical, because if informed and managed effectively and transparently, then transnational activity could garner support from donors, recipients, and the general population. Conversely, if left without address, it may suggest that unscrupulous activities are taking place. This may not be the case, but without address the public remains in the dark, trust could be compromised, and uncontested conjecture is left to define eye tissue services and the act of exportation.
A limitation of our research was our inability to analyze why exportation was excluded on some organization websites. We suspect some were not aware that exportation was something the public needed to know about, or they had simply not updated their website since the launch of The Barcelona Principles.13 Conversely, others may knowingly have decided to exclude exportation information, as they may not want to complicate the donation process, felt the information was unnecessary, or feared that its mention may dissuade some donors by promoting nationalist or racially-motivated decision-making within donors. It is noteworthy, however, that while such scenarios are plausible, there is insufficient data to indicate any specific motive, suggesting a need for further exploration of why donors may withdraw their donation from exportation.
With some nations only having one eye bank, in order to de-identify our review, this audit is presented with both country and organizations de-identified. While this may appear to detract from the research, it ensures that the presentation of this subject matter is provided on a level playing field, offering all export organizations and their importing partners the opportunity to review their own degree of transparency and make their own reform steps. Follow-up audits could be re-designed to publish organization and country names, after notice of the audit is provided.
Further studies could examine public access to information in the areas we excluded (for example, non-English websites, organizations without websites, social media, and university or government publications) and the public’s comprehension of information on existing websites and the cultural appropriateness of information sharing or withholding. Finally, interpretation of this study could be expanded to consider the degree of information provided by donation facilities and agencies, transplant facilities, and the information provided at the point-of-donation or during self-registration.
Finally, how websites were presented was challenging for our audit, with information about exportation not easy to locate, particularly if embedded in larger organizational websites (for example, if the exporter was part of a university, research institute or health department). Websites that implied participation in exportation and presented information about their international connections with other nations, were also difficult to follow. As a website visitor we were left to determine what their international connections meant. For example, did that include capacity building (infrastructure), grants, knowledge exchange, and was the activity just once or ongoing? We propose that exporters consider how they present information publicly about their export engagement alongside other forms of international collaboration and differentiate between the two. Additionally, to whom websites were targeted also differed. For example, some websites were targeted towards the general public and/or with a combined target audience towards donors, recipients, regulation/government agencies, other healthcare providers, investors, philanthropic funding providers, other eye bank/corneal providers, and corneal surgeons seeking corneas, consumables, medical devices, or training. Further examination is required to understand how an organization can effectively provide information on exportation when placed within a crowded website with competing audience needs.
To close, export organizations must strengthen their commitment to the public and donors by providing information regarding their export activity, in a manner appropriate to their donating public. This will enhance trust and safeguard access to donations for those requiring a corneal transplant domestically and internationally. We propose that export organizations and the wider eye tissue field evaluate their communication strategies and collaborate, preferably nationally, to develop frameworks on publicly-appropriate information and messaging regarding corneal tissue exportation.
Ethics Approval
This research did not involve human or animal participants. The audit was conducted on publicly available websites. Therefore, human ethics approval is not applicable for this study.
Acknowledgments
Damien McCarthy and Nicole Tindall at the Centre for Eye Research Australia for their data management support.
Author Contributions
All authors made a significant contribution to the work reported. This included the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
Heather Machin: Postgraduate Scholarship supported by the Australian National Health and Medical Research Council (NHMRC) ID: APP1150637.The Centre for Eye Research Australia receives Operational Infrastructure Support from the Victorian government.
Disclosure
The authors have no competing or conflicting interest.
References
1. King JH. International eye-bank and American ophthalmology. Am J Ophthalmol. 1964;5:873–876.
2. Machin H, Philippy B, Ross C, Sutton G, Baird PN. Part one: understanding the impact of COVID-19 on corneal transplant need and demand through the example of Australia. Int J Eye Bank. 2020;8:2.
3. Machin H, Philippy B, Ross C, Sutton G, Baird PN. Part 2. Defining surplus and waste in the pre- and post-COVID-19 era via Australian and USA examples. Int J Eye Bank. 2020;8:3.
4. Machin H, Sutton G, Baird PN. Do eye bank models and competitive practice affect international cornea allocation? Cornea. 2021;40(7):936–941. doi:10.1097/ICO.0000000000002693
5. Machin H, Sutton G, Baird PN. Examining the corneal tissue exportation fee and its impact on equitable allocation. Cornea. 2021. doi:10.1097/ico.0000000000002856
6. Martin DE, Kelly R, Jones GLA, Machin H, Pollock GA. Ethical issues in transnational eye banking. Cornea. 2017;36(2):252–256. doi:10.1097/ICO.0000000000001090
7. Machin H, Sutton G, Baird PN. Should nations with surplus donated corneal tissue export to those without? A review of sector opinion through the example of one Nation-Australia. Cornea. 2020;39(10):1334–1340. doi:10.1097/ICO.0000000000002390
8. Gain P, Jullienne R, He Z, et al. Global survey of corneal transplantation and eye banking. JAMA Ophthalmol. 2016;134(2):167–173. doi:10.1001/jamaophthalmol.2015.4776
9. Philippy B, Van Meter W, Dematteo J. 2019 Eye Banking Statistical Report. Eye Bank Association of America; 2020.
10. Machin H, Arslan J, Baird PN. Examining the impact of corneal tissue transnational activity, and transplantation, on import and export nations: a review of the literature. Cornea. 2020;39(6):795–800. doi:10.1097/ICO.0000000000002255
11. Machin H, Sutton G, Baird PN. Should donors consent to export their corneas? Examination of eye tissue and eye care sector opinion. Cornea. 2021;40(3):398–403. doi:10.1097/ICO.0000000000002559
12. Machin HM, Buckland L, Critchley C, Wiffen S, Sutton G, Baird PN. Determining the willingness of Australians to export their corneas on death. PLoS One. 2021;16(2):e0246622. doi:10.1371/journal.pone.0246622
13. The Global Alliance of Eye Bank Associations. The Barcelona principles: an agreement on the use of human donated tissue for ocular transplantation, research, and future technologies. Cornea. 2018;37(10):1213–1217. doi:10.1097/ICO.0000000000001675
14. Philippy B. By the numbers: notable trends and how to put the data to work for you.
15. Schulz-Blade A, Biller-Andorno N, Capron AM. International perspectives on the ethics and regulation of human cell and tissue transplantation. Bull WHO. 2007;85(12):941–948.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
