Back to Journals » Pharmacogenomics and Personalized Medicine » Volume 15
DNA Methylation Level of Transcription Factor Binding Site in the Promoter Region of Acyl-CoA Synthetase Family Member 3 (ACSF3) in Saudi Autistic Children
Authors Algothmi K, Alqurashi A, Alrofaidi A , Alharbi M, Farsi R , Alburae N, Ganash M , Azhari S, Basingab F, Almuhammadi A, Alqosaibi A , Alkhatabi H, Elaimi A , Jan M, Aldhalaan H, Alrafiah A , Alhazmi S
Received 25 October 2021
Accepted for publication 24 January 2022
Published 18 February 2022 Volume 2022:15 Pages 131—142
DOI https://doi.org/10.2147/PGPM.S346187
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Martin H Bluth
Khloud Algothmi,1 Amal Alqurashi,1 Aisha Alrofaidi,1 Mona Alharbi,1 Reem Farsi,1 Najla Alburae,1 Magdah Ganash,1 Sheren Azhari,1 Fatemah Basingab,1 Asma Almuhammadi,1 Amany Alqosaibi,2 Heba Alkhatabi,3,4 Aisha Elaimi,3,4 Mohammed Jan,5 Hesham Aldhalaan,6 Aziza Alrafiah,4 Safiah Alhazmi1
1Biological Sciences Department, Faculty of Science, King Abdulaziz University, Jeddah, Saudi Arabia; 2Department of Biology, College of Science, Imam Abdulrahman Bin Faisal University, Dammam, Saudi Arabia; 3King Abdulaziz University, Centre of Excellence in Genomic Medicine Research, Jeddah, Saudi Arabia; 4Medical LaboratorySciencesDepartment,Faculty of Applied Medical Sciences, Jeddah, Saudi Arabia; 5College of Medicine, King Abdulaziz University, Jeddah, Saudi Arabia; 6Center for Autism Research at King Faisal Specialist Hospital & Research Center (KFSH & RC), Riyadh, Saudi Arabia
Correspondence: Aziza Alrafiah, P.O Box 80200, Jeddah, 21589, Saudi Arabia, Tel +966 126401000 Ext. 23495, Fax +966 126401000 Ext. 21686, Email [email protected]
Background: DNA methylation (DNAm) is one of the main epigenetic mechanisms that affects gene expression without changing the underlying DNA sequence. Aberrant DNAm has an implication in different human diseases such as cancer, schizophrenia, and autism spectrum disorder (ASD). ASD is a neurodevelopmental disorder that affects behavior, learning, and communication skills. Acyl-CoA synthetase family member 3 (ACSF3) encodes malonyl-CoA synthetase that is involved in the synthesis and oxidation of fatty acids. The dysregulation in such gene has been reported in combined malonic and methylmalonic aciduria associated with neurological symptoms such as memory problems, psychiatric diseases, and/or cognitive decline. This research aims to study DNAm in the transcription factor (TF) binding site of ACSF3 in Saudi autistic children. To determine whether the DNAm of the TF-binding site is a cause or a consequence of transcription regulation of ACSF3.
Methods: RT-qPCR and DNA methylight qPCR were used to determine the expression and DNAm level in the promoter region of ACSF3, respectively. DNA and RNA were extracted from 19 cases of ASD children and 18 control samples from their healthy siblings.
Results: The results showed a significant correlation between the gene expression of ACSF3 and specificity protein 1 (SP1) in 17 samples of ASD patients, where both genes were upregulated in 9 samples and downregulated in 8 samples.
Conclusion: Although this study found no DNAm in the binding site of SP1 within the ACSF3 promoter, the indicated correlation highlights a possible role of ACSF3 and SP1 in ASD patients.
Keywords: autism, DNA methylation, SP1, ACSF3, Saudi autistic children, DNAm
Corrigendum for this paper has been published.
Introduction
Autism spectrum disorder (ASD) is a neurodevelopmental disorder characterized by impairment in social communication and interaction, restricted and repetitive interest, and behavior.1 However, attention-deficit hyperactivity disorder (ADHD), anxiety, bipolar disorder, and depression are comorbid psychiatric illnesses that appear in a high percent of ASD patients.2 It is not easy to find an accurate and updated record on the global prevalence of ASD because the numbers are unknown in many low- and middle-income countries, while the reported prevalence varies substantially across studies. However, one study (cited by the World Health Organization) estimates that about one in 160 children has ASD worldwide.3 Despite the fact, genetic and environmental factors were found to influence ASD4,5 but the relationships are still not well understood.6 According to King Salman’s Center for Disability Research (KSCDR), the Statistics of ASD were around 50,714 cases in Saudi Arabia in 2017.7
Gene expression can be regulated through various transcription factors (TFs) and DNA methylation (DNAm).8 The binding of TFs to the promoter of a particular gene is influenced by many factors such as mutation and DNAm.9–11 DNAm is one of the epigenetic mechanisms that alters the gene expression without changing the underlying DNA sequence.12 It plays a critical role in regulating normal development by affecting the expression of selected genes.13 Moreover, the abnormality in the DNAm process in the promoter region of a gene, especially around the TF binding site, has reported different diseases, including ASD.14–17 Evidence suggests that DNAm signatures can distinguish between pathogenic and benign mutations or copy number variants (CNVs) in various syndromic forms of ASD. This is because that pathogenic variants produce unique epi-signatures when combined with genetic or cytogenetic findings can provide an accurate diagnosis.18
ACSF3 is the gene encodes a mitochondrial enzyme that is involved in fatty acid synthesis. ACSF3 enzyme performs a chemical reaction to convert malonic acid (toxic metabolite that inhibits mitochondrial metabolism) to malonyl-CoA, which is the first step of fatty acid synthesis.19 Previous studies of exome sequencing showed that ACSF3 implicated in combined malonic and methylmalonic aciduria which associated with neurological symptoms such as memory problems, psychiatric disease and/or cognitive decline. Many studies revealed that methylation levels of the promoter regions of ACSF3 play a critical role on its expression.20 Moreover, the abundance of CpG sites in the ACSF3 promoter suggested that methylation could be a regulatory factor. In addition, ACSF3 displayed a differential hypermethylation in in region cg04033022 of ACSF3 in B-cells which is implicated in multiple sclerosis disease (MS), neurodegenerative diseases, Rheumatoid arthritis (RA), and in systemic lupus erythematosus (SLE).21 However, to the best of our knowledge no previous study has looked at the effect of DNAm on the TF binding site of this gene. There are few studies about the role of DNAm in the expression of the genes associated with ASD, specifically in Saudi Arabia. Therefore, this research aimed to determine the role of DNAm in the TF binding site of the ACSF3 gene in Saudi ASD children through bioinformatics tools and molecular techniques.
Methods
Study Population
The ethics of this study was approved by the Center of Excellence in Genomic Medicine Research (CEGMR). Parents or legal guardian of all participants provided informed consent to participate in the study after explaining the aim of research to them. The study group included 19 ASD children and 18 of their siblings, (see Table 1). The children were diagnosed based on Diagnostic and Statistical Manual of Mental Disorders version 5 (DSM-5) and showed no symptoms of malnutrition, active infection, or known genetic disease (such as Down syndrome). To detect chromosomal abnormalities and copy number variations, Agilent Cytogenomics version 4.0 software (Agilent Technologies, USA) was used to analyze array-CGH data (Agilent sure print G3 Hmn CGH 2x 400K array chips). Bioinformatics analysis of array-CGH data did not detect any alteration in the gene/loci that have been characterized as genetic factors for syndromic ASD. Blood samples were collected in EDTA tubes for DNA extraction.
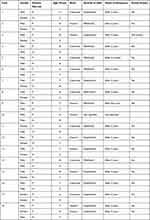 |
Table 1 Demographic Characteristics of ASD Children and Their Siblings |
DNA Extraction
Genomic DNA was extracted from a blood sample in vitro at the CEGMR using DNeasy Blood and Tissue Kit (Qiagen, UK) according to the manufacturer’s protocol. First, the purity and concentration of DNA were measured using a NanoDrop 2000c spectrophotometer (Thermo Scientific Inc.). Then, samples were stored at −20°C until use.
RNA Extraction
According to manufactures instructions, total RNA was extracted from blood using an RNeasy kit (Qiagen, UK). RNA concentration and purity were measured using a NanoDrop 2000c spectrophotometer (Thermo Scientific Inc.). Then, RNA reverse-transcribed into cDNA was applied using ImProm-II™ Reverse Transcription System (Promega, USA) according to the provided protocol.
Real-Time Quantitative PCR (RT-qPCR) for ACSF3 and SP1
For RT–qPCR, a master mix was prepared by mixing 5 µL of QuantiFast SYBR® Green 2x master mix buffer (Qiagen, USA), 0.5 µL of forward Primer, 0.5 µL of reverse Primer, and 2 µL of H2O. Master mix (8 µL) was loaded into 96-Well Reaction Plate (MicroAmp Fast Optical, Applied Biosystem) then cDNA sample (2 µL) was added. RT-qPCR was carried out using QuantiFast SYBR® Green PCR Kit (Qiagen, USA) on StepOnePlus Real-Time PCR System (Applied Biosystem). The PCR program consisted of an initial denaturation step of 5 min at 95°C, followed by 40 cycles of denaturation at 95°C for 10 sec, and annealing/extension at 58°C for 30 sec. All PCR reactions were applied in duplicate and actin beta (ACTB) was used as a reference gene to normalize the expression of target genes. The sequences of the forward and reverse primers used for RT–PCR were: ACTB (forward: 5′- AAAATCTGGCACCACACCTT-3′ and reverse: 5′- GCCTGGATAGCAACGTACAT-3′), ACSF3 (forward: 5′- CAG TGC TGG AGA AGT GGA AG-3′ and reverse: 5′- GGT TTT CTG AGA CAA TGC GC-3′) and SP1 (forward: 5’- AGTTGGTGGCAATAATGGGG-3’, and reverse: 5’- CTGGGAGTTGTTGCTGTTCT-3’). The PCR products were confirmed via 2% agarose gel electrophoresis.
Identification of Promoter Region of ACSF3
Eukaryotic Promoter Database was used to identify the promoter region of ACSF3, choosing the length of the promoter region ranges from −499 to 100. (https://epd.epfl.ch//cgi-bin/get_doc?db=hgEpdNew&format=genome&entry=ACSF3_1)
>FP022318 ACSF3_1:+U EU:NC; range −499 to 100.
ccagctaattgggaggttgaggcaggagaatcacttgaacccgggaggcggaggttgcgg
tgagccgagatcacaccactgcactctagtctgggcgacagagcgagactccatctcaaa
ataataataataataagaaaaagtaacaacaataataataataataaaatcacccaggag
ctcagcgtgcagacccccagccgcaaagcccagagagccccttttgggaggacggggcga
ggcccagcaatccgcgttgggagtggtcccgcgcgccgttctgatctgggcagccctggg
ctagcggagaccacggcgccttgcctcggccacggctcacgagacgcccgcggccccgac
cccgccacgacgggacgggcgggacaggggaacacgcgcgcccggaacccgccgccacgg
cgccgcgccggggggacgcgcttgggcgacggacgcgccgcgccggggaagctgttgggc
gccggaactggtccggcccGACTCACGACCCCGCGGGACCCGGCCGGAACCCGGCCCGAC
CCCGGCGCGCGCGCGGCGGAGGACGAGGAAGAGTTGTGGCGAGGCAGATCCTGCCCCGTG
Bisulfite Modification and MethyLight qPCR
According to manufactures instructions, all DNA samples were bisulfite-converted using EpiTect Bisulfite Kit (Qiagen, Germany). MethyLight assay (quantitative real-time PCR) was performed in a 10 µL reaction volume containing 5 µL 2x EpiTect MethyLight Master Mix, 1 µL ACSF3 primer/probe mix, 1 µL COL2A1 (collagen type II alpha one chain) primer/probe mix, 2 µL nuclease-free water and 1 µL of bisulfite-treated DNA template. COL2A1 was used as a reference gene for normalization. Reactions were performed in duplicate with the positive control (methylated human bisulfite-converted DNA), negative control (unmethylated human bisulfite-converted DNA), and unconverted human genomic DNA from healthy human blood samples. The MethyLight assay was performed in the StepOnePlus Real-Time PCR System (Applied Biosystems). The PCR program consisted of an initial denaturation step of 5 min at 95°C, followed by 40 cycles of denaturation at 95°C for 15s and annealing/extension at 58°C for 1 min. Two sets of primers and probes were designed specifically for bisulfite-converted DNA in this study for both (ACSF3, COL2A1). Collagen was used as the endogenous internal reference to normalize the amount of input DNA. The primers were purchased from (Integrated DNA Technologies, USA), and probes were purchased from (Macrogen, Korea). The primers and probes sequences used for Methylight qPCR were:
COL2A1: (forward: 5′-TCTAACAATTATAAACTCCAACCACCAA-3′, reverse: 5′GGGAA GATGGGATAGAAGGGAATAT-3′, and probe: 5’-CCTTCATTCTAACCCAATACCTAT CCC ACCTCTAAA-3’) and ACSF3: (forward: 5′-TTTTGGGTTAGCGGAGATTA −3′, reverse: 5′-TAACGACGAATTCCGAAC-3′, and probe: 5’-TTTTGTTTCGGTTACGGTTT-3’).
Bioinformatics Tools
Eukaryotic-Promoter-Database (EPD) (https://epd.epfl.ch//index.php) was used to identify the promoter region of ACSF3. Genomatix MatInspector (http://www.genomatix.de/matinspector.html) and GeneCards (https://www.genecards.org/) were used to determine the most common TFs that may influence the expression of ACSF3 gene. Moreover, Genomatix MatInspector was used to identify TF binding sites (TFBSs) of the TF (SP1). MethPrimer online software (http://www.urogene.org/methprimer/) was used to predict CpG islands in the promoter region of ACSF3.
Statistical Analysis
IBM SPSS Statistics for Windows, version 23 (IBM SPSS, IBM Corp., Armonk, NY, USA) was used to analyze the correlation between the expression level of ACSF3 and its TF (SP1) in ASD. The correlation between the two variables was determined using Pearson’s test and P values of < 0.05 were considered as significant.
Results
Prediction of CpG Island in the Promoter Region of ACSF3
MethPrimer online software (http://www.urogene.org/methprimer/) was used to predict CpG islands. One CpG island in the ACSF3 promoter was detected with size of 350 bp (196–545) shown in Figure 1.
 |
Figure 1 The predicted CpG Island in the promoter of ACSF3. The island appears in the light blue region. |
Identification of TFBSs of ACSF3
Genomatix MatInspector (http://www.genomatix.de/matinspector.html) was used to identify TFBSs found in the promoter region of ACSF3. The 600 bp of ACSF3 promoter was uploaded in MatInspector (Genomatix software) (April 2019). Numerous TFs families were found in ACSF3 promoter. According to GeneCards, one of the potential TFs that binds to ACSF3 promoter is SP1. The sequence of TFBS: gggacGGGCgggacagg.
Gene Expression Results of ACSF3 and Sp1
RT-qPCR was used to quantify the expression level of ACSF3 and SP1 and ACTB used as a reference gene to normalize the expression of target genes. The results showed that the expression level of ACSF3 was upregulated in 9 out of 19 ASD patients with fold change ranged from 1.3 and 34.6 while downregulated in 8 samples (fold change from −1.2 to −2.2, see Table 2. The analysis also showed thatSP1 was upregulated in 8 out of 19 ASD patients as fold change ranged between 1.5 and 245.1 while downregulated in 11 samples, see Table 2.
 |
Table 2 ACSF3 and Sp1 Expressions in ASD Children |
In order to assess whether the expression of ACSF3 and SP1 was corelated in ASD samples, a Pearson’s correlation was used to determine the relationship. The analysis indicated a significant correlation in the expression level between ACSF3 and SP1 (r-value = 0.454, P value = 0.05, Pearson’s correlation). The results is shown as scatter plot in Figure 2. This result suggests that SP1 may contribute to the regulation of ACSF3 expression.
 |
Figure 2 The positive correlation between expression of ACSF3 and transcription factor (SP1) by SPSS software. (r = 0.454, P = 0.05). |
MethyLight qPCR Results of ACSF3 Promoter Region
MethyLight technology was applied to detect the DNAm level in the promoter region of ACSF3 among autistics and controls. The methyLight PCR did not show any methylation pattern in all samples, indicating the absence of DNAm at the ACSF3 promoter. The amplification plot of methylated human bisulfite-converted DNA is shown in Figure 3, unmethylated human bisulfite-converted DNA (Figure 4), unconverted human genomic DNA (Figure 5). The specificity of the MethyLight PCR reaction was assessed by including control reactions to ensure that MethyLight PCR probes and primers specifically bind, also to exclude any false-negative results. The three DNA control templates were used in methylight qPCR to determine the accuracy and specify of primers and probes as follow: 100% methylated converted DNA (positive control), and 100% unmethylated converted DNA as well as 100% unmethylated unconverted DNA (negative controls). The amplification plots of positive control showed signals for both probes of two genes (ACSF3 and COL2A1) which indicated the probe’s specificity to detect only the methylated converted sequence. While the amplification plots of negative control (unmethylated converted DNA) showed signals for COL2A1 probe (reference) but no signals for ACSF3 probe because the reference probe does not have CpG sites. Furthermore, the amplification plots of negative control (unmethylated unconverted DNA) showed no signals for both probes which confirmed the conversion status of the sequence. These results of negative control confirmed that any negative reaction would not be a false negative result. However, the result of amplification plots of both probes showed no methylation in the ACSF3 at selected CpG sites for control and ASD samples (Figure 6).
 |
Figure 3 Five dilutions of positive control (methylated human bisulfite-converted DNA). |
 |
Figure 4 Negative control (unmethylated human bisulfite-converted DNA). |
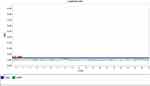 |
Figure 5 Unconverted human genomic DNA. |
 |
Figure 6 Converted DNA samples. (A) Sample (1–40). (B) Samples (41–42). |
Discussion
ASD is a serious condition related to a neurodevelopmental disorders that can cause behavioral challenges and impairment in communication and social interaction with others.22 According to Autism and Developmental Disabilities Monitoring (ADDM), the prevalence of ASD in the USA was higher than the previous estimation in 2014 (1 in 59), whereas in 2016 (1 in 54) among children aged 8 years old, where boys were four times likely to be affected than girls.23 In Saudi Arabia, the statistics of ASD in 2017 were around 50,714, whereas the actual prevalence has not been determined recently.7 The exact cause of ASD is still unclear. However, many studies suggested the implication of epigenetic mechanisms in ASD, specifically DNA methylation.24,25
This study aimed to increase the understanding of the biological role of ACSF3 promoter methylation in the binding ability of the TF by analyzing the correlation between the expression level of ACSF3 and its candidate TF (SP1) via using qPCR. Then, detecting and quantifying the methylation level of specific binding sites of SP1 in the promoter region of ACSF3 was analyzed by MethyLight qPCR. SP1 was chosen as a candidate TF because it has many recognitions binding sites in the promoter region of ACSF3 that are rich with CpG sites, thus DNAm may interfere with the binding of Sp1 to the promotor of ACSF3.
Our results indicated that transcriptional regulation of ACSF3 is associated with Sp1 in ASD. Thus, Sp1 could be essential to regulate transcription of ACSF3 and any dysregulation of SP1 is directly affect the ACSF3 expression. These variations in the expression levels of ACSF3 and SP1 in ASD cases could be related to the genetic pathway or environmental factors. In regarding to methylation analysis, the result revealed that the methylation of the CpG sites in ACSF3 does not affect the affinity of SP1 to bind and regulate the promoter of ACSF3 which is consistent with the finding of previous study which reported that the methylation level of CpG island does not affect the binding sites of Sp1.26 Furthermore, another possible explanation of this result might be related to multiple binding sites of SP1 within the promoter region and gene body. Supporting this assumption, Wang et al demonstrated that DNAm within ITPKA gene body modulates the binding of SP1 TF to the promoter region of ITPKA.27 Moreover, according to the study of Laubach et al, DNAm level that play role in expression of the ACSF3 gene was low in the body not in the promoter region of participants who have low socioeconomic status during the prenatal period.27
Despite the MethyLight qPCR result revealed no methylation pattern in the selected region of the promoter regions of ACSF3 in all autistic samples, there was a positive correlation between expression levels of ACSF3 and SP1 in the same samples of patients with ASD. This result is in the line with those of previous study which reported increasing in SP1 expression in autistic brains and the expression of related autistic candidate genes.28 Therefore, the dysregulation in the expression of ACSF3 and SP1 indited that these genes could have a role in ASD which correlated to specific symptom or mechanism of ASD.
Conclusions
This study did not confirm the role of DNAm on the binding site of SP1 within the ACSF3 promoter in autism Saudi children. The results partially substantiated the correlation between the expression of ACSF3 and SP1 in ASD Saudi children. The positive expression correlation between ACSF3 gene and its transcription factor (SP1) in all autistic samples pointed a potential role of SP1 as a target for ACSF3 in ASD. Furthermore, this correlation may be associated with certain mechanism or symptom of ASD etiology. However, studying the pathway mediated by the Sp1-ACSF3 could explain the complex phenotypes associated with autism.
In addition, although the methylation analysis technique that was used in this study was shown to be sequence-specific, it may not have been enough to cover all CpG sites in transcription binding sites of SP1 in ACSF3. Therefore, further studies are needed to analyze the potential interaction between ACSF3 and SP1 in ASD using advanced techniques and large samples to explore the pathophysiology of ASD.
Institutional Review Board Statement
The study was designed with correspondence to the codes of the guidelines for Ethics Committee of Biomedical Research-Centre of Excellence in Genomic Medicine Research at King Abdul Aziz University, ethical approval number (02-CEGMR-Bioeth-2018). The study was executed in consensus with the guidelines followed in King Fahd Center for Medical Research, KAU, Jeddah, Saudi Arabia, which were in accordance with declaration of Helsinki.
Informed Consent Statement
The informed consent was signed by boy’s parents for the publication of his case details.
Acknowledgment
Authors would like to acknowledge Center for Autism Research at King Faisal Specialist Hospital & Research Center (KFSH&RC) for supporting this study and the staff of Center of Excellence in Genomic Medicine Research specially Manal Shaabad and Lobna Mira for their assistance.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
This research was funded by a grant from the Center for Autism Research at King Faisal Specialist Hospital & Research Center (Grant No. CFAR/438/40).
Disclosure
The authors declare that they have no competing interests.
References
1. Dickerson AS, Pearson DA, Loveland KA, Rahbar MH, Filipek PA. Role of parental occupation in autism spectrum disorder diagnosis and severity. Res Autism Spectr Disord. 2014;8(9):997–1007. doi:10.1016/j.rasd.2014.05.007
2. Sharma SR, Gonda X, Tarazi FI. Autism spectrum disorder: classification, diagnosis and therapy. Pharmacol Ther. 2018;190:91–104. doi:10.1016/j.pharmthera.2018.05.007
3. Elsabbagh M, Divan G, Koh YJ, et al. Global prevalence of autism and other pervasive developmental disorders. Autism Res. 2012;5(3):160–179. doi:10.1002/aur.239
4. Weiss LA, Arking DE. A genome-wide linkage and association scan reveals novel loci for autism. Nature. 2009;461(7265):802–808. doi:10.1038/nature08490
5. Chaste P, Leboyer M. Autism risk factors: genes, environment, and gene-environment interactions. Dialogues Clin Neurosci. 2012;14(3):281.
6. Qiu S, Li Y, Li Y, et al. Association between SHANK3 polymorphisms and susceptibility to autism spectrum disorder. Gene. 2018;651:100–105. doi:10.1016/j.gene.2018.01.078
7. Alnemary FM, Aldhalaan HM, Simon-Cereijido G, Alnemary FM. Services for children with autism in the Kingdom of Saudi Arabia. Autism. 2017;21(5):592–602. doi:10.1177/1362361316664868
8. Liu ML, Su W, Wang JS, Yang YH, Yang H, Lin H. Predicting preference of transcription factors for methylated DNA using sequence information. Mol Ther Nucleic Acids. 2020;22:1043–1050. doi:10.1016/j.omtn.2020.07.035
9. Ziller MJ, Gu H, Müller F, et al. Charting a dynamic DNA methylation landscape of the human genome. Nature. 2013;500(7463):477–481. doi:10.1038/nature12433
10. Hu S, Wan J, Su Y, et al. DNA methylation presents distinct binding sites for human transcription factors. elife. 2013;2:e00726. doi:10.7554/eLife.00726
11. Yin Y, Morgunova E, Jolma A, et al. Impact of cytosine methylation on DNA binding specificities of human transcription factors. Science. 2017;356(6337). doi:10.1126/science.aaj2239.
12. Hamilton JP. Epigenetics: principles and practice. Dig Dis. 2011;29(2):130–135. doi:10.1159/000323874
13. Jin B, Li Y, Robertson KD. DNA methylation: superior or subordinate in the epigenetic hierarchy? Genes Cancer. 2011;2(6):607–617. doi:10.1177/1947601910393957
14. Tremblay MW, Jiang Y-H. DNA methylation and susceptibility to autism spectrum disorder. Annu Rev Med. 2019;70(1):151–166. doi:10.1146/annurev-med-120417-091431
15. Ghazanfari T, Tehrani GA, Maziri P. The relationship between the methylation of promoter regions of tumor suppressor genes PTEN and APC with endometrial cancer. Asian Pac J Cancer Prev. 2019;20(8):2259. doi:10.31557/APJCP.2019.20.8.2259
16. Hu Z, Ying X, Huang L, et al. Association of human serotonin receptor 4 promoter methylation with autism spectrum disorder. Medicine. 2020;99(4):e18838.
17. Bundo M, Ueda J, Nakachi Y, Kasai K, Kato T, Iwamoto K. Decreased DNA methylation at promoters and gene-specific neuronal hypermethylation in the prefrontal cortex of patients with bipolar disorder. Mol Psychiatry. 2021;26:3407–3418.
18. Aref-Eshghi E, Kerkhof J, Pedro VP, et al. Evaluation of DNA methylation episignatures for diagnosis and phenotype correlations in 42 Mendelian neurodevelopmental disorders. Am J Hum Genet. 2020;106(3):356–370. doi:10.1016/j.ajhg.2020.01.019
19. Bowman CE, Wolfgang MJ. Role of the malonyl-CoA synthetase ACSF3 in mitochondrial metabolism. Adv Biol Regul. 2019;71:34–40. doi:10.1016/j.jbior.2018.09.002
20. Sloan JL, Johnston JJ, Manoli I, et al. Exome sequencing identifies ACSF3 as a cause of combined malonic and methylmalonic aciduria. Nat Genet. 2011;43(9):883–886. doi:10.1038/ng.908
21. Maltby VE, Lea RA, Graves MC, et al. Genome-wide DNA methylation changes in CD19+ B cells from relapsing-remitting multiple sclerosis patients. Sci Rep. 2018;8(1):1–10. doi:10.1038/s41598-018-35603-0
22. Association AP. Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Publishing; 2013.
23. Maenner MJ, Shaw KA, Baio J. Prevalence of autism spectrum disorder among children aged 8 years—autism and developmental disabilities monitoring network, 11 sites, United States, 2016. MMWR Surveill Summ. 2020;69(4):1. doi:10.15585/mmwr.ss6904a1
24. Ladd-Acosta C, Hansen KD, Briem E, Fallin MD, Kaufmann WE, Feinberg AP. Common DNA methylation alterations in multiple brain regions in autism. Mol Psychiatry. 2014;19(8):862–871. doi:10.1038/mp.2013.114
25. Stathopoulos S, Gaujoux R, Lindeque Z, et al. DNA methylation associated with mitochondrial dysfunction in a South African autism spectrum disorder cohort. Autism Res. 2020;13(7):1079–1093.
26. Harrington MA, Jones PA, Imagawa M, et al. Cytosine methylation does not affect binding of transcription factor Sp1. Proc Natl Acad Sci U S A. 1988;85(7):2066–2070. doi:10.1073/pnas.85.7.2066
27. Wang Y-W, Ma X, Zhang Y-A, et al. ITPKA gene body methylation regulates gene expression and serves as an early diagnostic marker in lung and other cancers. J Thorac Oncol. 2016;11(9):1469–1481. doi:10.1016/j.jtho.2016.05.010
28. Hanseem I, Anitha A, Nakamura K, et al. Elevated transcription factor specificity protein 1 in autistic brains alters the expression of autism candidate genes. Biol Psychiatry. 2012;71(5):410–418. doi:10.1016/j.biopsych.2011.09.020
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
