Back to Journals » Infection and Drug Resistance » Volume 15
Diversity of glpK Gene and Its Effect on Drug Sensitivity in Mycobacterium bovis
Authors Dong Y , Ou X, Liu C, Fan W, Zhao Y, Zhou X
Received 11 November 2021
Accepted for publication 1 March 2022
Published 2 April 2022 Volume 2022:15 Pages 1467—1475
DOI https://doi.org/10.2147/IDR.S346724
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Yuhui Dong,1 Xichao Ou,2 Chunfa Liu,2 Weixing Fan,3 Yanlin Zhao,2 Xiangmei Zhou1
1Key Laboratory of Animal Epidemiology and Zoonosis, Ministry of Agriculture, National Animal Transmissible Spongiform Encephalopathy Laboratory, College of Veterinary Medicine, China Agricultural University, Beijing, 100193, People’s Republic of China; 2National Center for Tuberculosis Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing, 102206, People’s Republic of China; 3National Reference Laboratory for Animal Tuberculosis, China Animal Health and Epidemiology Center, Qingdao, 266032, People’s Republic of China
Correspondence: Xiangmei Zhou, Key Laboratory of Animal Epidemiology and Zoonosis, Ministry of Agriculture, National Animal Transmissible Spongiform Encephalopathy Laboratory, College of Veterinary Medicine, China Agricultural University, Beijing, 100193, People’s Republic of China, Email [email protected]
Background: Glycerol kinase (glpK) is essential for the first step of glycerol catabolism in Mycobacterium tuberculosis. However, Mycobacterium bovis has been known to grow poorly in glycerol media because of a base insertion in the glpK gene.
Methods: We analyzed the glpK gene sequences of 60 clinical M. bovis isolates, and determined the minimum inhibitory concentration of 14 drugs by microdilution method to evaluate the effect of frameshift mutations on drug sensitivity. The effect of M. bovis growth rate on its drug sensitivity was investigated using bacteria grown on glycerol or pyruvate.
Results: A total of 44 (73.33%) clinical M. bovis isolates have frameshift mutations in a homopolymeric tract of 7 cytosines in the glpK gene. 15.00% M. bovis isolates showed phenotypic drug resistance. Glycerol metabolism-deficient M. bovis showed reduced susceptibility to 9 out of 14 tested drugs. Mutations in the glpK gene can lead to impaired growth in glycerol-based media, while the minimal inhibitory concentration values of slow-growing M. bovis were higher.
Conclusion: Mutations in the glpK gene can lead to slowed growth and reduced susceptibility to drugs in M. bovis, which may contribute to the emergence of drug-resistant M. bovis and pose a threat to human health owing to the zoonotic capacity of M. bovis.
Keywords: Mycobacterium bovis, tuberculosis, glpK, drug resistance
Introduction
Tuberculosis (TB), a communicable disease, ranks as the second leading cause of death from a single infectious agent, after COVID-19 in 2020, with roughly a quarter of the world’s population infected with Mycobacterium tuberculosis.1 Since 1990, TB mortality has decreased; nevertheless, the rise of multidrug-resistant (MDR) and extremely drug-resistant (XDR) strains of M. tuberculosis represents a serious public health threat.1–3 Unlike drug-sensitive TB, which can be treated by 6 months of chemotherapy with the current four-drug frontline regimen, MDR-TB requires at least 18 to 24 months of therapy with four to six drugs, including fluoroquinolone and one injectable agent.2,4,5
The M. tuberculosis complex is a group of closely genetically related yet phenotypically diverse organisms.6 A range of in vitro characteristics can be used to distinguish the members of the complex; for example, unlike M. tuberculosis, M. bovis is unable to utilise glycerol as its sole carbon source.7 M. bovis primarily infects cattle, but can also infect a wide range of species, including humans.8 The genomes of M. bovis and M. tuberculosis are more than 99.95% identical, and the symptoms of human disease caused by M. bovis are similar to those of disease caused by M. tuberculosis.9,10 Treatment of disease caused by M. bovis usually includes rifampicin, isoniazid, and ethambutol.11 Due to the exclusion of pyrazinamide, since all strains of M. bovis are resistant to it, treatment duration is generally extended to 9 months.12 There have been some reports of infections caused by MDR M. bovis, including both sporadic cases and transcontinental outbreaks.13–17
The 2020 WHO global tuberculosis report estimated that there were 140,000 new human cases of zoonotic tuberculosis caused by M. bovis globally in 2019.3 The use of culture media such as glycerol, that inhibit M. bovis growth, has led to the number of human cases of M. bovis infection being underestimated and unreported.18–20 A frameshift mutant in the glpK gene that encodes the glycerol kinase enzyme of M. bovis causes the glycerol catabolic defect.7 Growing evidence suggests that frameshift mutations in a homopolymeric tract (HT) of 7 cytosines (7C) in the glpK gene lead to drug tolerance in M. tuberculosis.21,22
Here, we focus on the glpK frameshift mutations in the clinical isolates of M. bovis. We found that multigene mutation patterns in the 7C HT of the glpK gene in M. bovis and not all frameshift mutations caused glycerol kinase inactivation. Glycerol has a promotive effect on the growth of a part of M. bovis. Frameshift mutations that disrupt glycerol kinase activity contribute to an extensive reduction in antibiotic sensitivity in bovine tuberculosis.
Materials and Methods
Bacterial Strains and Culture Conditions
Clinical M. bovis strains are stored at the Chinese Center for Disease Control and Prevention. The clinical M. bovis isolates used in this study were isolated from a large cattle slaughterhouse in Xinjiang, China. Suspected M. bovis infected tissue samples were collected, and strain isolation and identification were all performed in the biosafety level 3 (BSL-3) laboratory. Farm animal welfare standards were met during transport and slaughter of the cattle. Transport drivers and escorts were trained in basic veterinary as well as animal welfare related knowledge. The slaughterhouse used humane slaughter, and the slaughterers were trained in animal welfare-related knowledge.
The clinical strains used in this study were isolated from slaughtered cattle. This study does not involve research studies on humans or animal experiments, so ethical approval for this study was not needed.
Unless otherwise stated, the M. bovis strains were cultivated at 37°C either in Middlebrook 7H9 broth (Difco) containing 0.05% Tween 80 or on Middlebrook 7H10 agar supplemented with 0.2% pyruvate, both enriched with 10% oleic acid-albumin-dextrose catalase (Difco). To test the growth of M. bovis in glycerol-based media, 0.2% glycerol was used instead of pyruvate.
The Minimal Inhibitory Concentration (MIC) Determination
MICs for clinical M. bovis strains were determined by the microdilution method, as described previously.23 Briefly, the first column of wells of a 96-well plate (Costar) was filled with 200 μL of 7H9 containing the drug at its maximum concentration to be tested. The remaining wells were filled with 100 μL 7H9 medium. This was used to perform 2-fold serial dilution of the drugs, Bedaquiline (BDQ; 0.0156 to 2 μg/mL), Amikacin (AMK; 0.25 to 32 μg/mL), Ethambutol (EMB; 0.125 to 16 μg/mL), Isoniazid (INH; 0.025 to 3.2 μg/mL), Levofloxacin (LEV; 0.0125 to 1.6 μg/mL), Moxifloxacin (MXF; 0.0625 to 8 μg/mL), Delamanid (DLM; 0.0156 to 2 μg/mL), Linezolid (LZD; 0.03125 to 4 μg/mL), Clofazimine (CFZ; 0.0625 to 8 μg/mL), Rifampin (RIF; 0.0625 to 8 μg/mL), Rifabutin (RFB; 0.0625 to 8 μg/mL), Para-aminosalicylic acid (PAS; 0.125 to 16 μg/mL), Ethionamide (ETH; 0.25 to 32 μg/mL), Kanamycin (KAN; 1 to 128 μg/mL). The plates were incubated at 37°C for 14 days and scored as either growth or no growth. The MIC was defined as the concentration at which no microbial growth was observed visually.
MIC for M. bovis C68004 was performed similarly as above, using 7H9 medium supplemented with 0.2% glycerol or pyruvate.
RNA Isolation and Real-Time Quantitative PCR
RNA was extracted as described previously.24 First-strand cDNA synthesis was performed by the HiScript III 1st Strand cDNA Synthesis Kit (Vazyme). Real-time quantitative PCR was performed on the CFX96 RealTime Thermal Cycler (Bio-Rad) with AceQ qPCR SYBR Green Master Mix (Vazyme). The thermal cycling conditions were initial denaturation for 5 min at 95°C, followed by 40 cycles of 95°C for 10s, 56°C for 30s and 72°C for 30s. Fluorescence measurements were recorded at each annealing step. A melting curve analysis was performed to ensure the specificity of the products.
Statistical Analysis
All assays were performed on 3 separate occasions. The results were expressed as means with standard errors of the mean. Statistical significance was determined by using Unpaired t-test (two-tailed).
Results
Multiple Mutation Patterns Exist in the glpK Gene of Clinical M. bovis
When glycerol is the sole carbon source, one of the key in vitro distinctions between M. bovis and M. tuberculosis is the requirement for pyruvate.25 M. bovis AF2122/97 has a single C insertion in the glpK 7C HT, causing a frameshift and leading to a truncated coding sequence.7 Noteworthy, in BCG, a 2 bp insertion in the glpK 8C HT of the M. bovis AF2122/97 glpK corrects the frameshift and results in an extra codon with respect to the M. tuberculosis glpK (Table 1). This extra codon was discovered in all M. bovis BCG strains tested (Pasteur, Tokyo, Danish, Russia, Tice, Frappier, Sweden), which can thrive solely on glycerol.6 Further analysis revealed that M. microti OV254, had the same frameshift mutation in glpK as M. bovis AF2122/97 (Table 1). M. microti, like M. bovis, requires the addition of pyruvate to glycerinated medium for growth.6
 |
Table 1 GlpK Slippage Site Sequences of Several Mycobacterial Species |
To explore the diversity of glpK, we sequenced 60 clinical isolates of M. bovis and grouped them according to glpK 7C HT (Table 2). GlpK frameshift mutations are common in M. bovis isolates. 40.00% of clinical M. bovis, like M. bovis AF2122/97, had glycerol kinase inactivation due to a single C insertion in the glpK 7C HT. Moreover, we detected 26.67% of clinical isolates with glpK 7C HT, the same as M. tuberculosis H37Rv. In addition, we detected 26.67% of glpK 9C HT isolates and 3.33% of glpK 10C HT and glpK 11C HT isolates (Table 2).
 |
Table 2 60 Clinical M. bovis Isolates Were Grouped According to the glpK Slippage Site Sequences |
Next, we evaluated the frequency of frameshift mutations in the glpK 7C HT in M. tuberculosis. We counted the genomic information on 2819 Mycobacterium tuberculosis strains in GMTV database26 and found that frameshift mutations in the glpK 7C HT are also present in M. tuberculosis, but at a lower frequency than in M. bovis (Table 3).
 |
Table 3 2816 Clinical M. tuberculosis Isolates Were Grouped According to the glpK Slippage Site Sequences |
Glycerol Catabolic Mutations are Associated with the Growth of M. bovis
Since the glpK gene encodes an enzyme involved in glycerol metabolism (Supplementary Figure 1), and early studies concluded that the growth of M. tuberculosis was strongly promoted by the addition of glycerol to the medium during in vitro culture,27,28 we monitored the growth curves of different M. bovis glpK HT strains. Similar to M. tuberculosis H37Rv, the growth of M. bovis glpK 7C HT strain was promoted in the glycerol-based medium compared to pyruvate (Figure 1A). In contrast, the M. bovis glpK 8C HT strain, representing the majority of M. bovis, grew slowly in the glycerol-based medium (Figure 1B). Furthermore, the M. bovis glpK 10C HT strain makes good use of glycerol, probably because this mutation only inserts an extra codon and does not cause extensive codon mismatches (Figure 1C).
To further verify the glycerol utilization ability of M. bovis glpK 10C HT strain, we next observed its growth in solid medium with glycerol or pyruvate as carbon sources. On the 12th day of incubation, lots of colonies were visible on 7H10 agar plates supplemented with 0.2% glycerol (Figure 1D), while a few colonies were visible on 7H10 agar plates supplemented with 0.2% pyruvate on the 14th day (Figure 1E).
M. bovis, an obligate aerobe, induces the expression of narX,29 narK2,30 dosR,31 hspX,32 and frdA33 in response to hypoxia. To measure the growth of the glpK 10C HT strain in both media, we monitored the transcript levels of these genes. Due to the fast growth rate in glycerol medium, the glpK 10C HT strain consumed oxygen more rapidly and the hypoxia-induced narX, narK2, dosR, hspX, and frdA expressions were elevated (Figure 1F).
glpK Frameshift Mutations Can Affect Anti-Tuberculosis Drug Sensitivity in M. bovis
To investigate the drug resistance of clinically popular M. bovis, we conducted drug susceptibility testing (DST) for 60 isolates from China. We detected 9 isolates (15% of total) showed phenotypic resistance to the tested drugs. Among these, 4 (6.67%) were resistant to delamanid, 4 (6.67%) were resistant to kanamycin, 2 (3.33%) were resistant to ethionamide, and 1 (1.67%) was resistant to isoniazid. Moreover, 2 (3.33%) isolates were resistant to two drugs at the same time (Table 4 and Supplementary Table 1). However, we did not find any known drug-resistance gene mutations in these phenotypic resistant isolates (Supplementary Table 2).
 |
Table 4 Qualitative Classification and MIC (Expressed in μg/mL, with Respective Cut-off Points) for DST in M. bovis Isolated from China |
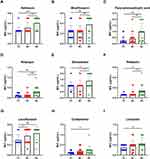
Frameshift mutations in glpK 7C HT leading to drug tolerance in Mycobacterium tuberculosis have been reported.21,22 We analyzed the MICs of different glpK HT M. bovis strains and found that glpK 9C HT strains showed lower drug sensitivity to 9/14 of the assessed drugs, including amikacin, moxifloxacin, para-aminosalicylic acid, rifampin, ethambutol, rifabutin, levofloxacin, clofazimine, and linezolid (Figure 2A–I). In addition, the levofloxacin sensitivity test showed a reduction in the sensitivity of glpK 8C HT strains (Figure 2G). However, we found no significant difference in the MIC level of five drugs, including isoniazid and bedaquiline (Supplementary Figure 2).
Since the MIC was measured in glycerol liquid medium, in which M. bovis glpK 8C HT strains and M. bovis glpK 9C HT strains grew slowly compared to M. bovis glpK 7C HT strains, next, we evaluated the effect of growth rate on drug susceptibility.
The Growth Rate of M. bovis Has an Impact on Drug Susceptibility
We used M. bovis C68004, a glpK 10C HT strain, and tested its drug MIC using glycerol or pyruvate-based medium. Among the 12 drugs tested, the MIC of 7 drugs in glycerol-based medium was lower than that in pyruvate-based medium (Table 5). This indicates that M. bovis C68004 is more sensitive to drugs due to its fast growth rate in glycerol-based medium.
 |
Table 5 MIC of M. bovis C68004 in Glycerol or Pyruvate-Based Medium MIC (μg/mL) |
Discussion
Unlike most bacterial pathogens, mycobacteria are capable of utilizing multiple carbon substrates.34,35 Early studies indicated that glycerol-fed M. tuberculosis grows faster and the bacilli reach a higher density than when metabolizing glucose or fatty acids, leading to the use of glycerol in virtually all standard mycobacterial growth media.27,36 However, due to frameshift mutation in a homopolymeric tract of 7 cytosines in the glpK gene, glycerol metabolism in M. bovis is defective and substituted it with pyruvate.7,25
Our sequence analysis of the glpK gene of clinical M. bovis revealed that 30% of M. bovis (glpK 7C HT strains and glpK 10C HT strains) can be subjected to the growth-promoting effect of glycerol. Therefore, we recommend testing for glpK gene status when performing M. bovis culture and selecting the appropriate medium.
M. bovis, the main cause of bovine tuberculosis (bTB), can cause major economic problems worldwide and can infect humans, posing a threat to public health.8 While the DSTs of M. tuberculosis isolated from human cases are generally assessed, research on antitubercular DSTs of M. bovis isolated from animals is limited.12 Our data showed that 15% of the M. bovis isolates are resistant to at least one drug. We detected phenotypic resistance to delamanid, kanamycin, ethionamide, and isoniazid in M. bovis, yet no drug-resistance gene mutations were found. In another study of drug resistance in M. bovis, a similar phenomenon was found. In Brazil, despite the government’s prohibition on the treatment of infected cattle, 31.3% of M. bovis showed resistance to the tested drugs, and 16% were classified as MDR M. bovis.18 Although it may be influenced by multiple factors, Mycobacteria sp. resistance to antimicrobials is often associated with mutations in target-encoding or related genes.35 However, the resistant strains were not subjected to sequencing of rpoB, katG, or the promoter region of inhA gene.18
The use of subtherapeutic doses of antibiotics as growth promoters, which is a commonly applied method to maximize yield in animal production, has been linked to the rise of antimicrobial resistance and cross-resistance.34 However, in many countries, first and second line antibiotics against TB are not approved for animal consumption.28 This further complicates the identification of the sources implicated in the acquisition of mutations conferring resistance to these drugs since multiple factors could participate in this process. Canonical mechanisms of resistance are generally grouped into three broad categories: target modification, drug inactivation, and drug transport.37–39 There is growing evidence that metabolic mutations also contribute to the evolution of bacterial drug resistance.39–41 Indeed, metabolic adaptation may represent a class of resistance mechanisms whereby, beyond conferring tolerance, cells alter their metabolic response to lessen antibiotic lethality’s downstream toxic effects.39
Our finding showed that fast-growing M. bovis is more sensitive to antibiotics. M. tuberculosis greatly reduces growth and metabolic activity in chronically infected animals.42–44 This means that mycobacteria that are sensitive to drugs in DSTs may not be effectively killed or limited by drugs in vivo, which undoubtedly promotes the emergence of drug-resistant strains. This is consistent with previous work in which virtually all antibiotics preferentially kill rapidly replicating bacteria.42,45,46
GlpK encodes glycerol kinase, which is involved in the first step of glycerol catabolism. However, most M. bovis is defective due to frameshift mutations in glpK 7C HT. The average sequence divergence between M. tuberculosis and M. bovis is less than 0.05%.47 In M. tuberculosis, similar frameshift mutations are present. Both in M. bovis and M. tuberculosis,21,22 glpK-deficient strains have lower drug sensitivity than glpK 7C HT strains. We hypothesize that the use of subtherapeutic doses of antibiotics as growth promoters in cattle production has allowed glpK-deficient strains to be screened, leading to the prevalence of clinical M. bovis glpK 8C HT strains and M. bovis glpK 9C HT strains. Glycerol assimilation can alter growth rate, metabolism, and cellular structure.21 We hypothesize that frameshift mutations that alter glycerol kinase activity may lead to decreased tricarboxylic acid cycle activity in favor of lipid anabolism. Increased lipid anabolism contributes to cell wall thickening, which reduces sensitivity to most anti-tuberculosis drugs.48 Furthermore, it has also been suggested that GlpK is a member of the ROK (repressor, open-reading frame, kinase) protein family; thus, GlpK (like other sugar kinases) may potentially act as a transcription regulator and is linked to the stress response.22
M. bovis TB is clinically, pathologically, and radiologically indistinguishable from M. tuberculosis.49 Moreover, there is growing evidence of the epidemic of MDR M. bovis, resulting in significant economic losses and threats to human health.13,14,28,50–52 Rifampicin, isoniazid, and ethambutol are used to treat disease due to M. bovis in humans. Treatment duration is generally extended to 9 months due to the exclusion of pyrazinamide, since all strains of M. bovis are resistant to it.11,12 Clinically prevalent M. bovis are mainly glpK 8C HT strains and glpK 9C HT strains with much lower susceptibility to drugs, which undoubtedly makes treatment more difficult.
In summary, we found that a proportion of M. bovis is subjected to the growth-promoting effect of glycerol, and fast-growing M. bovis is more sensitive to the drug. Frameshift mutations in M. bovis glpK HT are associated with lower drug sensitivity. Therefore, we need to thoroughly understand the relationship between bacterial metabolism and antibiotic function. Because M. bovis has the ability to infect both cattle and humans, the overuse of antibiotics in cattle can promote the development of drug resistance. Therefore, the use of antibiotics should be reduced to prevent human health threats from drug-resistant M. bovis.
Conclusion
In clinical M. bovis isolates, frameshift mutations in a homopolymeric tract of 7 cytosines in the glpK gene are prevalent, leading to slowed growth and reduced susceptibility to drugs in M. bovis, which may contribute to the emergence of drug-resistant M. bovis and pose a threat to human health owing to the zoonotic capacity of M. bovis.
Funding
This work was supported by the National Natural Science Foundation of China [32172800, 31873005].
Disclosure
The authors report no conflicts of interest in this work.
References
1. World Health Organization. Global Tuberculosis Report 2021. Geneva: World Health Organization; 2021.
2. Bald D, Villellas C, Lu P, Koul A. Targeting energy metabolism in Mycobacterium tuberculosis, a new paradigm in antimycobacterial drug discovery. mBio. 2017;8(2). doi:10.1128/mBio.00272-17
3. Harding E. WHO global progress report on tuberculosis elimination. Lancet Resp Med. 2020;8(1):E3. doi:10.1016/S2213-2600(19)30421-7
4. Phillips L. Infectious disease: TB’s revenge. Nature. 2013;493(7430):14–16. doi:10.1038/493014a
5. Dartois V. The path of anti-tuberculosis drugs: from blood to lesions to mycobacterial cells. Nat Rev Microbiol. 2014;12(3):159–167. doi:10.1038/nrmicro3200
6. Keating LA, Wheeler PR, Mansoor H, et al. The pyruvate requirement of some members of the Mycobacterium tuberculosis complex is due to an inactive pyruvate kinase: implications for in vivo growth. Mol Microbiol. 2005;56(1):163–174. doi:10.1111/j.1365-2958.2005.04524.x
7. Garnier T, Eiglmeier K, Camus JC, et al. The complete genome sequence of Mycobacterium bovis. Proc Natl Acad Sci USA. 2003;100(13):7877–7882. doi:10.1073/pnas.1130426100
8. Grange JM. Mycobacterium bovis infection in human beings. Tuberculosis. 2001;81(1–2):71–77. doi:10.1054/tube.2000.0263
9. Stermann M, Sedlacek L, Maass S, Bange FC. A promoter mutation causes differential nitrate reductase activity of Mycobacterium tuberculosis and Mycobacterium bovis. J Bacteriol. 2004;186(9):2856–2861. doi:10.1128/JB.186.9.2856-2861.2004
10. Michel AL, Muller B, van Helden PD. Mycobacterium bovis at the animal-human interface: a problem, or not? Vet Microbiol. 2010;140(3–4):371–381. doi:10.1016/j.vetmic.2009.08.029
11. Blumberg HM, Burman WJ, Chaisson RE, et al. American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: treatment of tuberculosis. Am J Respir Crit Care Med. 2003;167(4):603–662. doi:10.1164/rccm.167.4.603
12. Lan ZY, Bastos M, Menzies D. Treatment of human disease due to Mycobacterium bovis: a systematic review. Eur Respir J. 2016;48(5):1500–1503. doi:10.1183/13993003.00629-2016
13. Schultsz C, Kuijper EJ, vanSoolingen D, Prins JM. Disseminated infection due to multidrug-resistant Mycobacterium bovis in a patient who was seropositive for human immunodeficiency virus. Clin Infect Dis. 1996;23(4):841–843. doi:10.1093/clinids/23.4.841
14. Palenque E, Villena V, Rebollo J, Jimenez S, Samper S. Transmission of multidrug-resistant Mycobacterium bovis to an immunocompetent patient. Clin Infect Dis. 1998;26(4):995–996. doi:10.1086/517645
15. Bobadilla-del Valle M, Torres-Gonzalez P, Cervera-Hernandez ME, et al. Trends of Mycobacterium bovis isolation and first-line anti-tuberculosis drug susceptibility profile: a fifteen-year laboratory-based surveillance. PLoS Negl Trop Dis. 2015;9(9):e0004124. doi:10.1371/journal.pntd.0004124
16. Vazquez-Chacon CA, Martinez-Guarneros A, Couvin D, et al. Human multidrug-resistant Mycobacterium bovis infection in Mexico. Tuberculosis. 2015;95(6):802–809. doi:10.1016/j.tube.2015.07.010
17. Khattak I, Mushtaq MH, Ayaz S, et al. Incidence and drug resistance of zoonotic Mycobacterium bovis infection in Peshawar, Pakistan. Adv Microbiol Infect Dis Public Health. 2018;1057:111–126. doi:10.1007/5584_2018_170
18. Franco MMJ, Ribeiro MG, Pavan FR, et al. Genotyping and rifampicin and isoniazid resistance in Mycobacterium bovis strains isolated from the lymph nodes of slaughtered cattle. Tuberculosis. 2017;104:30–37. doi:10.1016/j.tube.2017.02.006
19. Valle MBD, Torres-Gonzalez P, Cervera-Hernandez ME, et al. Trends of Mycobacterium bovis isolation and first-line anti-tuberculosis drug susceptibility profile: a fifteen-year laboratory-based surveillance. PLoS Negl Trop Dis. 2015;9(9). doi:10.1371/journal.pntd.0004124
20. Kaneene JB, Kaplan B, Steele JH, Thoen CO. One health approach for preventing and controlling tuberculosis in animals and humans. Zoonotic Tuberculosis. 2014;9–20. doi:10.1002/9781118474310
21. Bellerose MM, Baek SH, Huang CC, et al. Common variants in the glycerol kinase gene reduce tuberculosis drug efficacy. mBio. 2019;10(4). doi:10.1128/mBio.00663-19
22. Safi H, Gopal P, Lingaraju S, et al. Phase variation in Mycobacterium tuberculosis glpK produces transiently heritable drug tolerance. Proc Natl Acad Sci USA. 2019;116(39):19665–19674. doi:10.1073/pnas.1907631116
23. Wiegand I, Hilpert K, Hancock REW. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat Protoc. 2008;3(2):163–175. doi:10.1038/nprot.2007.521
24. Sherman DR, Voskuil M, Schnappinger D, Liao RL, Harrell MI, Schoolnik GK. Regulation of the Mycobacterium tuberculosis hypoxic response gene encoding alpha-crystallin (vol 98, pg 7534, 2001). Proc Natl Acad Sci USA. 2001;98(26):15393.
25. Kubica GP, Wayne LG. The Mycobacteria: A Sourcebook. Marcel Dekker Incorporated; 1984.
26. Chernyaeva EN, Shulgina MV, Rotkevich MS, et al. Genome-wide Mycobacterium tuberculosis variation (GMTV) database: a new tool for integrating sequence variations and epidemiology. BMC Genom. 2014;15(1):308. doi:10.1186/1471-2164-15-308
27. Dubos RJ, Middlebrook G. Media for Tubercle Bacilli. Am Rev Tuberc Pulm. 1947;56(4):334–345.
28. Vazquez-Chacon CA, Rodriguez-Gaxiola FD, Lopez-Carrera CF, et al. Identification of drug resistance mutations among Mycobacterium bovis lineages in the Americas. PLoS Negl Trop Dis. 2021;15(2):e0009145. doi:10.1371/journal.pntd.0009145
29. Hutter B, Dick T. Up-regulation of narX, encoding a putative ‘fused nitrate reductase’ in anaerobic dormant Mycobacterium bovis BCG. FEMS Microbiol Lett. 1999;178(1):63–69. doi:10.1111/j.1574-6968.1999.tb13760.x
30. Giffin MM, Raab RW, Morganstern M, Sohaskey CD. Mutational analysis of the respiratory nitrate transporter NarK2 of Mycobacterium tuberculosis. PLoS One. 2012;7(9):e45459. doi:10.1371/journal.pone.0045459
31. Park HD, Guinn KM, Harrell MI, et al. Rv3133c/dosR is a transcription factor that mediates the hypoxic response of Mycobacterium tuberculosis. Mol Microbiol. 2003;48(3):833–843. doi:10.1046/j.1365-2958.2003.03474.x
32. Yuan Y, Crane DD, Simpson RM, et al. The 16-kDa alpha-crystallin (Acr) protein of Mycobacterium tuberculosis is required for growth in macrophages. Proc Natl Acad Sci USA. 1998;95(16):9578–9583. doi:10.1073/pnas.95.16.9578
33. Watanabe S, Zimmermann M, Goodwin MB, Sauer U, Barry CE, Boshoff HI. Fumarate reductase activity maintains an energized membrane in anaerobic Mycobacterium tuberculosis. PLoS Pathog. 2011;7(10):e1002287. doi:10.1371/journal.ppat.1002287
34. Evangelista AG, Correa JAF, Pinto AC, Luciano FB. The impact of essential oils on antibiotic use in animal production regarding antimicrobial resistance - a review. Crit Rev Food Sci Nutr. 2021;1–17. doi:10.1080/10408398.2021.1883548
35. Koch A, Mizrahi V, Warner DF. The impact of drug resistance on Mycobacterium tuberculosis physiology: what can we learn from rifampicin? Emerg Microbes Infect. 2014;3(1):1–11. doi:10.1038/emi.2014.17
36. Edson NL. The intermediary metabolism of the mycobacteria. Bacteriol Rev. 1951;15(3):147–182. doi:10.1128/Mmbr.15.3.147-182.1951
37. Woodford N, Ellington MJ. The emergence of antibiotic resistance by mutation. Clin Microbiol Infect. 2007;13(1):5–18. doi:10.1111/j.1469-0691.2006.01492.x
38. Blair JM, Webber MA, Baylay AJ, Ogbolu DO, Piddock LJ. Molecular mechanisms of antibiotic resistance. Nat Rev Microbiol. 2015;13(1):42–51. doi:10.1038/nrmicro3380
39. Lopatkin AJ, Bening SC, Manson AL, et al. Clinically relevant mutations in core metabolic genes confer antibiotic resistance. Science. 2021;371(6531):6531. doi:10.1126/science.aba0862
40. Zampieri M, Zimmermann M, Claassen M, Sauer U. Nontargeted metabolomics reveals the multilevel response to antibiotic perturbations. Cell Rep. 2017;19(6):1214–1228. doi:10.1016/j.celrep.2017.04.002
41. Zampieri M, Enke T, Chubukov V, Ricci V, Piddock L, Sauer U. Metabolic constraints on the evolution of antibiotic resistance. Mol Syst Biol. 2017;13(3):917. doi:10.15252/msb.20167028
42. Baek SH, Li AH, Sassetti CM. Metabolic regulation of mycobacterial growth and antibiotic sensitivity. PLoS Biol. 2011;9(5):e1001065. doi:10.1371/journal.pbio.1001065
43. Munoz-Elias EJ, Timm J, Botha T, Chan WT, Gomez JE, McKinney JD. Replication dynamics of Mycobacterium tuberculosis in chronically infected mice. Infect Immun. 2005;73(1):546–551. doi:10.1128/IAI.73.1.546-551.2005
44. Gill WP, Harik NS, Whiddon MR, Liao RP, Mittler JE, Sherman DR. A replication clock for Mycobacterium tuberculosis. Nat Med. 2009;15(2):211–214. doi:10.1038/nm.1915
45. Tomasz A, Albino A, Zanati E. Multiple antibiotic resistance in a bacterium with suppressed autolytic system. Nature. 1970;227(5254):138–140. doi:10.1038/227138a0
46. Gomez JE, McKinney JD. M. tuberculosis persistence, latency, and drug tolerance. Tuberculosis. 2004;84(1–2):29–44. doi:10.1016/j.tube.2003.08.003
47. Smith NH, Gordon SV, de la Rua-domenech R, Clifton-Hadley RS, Hewinson RG. Bottlenecks and broomsticks: the molecular evolution of Mycobacterium bovis. Nat Rev Microbiol. 2006;4(9):670–681. doi:10.1038/nrmicro1472
48. Goossens SN, Sampson SL, Van Rie A. Mechanisms of drug-induced tolerance in Mycobacterium tuberculosis. Clin Microbiol Rev. 2020;34(1). doi:10.1128/CMR.00141-20
49. Tazerart F, Saad J, Niar A, Sahraoui N, Drancourt M. Mycobacterium bovis pulmonary tuberculosis, Algeria. Emerg Infect Dis. 2021;27(3):972–974. doi:10.3201/eid2703.191823
50. Rivero A, Marquez M, Santos J, et al. High rate of tuberculosis reinfection during a nosocomial outbreak of multidrug-resistant tuberculosis caused by Mycobacterium bovis strain B. Clin Infect Dis. 2001;32(1):159–161. doi:10.1086/317547
51. Esteban J, Robles P, Jimenez MS, Guerrero MLF. Pleuropulmonary infections caused by Mycobacterium bovis: a re-emerging disease. Clin Microbiol Infect. 2005;11(10):840–843. doi:10.1111/j.1469-0691.2005.01225.x
52. Long R, Nobert E, Chomyc S, et al. Transcontinental spread of multidrug-resistant Mycobacterium bovis. Am J Respir Crit Care Med. 1999;159(6):2014–2017. doi:10.1164/ajrccm.159.6.9809076
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.