Back to Journals » Vascular Health and Risk Management » Volume 17
Distribution Pattern of Atherosclerosis in the Abdomen and Lower Extremities and Its Association with Clinical and Hematological Factors
Authors Park JK , Jung WB, Yoon JH
Received 16 October 2020
Accepted for publication 31 December 2020
Published 14 January 2021 Volume 2021:17 Pages 13—21
DOI https://doi.org/10.2147/VHRM.S287194
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Harry Struijker-Boudier
Jong Kwon Park,1 Won Beom Jung,1 Jung-Hee Yoon2
1Department of Surgery, Haeundae Paik Hospital, College of Medicine, Inje University, Busan, Republic of Korea; 2Department of Radiology, Haeundae Paik Hospital, College of Medicine, Inje University, Busan, Republic of Korea
Correspondence: Jong Kwon Park Email [email protected]
Purpose: Abdominal arteries differ from the arteries located at the extremities in histological composition and clinical features. This study investigated the distributional pattern of atherosclerosis in arteries of the abdomen and lower extremities and its association with clinical and hematologic factors.
Patients and Methods: This retrospective study included 227 patients with atherosclerosis who underwent computed tomography angiography (CTA) of the abdomen and lower extremities. The distributional pattern of atherosclerosis was categorized into type 1 (suprainguinal elastic), type 2 (infrainguinal muscular), and type 3 (both arterial involvement). Chi-square tests, Mann–Whitney U-tests, and logistic regression analysis were used to investigate the data.
Results: Of the 227 patients, 132 (58%) had type 1 and 95 (42%) had type 3 atherosclerosis. None had type 2. Older age, heavier smoking, and higher levels of HbA1c and homocysteine were the significant risk factors for type 3 atherosclerosis (odds ratio: 1.076, 1.023, 1.426, and 1.130, respectively). Patients with type 3 showed significantly lower right and left ankle and toe brachial indices compared to type 1 (P: 0.029, 0.023, 0.003, and < 0.001, respectively).
Conclusion: In arteries of the abdomen and lower extremities, atherosclerosis may occur initially at suprainguinal elastic arteries. In addition, the significant risk factors for type 3 atherosclerosis may contribute to the development of atherosclerosis at infrainguinal muscular arteries and deteriorate the peripheral arterial circulation. Therefore, if atherosclerotic lesions are found at the suprainguinal elastic arteries on CTA, to prevent atherosclerosis at infrainguinal muscular arteries and subsequent peripheral arterial ischemic disease, cessation of smoking and control of blood glucose and homocysteine may be recommended, especially in elderly patients.
Keywords: atherosclerosis, elastic arteries, muscular arteries, risk factors
Introduction
Although individual risk factors for atherosclerosis are still being elucidated, aging, diabetes mellitus, a history of smoking, hyperlipidemia, hypertension, and a family history of peripheral arterial disease or antiphospholipid syndrome are well-known factors for an increased risk.1–3 In addition, it is known that aging progressively deteriorates elastin through proteolytic degradation and mechanical fatigue fracture4–6 and the subsequent elastin-derived peptides (EDPs), released by elastin degradation, are known to accelerate atherosclerosis.6 The ratio of elastin to collagen decreases with increasing distance from the heart, while the relative number of vascular smooth muscle cells (VSMCs) increases, continuing to the distal end of the leg arteries.7,8 Thus, arteries can be classified into two main types, elastic and muscular arteries, of which aorta and femoral arteries are typical examples, respectively.9
Computed tomography angiography (CTA) is a useful method to reveal the overall distribution of atherosclerotic plaque and calcification throughout the body and is frequently used for the diagnosis of arterial diseases. In the present study, we aimed to investigate the distribution pattern of atherosclerosis between the suprainguinal elastic and infrainguinal muscular arteries of the abdomen and lower extremities via CTA and its association with concurrent clinical and hematologic factors.
Patients and Methods
Patients and Computed Tomography Angiography
Between January 1, 2018, and July 31, 2019, 289 consecutive patients with pain or discomfort in the lower extremities who opted for a vascular health screening exam underwent CTA of the abdomen and lower extremities, hematology testing, and clinical examination in the division of vascular surgery at our hospital. Male and female patients with atherosclerosis in the arteries of the abdomen and lower extremities were eligible for the study.
The CTA images (Definition AS+, Siemens Healthcare, Forchheim, Germany) of the peripheral arteries were acquired during continuous infusion of a contrast agent (2mL/kg of Ultravist 300, Bayer Health Care, Wayne, NJ, USA) and the acquisition was initiated by bolus tracking software (DynEVA software, Siemens Medical Solution, Forchheim, Germany). The scan acquisition was started when the density within the vascular lumen reached more than 150 Hounsfield units (HU) at a region of interest in the abdominal aorta at the level of the celiac origin. The contrast agent was injected at 4~5 mL/s via a large bore (22 G) intravenous cannula. Images were acquired in a cranial to caudal direction with a slice thickness of 1 mm or less and collimator thickness of 0.4–0.6 mm. The 3D dataset was reconstructed with multiplanar reformatted (MPR), maximum intensity projection (MIP), and volume-rendered (VR) algorithms.
Coronal MPR and axial images were reconstructed automatically immediately following the CTA image acquisition. Image data was then transferred to dedicated post-processing software where specific MIP and VR images were created. The presence of any atherosclerotic plaques or calcifications in the arteries was diagnosed as atherosclerosis, regardless of the size of the lesions or the hemodynamic status of the arteries. To minimize artifacts by dense calcifications or calcific plaque, a window level of 200 HU and width of 1000 HU were used. Both the reconstruction and source images were analyzed to improve the detection of calcified arteries.
There was no case of exclusion due to poor imaging quality. Among the 289 patients, 62 with normal arteries were excluded from the study. The remaining 227 patients with atherosclerosis were included. Of the 227 patients, 22 underwent interventional or surgical treatment, including 14 endovascular interventions, 6 bypass surgeries, and 2 hybrid procedures including both endovascular interventions and bypass surgery. The remaining 205 patients were treated conservatively.
Demographic and Clinical Data
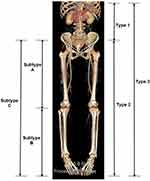
The demographic and clinical factors included in this study were age, sex, hypertension, diabetes mellitus, smoking status, and alcohol consumption. Based on the ratio of elastin and VSMCs in the arterial wall, arteries of the abdomen and lower extremities were categorized into suprainguinal elastic and infrainguinal muscular arteries.7–9 The arteries at or above the inguinal ligament, except the splanchnic arteries, were defined as suprainguinal elastic arteries, and those below the inguinal ligament were defined as infrainguinal muscular arteries. In the arteries of the abdomen and lower extremities, atherosclerosis was classified into 3 types according to the location of the atherosclerotic lesions. Specifically, type 1 atherosclerosis was defined by atherosclerotic lesions located within the suprainguinal elastic arteries, type 2 was defined by lesions within the infrainguinal muscular arteries, and type 3 was characterized by lesions within both the suprainguinal elastic and infrainguinal muscular arteries. In type 3 atherosclerosis, the atherosclerosis of the infrainguinal muscular arteries was classified into 3 subtypes without considering the suprainguinal elastic arteries. Subtype A was defined by the atherosclerotic lesions located within the above-knee arteries, subtype B was defined by the lesions within the below-knee arteries, and subtype C was characterized by the lesions within both the above-knee and below-knee arteries (Figure 1). Smoking was calculated by the number of packs smoked per day multiplied by the years as a smoker. The days of alcohol consumption were calculated by the following formula: (average number of days of alcohol consumption/1 month) × (12 months) × (number of years of alcohol consumption). For the evaluation of peripheral arterial circulation in the lower extremities, right and left ankle brachial indices (ABIs) and toe brachial indices (TBIs) were measured.
Hematologic Data
Blood tests were carried out as previously described.10 All diagnostic tests were performed according to the manufacturers’ instructions. Serum total cholesterol, high-density lipoprotein cholesterol (HDL), and low-density lipoprotein cholesterol (LDL) were measured using the appropriate enzyme kits (Cholesterol Gen.2, HDL-Cholesterol Gen.4, and LDL- Cholesterol Gen.3, respectively [Roche Diagnostics, Mannheim, Germany]) and the Roche/Hitachi Cobas C 701 analyzer (Roche Diagnostics, Mannheim, Germany). Serum homocysteine was measured using an enzymatic assay (Homocysteine Enzymatic Assay, Roche Diagnostics, Mannheim, Germany) and the Roche/Hitachi Cobas C 501 analyzer (Roche Diagnostics, Mannheim, Germany). Protein C, protein S, antithrombin III, factor VIII, fibrinogen, and lupus anticoagulant levels were measured using a Sysmex CA-7000 System (Sysmex, Kobe, Japan). All reagents were purchased from Siemens Healthcare Diagnostics Products GmbH (Marburg, Germany) and used according to the manufacturer’s instructions. Serum concentrations of IgG and IgM antibodies to β2 glycoprotein I were measured using Triturus (Grifols, Marina, Spain) with the REAADS IgG Anti-B2GPI test kit and the REAADS IgM Anti-B2GPI test kit (Corgenix, Inc., Colorado, USA), which are enzyme-linked immunosorbent assays (ELISA) for the semiquantitative determination of anti-β2 glycoprotein I antibodies in human serum. With serum samples, anticardiolipin antibody IgG and anticardiolipin antibody IgM were evaluated by ELISA using an autoimmune anticardiolipin (IgG/IgM) test kit (Bio-Rad Laboratories, Hercules, CA, USA). Results are expressed in IgG phospholipid (GPL) units for IgG anticardiolipin antibodies and IgM phospholipid (MPL) units for IgM anticardiolipin antibodies, with 1 GPL or MPL unit equivalent to 1 mg/mL of an affinity-purified standard IgG or IgM anticardiolipin antibody sample, respectively.
Statistical Analysis
The nominal variables are summarized and presented as the number of patients per total number of patients and the percentage. The continuous variables are summarized and presented as mean ± standard deviation. The nominal variables, including sex, hypertension, and diabetes mellitus, were compared using the chi-square test. The continuous variables, including age, body mass index (BMI), smoking, alcohol consumption, and the hematologic factors were compared using the nonparametric Mann–Whitney U-test, because not all the continuous variables revealed normal distribution by Kolmogorov–Smirnov test. Finally, the difference between type 1 and type 3 atherosclerosis was analyzed via a multivariate analysis using binary logistic regression analysis with a forward stepwise method. For the analysis, the dependent variable was the type of atherosclerosis (type 1 was designated number 1, and type 3 was designated number 2). All nominal and continuous variables, including age, sex, hypertension, diabetes, BMI, smoking, alcohol consumption, anti-β2 glycoprotein I antibody IgG, anti-β2 glycoprotein I antibody IgM, anticardiolipin antibody IgG, anticardiolipin antibody IgM, lupus anticoagulant, protein C, protein S, fibrinogen, antithrombin III, factor 8, HbA1c, total cholesterol, HDL cholesterol, LDL cholesterol, triglyceride and homocysteine, were included as independent variables. Right and left ABIs and TBIs were compared using the Mann–Whitney U-test as they did not reveal normal distribution. Significance was defined as P <0.05. Statistical analyses were performed using PASW Statistics 18 release 18.0.0 (WinWrap Basic, Polar Engineering and Consulting, USA).
Results
Type and Subtype of Atherosclerosis
Of the 227 patients with atherosclerosis, there were 132 (58%) type 1 and 95 (42%) type 3 cases. None was diagnosed with type 2 atherosclerosis (Table 1). Of the 95 patients with type 3 atherosclerosis, there were 34 (36%) subtype A, 7 (7%) subtype B, and 54 (57%) subtype C patients (Table 2).
 |
Table 1 Three Types of Atherosclerosis in 227 Patients |
 |
Table 2 Three Subtypes of Atherosclerosis in the Infrainguinal Arteries of the 95 Patients with Type 3 Atherosclerosis without Considering the Suprainguinal Arteries |
Comparison of Nominal Variables
Male, hypertensive, and diabetic patients showed significantly higher rates of type 3 atherosclerosis compared to type 1 (OR [95% CI]: 0.258 [0.148–0.451], 2.714 [1.571–4.688] and 2.269 [1.253–4.108], respectively) (Table 3).
 |
Table 3 Comparison of Nominal Variables Between Type 1 and 3 Atherosclerosis Patients |
Comparison of Continuous Variables
Patients with type 3 atherosclerosis were significantly older, smoked more cigarettes, had consumed alcohol longer, had higher levels of lupus anticoagulants, HbA1c, and homocysteine, and showed significantly lower levels of total, HDL and LDL cholesterols than those with type 1 atherosclerosis (P: <0.001, <0.001, 0.004, 0.025, 0.001, <0.001, 0.005, 0.001 and 0.006, respectively) (Table 4).
 |
Table 4 Comparison of Continuous Variables Between Type 1 and 3 Atherosclerosis Patients |
Multivariate Analysis
Binary logistic regression analysis with forward stepwise method revealed that older age, smoking more cigarettes, and higher blood levels of HbA1c and homocysteine were significant risk factors for type 3 atherosclerosis (OR [95% CI]:1.076 [1.039–1.114], 1.023 [1.008–1.038], 1.426 [1.002–2.031] and 1.130 [1.036–1.233], respectively) (Table 5).
 |
Table 5 Multivariate Analysis Between Type 1 and 3 Atherosclerosis Patients and Risk Factors for Type 3 Atherosclerosis |
Peripheral Blood Circulation in the Lower Extremities
Comparing ABIs and TBIs between patients with type 1 and type 3 atherosclerosis, patients with type 3 atherosclerosis showed significantly lower right and left ABI and TBI scores than those with type 1 (P: 0.029, 0.023, 0.003 and <0.001, respectively) (Table 6).
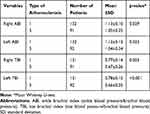 |
Table 6 Comparison of ABI and TBI Scores Between Type 1 and 3 Atherosclerosis Patients |
Discussion
In our study, there were many patients with isolated atherosclerosis at the suprainguinal elastic arteries (type 1 atherosclerosis), but none of the patients displayed isolated atherosclerosis at the infrainguinal muscular arteries (type 2 atherosclerosis). The atherosclerosis of the infrainguinal muscular arteries existed only as part of type 3 atherosclerosis. Meanwhile, in 95 patients with type 3 atherosclerosis, the percentage of below-knee atherosclerosis (subtype B) was only 7%, while that of above-knee atherosclerosis (subtype A) was 36%. This finding suggests that even within the infrainguinal muscular arteries of patients with type 3, atherosclerosis may occur more frequently at the proximal arteries, which contain a relatively higher level of elastin than the distal arteries.
Thus, we hypothesize that in arteries of the abdomen and lower extremities, atherosclerosis may occur initially at the suprainguinal arteries where there is higher elastin content, and then progresses to the infrainguinal arteries which have lower elastin content in certain physiological or pathological conditions. Meantime, contrary to our results, in a study of 286 patients with critical limb ischemia (CLI), there were 43 (15%) patients with isolated infrapopliteal diseases.11 However, regarding the suprapopliteal arteries of the aforementioned 43 patients, there was no clear description about the presence or absence of small atherosclerotic plaques or calcifications unrelated to hemodynamic abnormalities. There have been other studies reporting isolated tibial disease in 36% of CLI patients, and isolated popliteal and infrapopliteal disease in 49.5% of diabetic foot ulcer patients.12,13 However, these studies examined iliac or extremity arteries only, omitting the abdominal aorta from their investigations. In our study, we investigated both the suprainguinal elastic and infrainguinal muscular arteries simultaneously and diagnosis of atherosclerosis was confirmed by the presence of any atherosclerotic plaques or calcifications on the arteries, no matter how small the lesions were and regardless of the hemodynamic status of the arteries. We therefore believe that our study presents a novel finding, as it is a clinical and anatomic study about the distribution pattern of atherosclerosis between the elastic and muscular arteries in the abdomen and lower extremities, regardless of the size of the atherosclerotic lesions and the hemodynamic status.
It is known that VSMCs can switch between a contractile and synthetic phenotype in response to physiological and pathological stimuli and that the transition of the arterial SMC phenotype from a contractile to a synthetic state is a mechanism in the pathogenesis of atherosclerosis.14,15 In this study, aging, heavier smoking, and higher blood levels of HbA1c and homocysteine were significant risk factors for type 3 atherosclerosis in which both the suprainguinal elastic and infrainguinal muscular arteries were involved. Thus, we speculate that these risk factors may be associated with the development of atherosclerosis at the infrainguinal muscular arteries via the phenotypic switch of arterial SMCs from a contractile to a synthetic state.
It has been reported that senescent human SMCs are not bystanders in atherosclerotic plaque progression but are involved through a proinflammatory senescence-associated secretion of multiple cytokines and chemokines.16 Since aging causes the phenotype of VSMCs to switch from contractile to synthetic type,17 it may further enhance the development of atherosclerosis in the infrainguinal muscular arteries as well as in the suprainguinal elastic arteries. It is known that of the 4800 chemical compounds in cigarettes, nicotine is the major constituent.3,18 In one study, nicotine-induced autophagy promoted the phenotypic switching of VSMCs in mice to the synthetic type, accelerating the development of atherosclerosis.19 Therefore, it is plausible to assume that smoking significantly enhances the development of atherosclerosis in the infrainguinal muscular arteries.
In a study with cultured aortic VSMCs from streptozotocin-induced diabetic rats and nondiabetic control rats, VSMCs from the diabetic rats showed a higher ratio of the synthetic phenotype than those from nondiabetic rats.20 Thus, diabetes mellitus and subsequently high levels of HbA1c may also be significant factors that enhance the development of atherosclerosis in the infrainguinal muscular arteries. Meanwhile, there is controversy on the role of homocysteine in the development of atherosclerosis. In a meta-analysis study with diabetic patients, there was no causal association between plasma homocysteine levels and coronary artery disease.21 However, it has been reported that homocysteine causes oxidative stress, endothelial dysfunction, vascular remodeling, and atherosclerosis by inducing the phenotypic switch of VSMCs from a contractile to a synthetic phenotype.7,22,23 Although the relevance between homocysteine and the development of atherosclerosis is not clear, the present study suggests that the high level of homocysteine in the blood may be associated with atherosclerosis in the infrainguinal muscular arteries. To elucidate more precise information on the relationship between homocysteine and atherogenesis, further study is needed.
From the findings of our study, we hypothesize that aging, heavier smoking, and higher blood levels of HbA1c and homocysteine may be the significant risk factors for the development of atherosclerosis in the infrainguinal muscular arteries. In addition, although the ABI and TBI scores were within the clinically normal range, patients with type 3 atherosclerosis compared to those with type 1 exhibited significantly lower ABI and TBI scores in both the right and left legs. This result implies that in advanced cases of the disease, the peripheral arterial circulation of type 3 patients may be more jeopardized than that of type 1 patients. Thus, to prevent the peripheral arterial ischemic diseases of the lower extremities, cessation of smoking, and control of blood glucose and homocysteine levels may be required, especially in elderly patients.
This study is limited due to the relatively small number of patients and its retrospective design. Thus, further studies with an increased number of patients and experimental research regarding the role of elastin and VSMC in the pathogenesis of atherosclerosis will help elucidate a more precise mechanism of the development and progression of atherosclerosis in elastic and muscular arteries.
Conclusion
Our results indicate that in arteries of the abdomen and lower extremities, atherosclerosis may occur initially at the suprainguinal elastic arteries and the significant risk factors for type 3 atherosclerosis, including aging, heavier smoking, and high blood levels of HbA1c and homocysteine, may contribute to the development of atherosclerosis at infrainguinal muscular arteries, deteriorating the peripheral arterial circulation. Therefore, if atherosclerotic lesions are found at the suprainguinal elastic arteries on CTA, to prevent atherosclerosis at infrainguinal muscular arteries and subsequent peripheral arterial ischemic disease, cessation of smoking and control of blood glucose and homocysteine may be recommended, especially in elderly patients.
Data Sharing Statement
The data generated and analyzed during this study are available on appropriate request from the corresponding author JKP. The data are not publicly available due to Korean law on personal data.
Ethics Approval and Informed Consent
This study protocol was approved by the Inje University Haeundae Paik Hospital’s Institutional Review Board (HPIRB 2019-12-021-001). This study was conducted in accordance with the Declaration of Helsinki. The need for consent was waived by the IRB owing to minimal risk to the patients. However, the data was anonymized and maintained with confidentiality.
Funding
The authors received no financial support for this article.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Asanuma Y, Oeser A, Shintani AK, et al. Premature coronary-artery atherosclerosis in systemic lupus erythematosus. N Engl J Med. 2003;349(25):2407–2415. doi:10.1056/NEJMoa035611
2. Jara LJ, Medina G, Vera-Lastra O, Shoenfeld Y. Atherosclerosis and antiphospholipid syndrome. Clin Rev Allergy Immunol. 2003;25(1):79–88. doi:10.1385/CRIAI:25:1:79
3. Gerhard-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation. 2017;135(12):e726-e779.
4. Fhayli W, Boete Q, Harki O, Briancon-Marjollet A, Jacob MP, Faury G. Rise and fall of elastic fibers from development to aging. Consequences on arterial structure-function and therapeutical perspectives. Matrix Biol. 2019;84:41–56. doi:10.1016/j.matbio.2019.08.005
5. Maurice P, Blaise S, Gayral S, et al. Elastin fragmentation and atherosclerosis progression: the elastokine concept. Trends Cardiovasc Med. 2013;23(6):211–221. doi:10.1016/j.tcm.2012.12.004
6. Gayral S, Garnotel R, Castaing-Berthou A, et al. Elastin-derived peptides potentiate atherosclerosis through the immune Neu1-PI3Kgamma pathway. Cardiovasc Res. 2014;102(1):118–127. doi:10.1093/cvr/cvt336
7. Greenwald SE. Ageing of the conduit arteries. J Pathol. 2007;211(2):157–172. doi:10.1002/path.2101
8. Basu P, Sen U, Tyagi N, Tyagi SC. Blood flow interplays with elastin: collagen and MMP: TIMP ratios to maintain healthy vascular structure and function. Vasc Health Risk Manag. 2010;6:215–228. doi:10.2147/vhrm.s9472
9. Tucker WD, Arora Y, Mahajan K. Anatomy, blood vessels. Statpearls Treasure Island. 2020.
10. Knibb G, Jones A, Christiansen P. Pour guess: the effect of glass shape and an ice substitute on alcohol pouring and estimation. Alcohol Clin Exp Res. 2018;42(7):1228–1236. doi:10.1111/acer.13767
11. Chung J, Modrall JG, Knowles M, et al. Arteriographic patterns of atherosclerosis and the association between diabetes mellitus and ethnicity in chronic critical limb ischemia. Ann Vasc Surg. 2017;40:198–205. doi:10.1016/j.avsg.2016.11.003
12. Faglia E, Favales F, Quarantiello A, et al. Angiographic evaluation of peripheral arterial occlusive disease and its role as a prognostic determinant for major amputation in diabetic subjects with foot ulcers. Diab Care. 1998;21(4):625–630. doi:10.2337/diacare.21.4.625
13. Gray BH, Grant AA, Kalbaugh CA, et al. The impact of isolated tibial disease on outcomes in the critical limb ischemic population. Ann Vasc Surg. 2010;24(3):349–359. doi:10.1016/j.avsg.2009.07.034
14. Thyberg J, Hedin U, Sjolund M, Palmberg L, Bottger BA. Regulation of differentiated properties and proliferation of arterial smooth muscle cells. Arteriosclerosis. 1990;10(6):966–990. doi:10.1161/01.ATV.10.6.966
15. Chappell J, Harman JL, Narasimhan VM, et al. Extensive proliferation of a subset of differentiated, yet plastic, medial vascular smooth muscle cells contributes to neointimal formation in mouse injury and atherosclerosis models. Circ Res. 2016;119(12):1313–1323. doi:10.1161/CIRCRESAHA.116.309799
16. Majesky MW. Vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2016;36(10):e82–86. doi:10.1161/ATVBAHA.116.308261
17. Tesauro M, Mauriello A, Rovella V, et al. Arterial ageing: from endothelial dysfunction to vascular calcification. J Intern Med. 2017;281(5):471–482. doi:10.1111/joim.12605
18. Siasos G, Tsigkou V, Kokkou E, et al. Smoking and atherosclerosis: mechanisms of disease and new therapeutic approaches. Curr Med Chem. 2014;21(34):3936–3948. doi:10.2174/092986732134141015161539
19. Wang Z, Liu B, Zhu J, Wang D, Wang Y. Nicotine-mediated autophagy of vascular smooth muscle cell accelerates atherosclerosis via nAChRs/ROS/NF-kappaB signaling pathway. Atherosclerosis. 2019;284:1–10. doi:10.1016/j.atherosclerosis.2019.02.008
20. Etienne P, Pares-Herbute N, Mani-Ponset L, et al. Phenotype modulation in primary cultures of aortic smooth muscle cells from streptozotocin-diabetic rats. Differentiation Res Biol Diversity. 1998;63(4):225–236. doi:10.1111/j.1432-0436.1998.00225.x
21. Xu T, Chen S, Yang F, et al. The impact of homocysteine on the risk of coronary artery diseases in individuals with diabetes: a Mendelian randomization study. Acta Diabetol. 2020. doi:10.1007/s00592-020-01608-3
22. Steed MM, Tyagi SC. Mechanisms of cardiovascular remodeling in hyperhomocysteinemia. Antioxid Redox Signal. 2011;15(7):1927–1943. doi:10.1089/ars.2010.3721
23. Lin H, Ni T, Zhang J, et al. Knockdown of Herp alleviates hyperhomocysteinemia mediated atherosclerosis through the inhibition of vascular smooth muscle cell phenotype switching. Int J Cardiol. 2018;269:242–249. doi:10.1016/j.ijcard.2018.07.043
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.