Back to Journals » Infection and Drug Resistance » Volume 14
Distribution and Antibiotic Susceptibility Pattern of Multidrug-Resistant Bacteria and Risk Factors Among Kidney Transplantation Recipients with Infections Over 13 Years: A Retrospective Study
Authors Gong L, Zhang L, Liu X, Odilov B, Li S, Hu Z, Xiao X
Received 6 May 2021
Accepted for publication 11 November 2021
Published 24 December 2021 Volume 2021:14 Pages 5661—5669
DOI https://doi.org/10.2147/IDR.S318941
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Liying Gong,1 Luwei Zhang,2 Xiaoli Liu,3 Bekzod Odilov,4 Shengnan Li,1 Zhao Hu,1 Xiaoyan Xiao1
1Department of Nephrology, Qilu Hospital of Shandong University, Jinan, People’s Republic of China; 2Department of Organ Transplantation, Qilu Hospital of Shandong University, Jinan, People’s Republic of China; 3Department of Kidney Transplantation, The Second Hospital, Cheeloo College of Medicine, Shandong University, Jinan, People’s Republic of China; 4Department of Endocrinology, Qilu Hospital of Shandong University, Cheeloo College of Medicine, Shandong University, Jinan, People’s Republic of China
Correspondence: Xiaoyan Xiao
Department of Nephrology, Qilu Hospital of Shandong University, 107 Wenhua Xi Road, Jinan, 250012, Shandong Province, People’s Republic of China
Tel/Fax +86-531-82169316
Email [email protected]
Background: Infection ranks as the most common complication after kidney transplantation (KT) and threatens outcomes of kidney transplantation recipients (KTR). This study aimed to investigate the microbiological profile of infection, assess bacterial resistance and identify risk factors for multidrug-resistant (MDR) bacterial infection among KTR.
Methods: During the study period, 866 recipients underwent kidney transplant surgery. We studied the distribution of pathogens, resistance rate of MDR bacteria and the risk factors of MDR bacterial infection.
Results: Totally, 214 species of pathogens (110 species were MDR bacteria) were isolated in 119 KTR. Escherichia coli (E. coli) was the most common bacteria of the infection. MDR extended spectrum β-lactamase (ESBL)-producing Enterobacteriaceae (ESBL-E) were most resistant to ampicillin, cefazolin, ciprofloxacin and complex sulfamethoxazole, while quite sensitive to imipenem, amikacin and piperacillin/tazobactam (PIT). All MDR gram-positive bacteria were quite sensitive to linezolid and vancomycin, except that MDR Staphylococcus was also susceptible to rifampicin. Female gender (OR = 3.497, 95% CI = 1.445– 8.467, P = 0.006), pathogen types > 1 (OR = 3.832, 95% CI = 1.429– 10.273, P = 0.008) and postoperative time < 3 months (OR = 0.331, 95% CI = 0.137– 0.799, P = 0.014) were independent risk factors for MDR bacterial infection.
Conclusion: PIT and amikacin may be an alternative choice of ESBL-E infection. Rifampicin can also be prescribed for MDR Staphylococcus infection. MDR bacterial infection was associated with female gender, pathogen types more than 1 and 3 months postoperative period.
Keywords: kidney transplantation, risk factor, MDR, ESBL, piperacillin/tazobactam, multidrug-resistant, KT
Introduction
Kidney transplantation (KT), as the only curable option of treatment for chronic kidney disease, is threatened by all kinds of infections since they can pose a risk of acute rejection, delayed graft function, endanger allograft and patients’ survival, and result in longer hospital duration.1–4 Moreover, they accounts for approximately 18% to 23% of death after KT.5 The vast majority of infections occur within 6 months after surgery, especially within the first month. The incidence rate of infections following renal transplantation tends to follow a predictable temporal pattern that depends on the intensity of immunosuppression, female gender, advanced age and various implants to a large extent.6 During this interval, urinary tract infection (UTI) is the most prevalent infections with Escherichia coli (E. coli) being the predominant bacteria.6
In recent decades, the incidence of infection caused by multidrug-resistant (MDR) bacteria including extended spectrum β-lactamase (ESBL)-producing bacteria has been increasing worldwide. It has also become a global health priority since its several limitations including less treatment experience, higher incidence of adverse effects and limited therapeutic options are still present.7,8 The use of broad-spectrum antibiotics, antibiotic abuse and more potent immunosuppressive regimens as well as other risk factors may contribute to the increase.1,9 KTR are highly vulnerable to the threat of MDR bacterial infections and face a three times higher recurrence risk compared to those infected with non-MDR infection.10,11
Thus, in order to improve the quality life of KTR and curtails the development of MDR bacteria, the surveillance of infection is very critical especially for MDR bacterial infection. This study was conducted to investigate the microbiologic profile of bacteria, to assess the drug resistance of MDR bacteria, and verify the risk factors of MDR bacterial infection.
Materials and Methods
Study Design and Patients
This retrospective study was performed based on reviewing medical records of KTR in Qilu hospital of Shandong university, Jinan, China from January 1, 2007 to December 31, 2019. ALL recipients received anti-thymocyte globulin (ATG) or anti-interleukin-2 receptor antibodies as an induction therapy. All KTR who diagnosed as infection with positive specimens were included in the study. All the following information was gathered for each patient: the date of infection, site of the infection, specimen type, pathogen, clinical characteristics, sensitivity to antibiotics and laboratory findings. Duplicate strains from the same site during hospitalization of the same patient, patients with absence of drug sensitivity test, contaminated specimens and negative cultures were excluded. All source of the donated organs were from living relative donor, cadaveric kidneys or donation after cardiac death donors with written informed consent, which was conducted in accordance with the Declaration of Istanbul. The study was approved by Medical Ethics Committee for clinical Studies of Qilu Hospital of Shandong University in adherence to the Declaration of Helsinki. Patient consent to review their medical records was required by the ethics committee.
Microbiologic Analysis
Antimicrobial susceptibility was determined by the Kirby-Bauer disk diffusion method, and the minimum inhibitory concentration was tested by agar dilution. Determining whether the bacteria was resistant, intermediate or sensitive was according to Clinical and Laboratory Standards Institute. In this study intermediate susceptibility was considered as resistant.
Statistical Analysis
Data were analyzed by IBM SPSS statistics version 22.0 software (IBM SPSS, Armonk, NY, USA). Categorical variables were compared by Pearson’s χ2 test or Fisher’s exact test when appropriate. Continuous variables were presented as mean ± SD. We use logistic regression to analyze univariate and multivariate analysis. Variables with a P-value < 0.05 in the univariate analysis were introduced into the subsequent multivariate analysis based on enter logistic regression. Associations were displayed as odds ratios (OR) with a 95% CI. All P values < 0.05 were considered as statistically significant.
Results
Basic Characteristics of KTR with Infection
There were 866 patients undergone KT surgery during the study period. Totally, 119 patients (40 females and 79 males, mean age 39.5 ± 12.3 years) were diagnosed with 142 episodes of culture-proven infection and a total of 214 species of pathogens were isolated. Primary glomerulonephritis was the most common cause of end-stage renal disease leading to transplantation. More than 70% recipients received tacrolimus as main immune maintenance therapy. During the study period, 8 recipients died during hospitalization and 7 recipients’ deaths were caused by infection with 5 deaths of MDR bacterial infection. The basic characteristics of the recipients were shown in Table 1.
 |
Table 1 The Clinical Characteristics of Patients with Culture-Proven Infections |
Distribution of Pathogens and Infection Types by Time Course
Among the 214 species of pathogens, 193 were bacteria (146 were gram-negative bacteria and 47 were gram-positive bacteria) and 21 were fungus (Table 2). The top 3 pathogens were E. coli, Pseudomonas aeruginosa (P. aeruginosa) and Enterococcus Faecium (E. Faecium). Among the gram-negative bacteria, 72 species were from Enterobacteriaceae family with 52 species of MDR bacteria and 65 species from non-fermentative bacteria with 19 species of MDR bacteria, respectively. Compared with Staphylococcus, genus of Enterococcus was more common, especially E. Faecium. KTR were also in the risk of fungus infection. Candida glabrata and Candida tropicalis were the most common fungus. Although the incidence of MDR bacteria fluctuated by time course, in overall, a clear upward trend could be observed in subjects. The overall MDR bacteria detection rate among the three intervals represented a significant difference (P = 0.02) and was mainly caused by the gram-negative bacteria.
 |
Table 2 Distribution of Micrograms and Changes in the Incidence Rate of MDR Bacteria Types by Year (MDR Number/Total Number) |
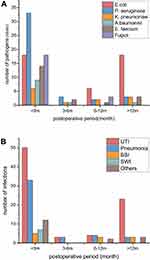
We studied the distribution of pathogens in 3 different aspects depending on postoperative time, infection type and source of specimens. In 4 different intervals (postoperative 3 months, within the 3 to 6 months, within the 6 months to 1 year and after the 1 year postoperatively), the majority of pathogens were isolated within the 3 months postoperatively. P. aeruginosa was the most identified pathogen during the initial postsurgical 3 months followed by E. coli, fungus and E. Faecium. After the initial postoperative 3 months, E. coli became the most common pathogen, especially 1 year postoperatively (Figure 1A).
As depicted in Figure 1B, the onset of various infections was concentrated in the first 3 months after surgery, especially in the first month. In our study, 94 (66.2%) episodes of infection occurred within 3 months postoperatively, of which 72 (50.1%) were found in initial month. UTI was the most common infection complication after KT surgery in all intervals. P. aeruginosa and E. coli were the most frequent isolated bacteria within the postoperative 3 months and after the 1 year postoperatively, respectively. For pneumonia, non-fermentative bacteria comprised the majority of pathogens, P. aeruginosa in particular. Among bloodstream infection (BSI), gram-negative bacteria were in abundance with E. coli being the most common bacteria. Interestingly, nearly all of BSI were unknown for primary source (81.8%). Gram-positive bacteria were most frequent bacteria among surgical wound infection (SWI). Other types of infection mainly included abdominal wall abscess, graft perirenal abscess and peritonitis. Most of the pathogens were MDR bacteria.
Among the sources of pathogens, 103 species were from urine, 65 species were from sputum, 12 species from blood, and 34 species from other specimens including secretions, venous catheter tip respectively. E. coli was the most common detected bacteria in urine sample, blood and others (secretions) sample, while P. aeruginosa was most common isolated bacteria in sputum. The detection rate of MDR bacteria in each sample was 30.8% (sputum), 59.2% (urine), 61.8% (others) and 66.7% (blood), correlatively.
Drug Resistance Rate of MDR Bacteria
Among the 193 species of bacteria, 110 species were MDR bacteria with Enterobacteriaceae being the dominant MDR bacteria. E. coli (34.5%, n = 38) ranked as the most frequently isolated MDR bacteria with rates of ESBL-producing and carbapenem-resistant isolates of 69.2% and 3.8%, respectively, following by E. faecium (15.5%, n = 17) and Klebsiella pneumoniae (8.2%, n = 9). MDR ESBL-Enterobacteriaceae (ESBL-E) showed high resistant rate to ampicillin (100%), cefazolin (100%), trimethoprim/sulfamethoxazole (SMZ) (97.1%) and ciprofloxacin (95.8%), were quite sensitive to imipenem, amikacin and piperacillin/tazobactam (PIT), with the resistant rate of 0.0%, 15.2% and 15.6%, correlatively. MDR non-fermentative bacteria mainly including Acinetobacter and Pseudomonas accounted for 17.3% of MDR bacteria. They were most resistant to ceftazidime, imipenem and ciprofloxacin. On the other hand, they showed less resistance rate to amikacin (Table 3).
 |
Table 3 Resistance Rate of MDR Gram-Negative Bacteria of KTR (Resistant Number/Test Number/Resistant Rate %) |
MDR gram-positive bacteria showed high susceptibility to linezolid (100%), followed by vancomycin (97.1%) and rifampicin (68.4%). The tested species showed low susceptibility to ciprofloxacin, gentamicin, clindamycin and carbapenem (< 30%). Less than 9% of the MDR gram-positive bacteria were susceptible to erythromycin and ampicillin. For MDR Enterococcus, they were highly resistant to penicillin, ciprofloxacin and high level of gentamicin, while quite sensitive to linezolid and vancomycin. Among Staphylococcus, 85.7% were resistant to erythromycin and 86.7% were resistant to oxacillin which means they were almost resistant to all β-lactam antibiotics. They were certainly susceptible to linezolid, vancomycin and rifampicin (Table 4).
 |
Table 4 Resistance Rate of Gram-Positive MDR Bacteria for KT Patients (Resistant Number/Test Number/ Resistant Rate [%]) |
The Risk Factors of MDR Bacterial Infection
In order to find out the risk factors, we analyzed the characteristics of MDR bacterial infection group and non-MDR bacterial infection group by univariate and multivariate analysis. Female, age > 46 years old, pathogen types > 1 and postoperative time < 3 months were significant variable in univariate analysis. No significance was demonstrated in age, donor type, and length of hospitalization or clinical characteristics including albumin, creatinine and others. In multivariable analysis, female gender (OR = 3.497, 95% CI = 1.445–8.467, P = 0.006), pathogen types > 1 (OR = 3.832, 95% CI = 1.429–10.273, P = 0.008) and postoperative time < 3 months (OR = 0.331, 95% CI = 0.137–0.799, P = 0.014) were independent risk factors for MDR bacterial infection (Table 5).
 |
Table 5 Univariate and Multivariate Analysis of Risk Factors Associated with the Incidence of MDR Infection |
Discussion
Most of the infection occurred within 3 month postoperatively, especially the first month with UTI being the most common infection which is similar to other studies around the world.12,13 Immunosuppressive therapy as well as present catheters, advanced age, delayed graft function, deceased donor and other risk factors contribute to increase the infection rate.6,14–16 Recent researches also revealed that gut uropathogens abundance could be a risk factor for UTI.17 P. aeruginosa and E. coli were the most frequent isolated bacteria in UTI within the 1st operative 3 months and after the 1 year postoperatively, respectively. However, Alangaden et al found that Enterococcus species were the most common uropathogens during the first month post-transplant, and E. coli was isolated mostly after 6 months.9 It is critical to figure out the microbiological diagnostics of these infections, as it determines the targeted treatment and reduces the excessive use of antimicrobial agents.18 Prevention and proper management of UTI in kidney recipients is essential to reduce the risk of more serious complications, including gram-negative BSI and invasive fungal infection, which associated with reduced allograft survival and all-cause mortality.2,19
Data related to BSI among KTR are limited, BSI with the incidence rate of 3.9–7.3% can lead to reduced allograft and mortality.2,20 However compared with non-transplant patients, transplantation recipients presented decreased mortality since immunosuppressive therapy in transplantation may provide a survival advantage to transplant recipients with sepsis through modulation of the inflammatory response.21 According to recent studies, UTI was most common source of BSI among KTR, while in other transplant recipients central venous catheters were most common source.20,22 Interestingly, results were quite different in our study which indicated that nearly all of BSI came without identified source. This can be explained by the reason that specimens were collected after antibiotic application and other cultures was not performed since the recipients did not have classical symptoms due to immunosuppressed state. Prevention and management of other site infection, in particular UTI, can be a key point in BSI reduction.
In the present study, ESBL-E infection accounted for 23.2% of infections. A meta-analysis yielded regional variations that the proportion of KTR affected by an ESBL-E UTI was 2% in North America, 5% in Europe, 17% in South America, and 33% in Asia.10 Compared with non-KTR, KTR are more prone to suffer from infections caused by ESBL-producing strains.23 Age, acute rejection, higher level of immunosuppressant and antibiotics use are found to be associated with the incidence of ESBL-E infection.3,24 Besides that, gastrointestinal colonization with ESBL-E is also an independent risk factor for infection, especially UTI.25 O25 serotype was exhibited high prevalence among KTR around 28.6%, while it was less than 2% in non-immuosuppressed patients. Moreover, the O25 serotype was associated with ESBL-production and quinolone resistance.26–28 ESBL-E infections are accompanied by resistance to a broad range of antibiotics such as β-lactam and carbapenem that are drugs of choice in the treatment of ESBL-E infection. However with the increased usage of carbapenem, it might be partly associated with the spread of carbapenem resistance.29 Carbapenem-sparing regimens mainly including classic and newer β-lactam/β-lactamase inhibitor (BL/BLI) have been proposed to reduce the carbapenem administration, although the mortality and efficacy remained controversial. Several large-scale studies conducted in general population have illustrated that BL/BLI appeared to be as effective as carbapenem both in definitive and empirical therapies of ESBL-E BSI, as well as BL/BLI were associated with fewer MDR and fungal infection.30–32 On the contrast, Tamma et al showed that PIT appeared inferior to carbapenem for the treatment of ESBL bacteremia since there was higher mortality rate among empirical PIT group.33 Currently, the studies focused on kidney transplant setting are limited. According to a study, high effectiveness of PIT, carbapenem and amikacin were reported in KTR. What’s more, it also revealed that ESBL-E isolated from KTR harbored higher resistance genes such as blaCTX-M and blaTEM.23 Two meta-analysis including organ transplant recipients demonstrated that BL/BLI may provide an appropriate and alternative treatment option at some settings.34,35 Recent a multinational retrospective study based on ESBL-E BSI secondary to UTI conducted in KTR has reinforced the efficacy of PIT in ESBL-E infection treatment.36 Besides that, faecal microbiota transplantation has been reported to treat different sites of infections among KTR including carbapenem-resistant Klebsiella pneumoniae infection and ESBL-Klebsiella pneumoniae, which also indicated that it could shift the microbial composition and increased the diversity and abundance of gut microbiota.37,38 According to our results, antibiotics including PIT and amikacin are quite effective against ESBL-E and can be an alternative for ESBL-E infection, but the nephrotoxicity of amikacin may limits its use. However most of these studies are based only on BSI secondary to UTI or other source of infection, and its effectiveness in other site of infection remains unclear.
In our center, rifampicin showed relatively great anti-bacterial effect against MDR Staphylococcus. Rifampicin has been mostly known to treat tuberculosis in combination with other drugs. Besides that, rifampicin as adjunctive therapy has been reported successfully in treating or preventing Staphylococcus infections.39–41 It also exhibits an independent protective effect and more efficiency in treating MRSA infection.40,42 In addition to tuberculosis, there are few researches regarding the role of rifampicin in treating in non-tuberculosis infection in KTR or even organ transplant recipients. More trials are needed to assess its role.
Regarding risk factors associated with MDR bacterial infection, female, older age and diabetes mellitus were prevalent independent risk factors associated MDR bacterial infection.1,43,44 In our survey, we proved that female gender was a risk factor for MDR bacterial infection while no relationship has been found either with diabetes mellitus or older age. This may due to the small number of diabetes mellitus recipients and patients over 60 years old rarely receive kidney transplant surgery in China. Moreover, 3 months postoperative period was found to be associated with MDR bacterial infection in our center. Since during this interval, delayed graft function, acute rejection, induction of ATG therapy, high levels of immunosuppressant, prolonged use of urinary catheter contribute to the occurrence of MDR bacterial infection.1,3,43 Another study has pointed out that creatinine above 1.5 mg/d, non-fermentative bacteria and polycystic kidney disease were also contributed to higher rate of MDR bacterial infection and even more extensively drug resistant infection.45 However we failed to demonstrated the relationship between creatinine level and MDR bacterial infection. In addition we found recipients who were diagnosed more than 1 pathogen were susceptible to MDR bacterial infection.
This study has several limitations. Firstly, it is a single center retrospective study, which limits the applicability of our findings to other populations with different antimicrobial regimens or microbial prevalence. Secondly, since this retrospective study covers a long period, the antibiotics used for drug sensitivity test was a little bit different between earlier years and recent years, some antibiotics such as cefoperazone-sulbactam were not included in the resistance analysis. Thirdly, for some antibiotics such as polymyxin, tigecycline and daptomycin were seldom used in drug sensitivity test in our center particularly in earlier years, so we could not assess resistance rate.
Conclusions
In conclusion, our study identified that E. coli accounted for the most common pathogens and UTI was the most prevalent infection among KTR. In addition, female gender, pathogen types > 1 and 3 months postoperative period were considered as an independent risk factors for MDR bacterial infection. Since MDR infection is increasing, it may be noteworthy to find alternative antimicrobial regimens against MDR infection. Thus, PIT and amikacin may be an alternative choice of ESBL-E infection. Besides that, rifampicin may be effective in treating MDR Staphylococcus infection. Further study is warranted so that we can better prevent infection and optimize empirical treatment protocols while reducing the incidence of MDR bacterial infection.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Freire MP, Mendes CV, Piovesan AC, et al. Does the urinary tract infection caused by carbapenem-resistant gram-negative bacilli impact the outcome of kidney transplant recipients? Transpl Infect Dis. 2018;20(4):e12923. doi:10.1111/tid.12923
2. Al-Hasan MN, Razonable RR, Kremers WK, Baddour LM. Impact of Gram-negative bloodstream infection on long-term allograft survival after kidney transplantation. Transplantation. 2011;91(11):1206–1210. doi:10.1097/TP.0b013e3182180535
3. Brakemeier S, Taxeidi SI, Zukunft B, et al. Extended-spectrum beta-lactamase-producing enterobacteriaceae-related urinary tract infection in kidney transplant recipients: risk factors, treatment, and long-term outcome. Transplant Proc. 2017;49(8):1757–1765. doi:10.1016/j.transproceed.2017.06.033
4. Jackson KR, Motter JD, Bae S, et al. Characterizing the landscape and impact of infections following kidney transplantation. Am J Transplant. 2020;21(1):198–207.
5. Kinnunen S, Karhapaa P, Juutilainen A, Finne P, Helantera I. Secular trends in infection-related mortality after kidney transplantation. Clin J Am Soc Nephrol. 2018;13(5):755–762. doi:10.2215/CJN.11511017
6. Aydin S, Patil A, Desai M, Simforoosh N. Five compelling UTI questions after kidney transplant. World J Urol. 2020;38(11):2733–2742. doi:10.1007/s00345-020-03173-4
7. Bergamasco MD, Barroso Barbosa M, de Oliveira Garcia D, et al. Infection with Klebsiella pneumoniae carbapenemase (KPC)-producing K. pneumoniae in solid organ transplantation. Transpl Infect Dis. 2012;14(2):198–205. doi:10.1111/j.1399-3062.2011.00688.x
8. Dowzicky M, Park C. Update on antimicrobial susceptibility rates among gram-negative and gram-positive organisms in the United States: results from the Tigecycline Evaluation and Surveillance Trial (TEST) 2005 to 2007. Clin Ther. 2008;30(11):2040–2050. doi:10.1016/j.clinthera.2008.11.006
9. Alangaden GJ, Thyagarajan R, Gruber SA, et al. Infectious complications after kidney transplantation: current epidemiology and associated risk factors. Clin Transplant. 2006;20(4):401–409. doi:10.1111/j.1399-0012.2006.00519.x
10. Alevizakos M, Nasioudis D, Mylonakis E. Urinary tract infections caused by ESBL-producing Enterobacteriaceae in renal transplant recipients: a systematic review and meta-analysis. Transpl Infect Dis. 2017;19(6):e12759. doi:10.1111/tid.12759
11. Bodro M, Sabé N, Tubau F, et al. Risk factors and outcomes of bacteremia caused by drug-resistant ESKAPE pathogens in solid-organ transplant recipients. Transplantation. 2013;96(9):843–849. doi:10.1097/TP.0b013e3182a049fd
12. Illesy L, Szabo-Pap M, Toth F, et al. Bacterial infections after kidney transplantation: a single-center experience. Transplant Proc. 2016;48(7):2540–2543. doi:10.1016/j.transproceed.2016.07.011
13. Hemmersbach-Miller M, Alexander BD, Sudan DL, Pieper C, Schmader KE. Single-center analysis of infectious complications in older adults during the first year after kidney transplantation. Eur J Clin Microbiol Infect Dis. 2019;38(1):141–148. doi:10.1007/s10096-018-3405-5
14. Yalci A, Celebi ZK, Ozbas B, et al. Evaluation of infectious complications in the first year after kidney transplantation. Transplant Proc. 2015;47(5):1429–1432. doi:10.1016/j.transproceed.2015.04.056
15. Lee JR, Bang H, Dadhania D, et al. Independent risk factors for urinary tract infection and for subsequent bacteremia or acute cellular rejection: a single-center report of 1166 kidney allograft recipients. Transplantation. 2013;96(8):732–738. doi:10.1097/TP.0b013e3182a04997
16. Wu X, Dong Y, Liu Y, et al. The prevalence and predictive factors of urinary tract infection in patients undergoing renal transplantation: a meta-analysis. Am J Infect Control. 2016;44(11):1261–1268. doi:10.1016/j.ajic.2016.04.222
17. Magruder M, Sholi AN, Gong C, et al. Gut uropathogen abundance is a risk factor for development of bacteriuria and urinary tract infection. Nat Commun. 2019;10(1):5521. doi:10.1038/s41467-019-13467-w
18. Fishman JA. Introduction: infection in solid organ transplant recipients. Am J Transplant. 2009;9(Suppl 4):S3–6. doi:10.1111/j.1600-6143.2009.02887.x
19. Leitheiser S, Harner A, Waller JL, et al. Risk factors associated with invasive fungal infections in kidney transplant patients. Am J Med Sci. 2020;359(2):108–116. doi:10.1016/j.amjms.2019.10.008
20. Moreno A, Cervera C, Gavalda J, et al. Bloodstream infections among transplant recipients: results of a nationwide surveillance in Spain. Am J Transplant. 2007;7(11):2579–2586. doi:10.1111/j.1600-6143.2007.01964.x
21. Kalil A, Syed A, Rupp M, et al. Is bacteremic sepsis associated with higher mortality in transplant recipients than in nontransplant patients? A matched case-control propensity-adjusted study. Clin Infect Dis. 2015;60(2):216–222. doi:10.1093/cid/ciu789
22. Shendi AM, Wallis G, Painter H, Harber M, Collier S. Epidemiology and impact of bloodstream infections among kidney transplant recipients: a retrospective single-center experience. Transpl Infect Dis. 2018;20(1):e12815. doi:10.1111/tid.12815
23. Halaji M, Shahidi S, Atapour A, Ataei B, Feizi A, Havaei SA. Characterization of extended-spectrum beta-lactamase-producing uropathogenic Escherichia coli among Iranian kidney transplant patients. Infect Drug Resist. 2020;13:1429–1437. doi:10.2147/IDR.S248572
24. Anesi JA, Lautenbach E, Tamma PD, et al. Risk factors for extended-spectrum beta-lactamase-producing Enterobacterales bloodstream infection among solid organ transplant recipients. Clin Infect Dis. 2020;72(6):953–960.
25. Alevizakos M, Kallias A, Flokas ME, Mylonakis E. Colonization with extended-spectrum beta-lactamase-producing Enterobacteriaceae in solid organ transplantation: a meta-analysis and review. Transpl Infect Dis. 2017;19(4):e12718. doi:10.1111/tid.12718
26. Sadeghi A, Halaji M, Fayyazi A, Havaei SA. Characterization of plasmid-mediated quinolone resistance and serogroup distributions of uropathogenic Escherichia coli among Iranian kidney transplant patients. Biomed Res Int. 2020;2020:2850183. doi:10.1155/2020/2850183
27. Johnson JR, Orskov I, Orskov F, et al. O, K, and H antigens predict virulence factors, Carboxylesterase B pattern, antimicrobial resistance, and host compromise among Escherichia coli strains causing urosepsis. J Infect Dis. 1994;169(1):119–126. doi:10.1093/infdis/169.1.119
28. Overdevest IT, Bergmans AM, Verweij JJ, et al. Prevalence of phylogroups and O25/ST131 in susceptible and extended-spectrum beta-lactamase-producing Escherichia coli isolates, the Netherlands. Clin Microbiol Infect. 2015;21(6):570 e571–574. doi:10.1016/j.cmi.2015.02.020
29. Rodriguez-Bano J, Gutierrez-Gutierrez B, Machuca I, Pascual A. Treatment of infections caused by extended-spectrum-beta-lactamase-, AmpC-, and carbapenemase-producing Enterobacteriaceae. Clin Microbiol Rev. 2018;31(2). doi:10.1128/CMR.00079-17
30. Ng TM, Khong WX, Harris PN, et al. Empiric piperacillin-tazobactam versus carbapenems in the treatment of bacteraemia due to extended-spectrum beta-lactamase-producing enterobacteriaceae. PLoS One. 2016;11(4):e0153696. doi:10.1371/journal.pone.0153696
31. Sharara SL, Amoah J, Pana ZD, Simner PJ, Cosgrove SE, Tamma PD. Is piperacillin-tazobactam effective for the treatment of pyelonephritis caused by extended-spectrum beta-lactamase-producing organisms? Clin Infect Dis. 2020;71(8):e331–e337. doi:10.1093/cid/ciz1205
32. Gutierrez-Gutierrez B, Perez-Galera S, Salamanca E, et al. A multinational, preregistered cohort study of beta-lactam/beta-lactamase inhibitor combinations for Treatment of bloodstream infections due to extended-spectrum-beta-lactamase-producing enterobacteriaceae. Antimicrob Agents Chemother. 2016;60(7):4159–4169. doi:10.1128/AAC.00365-16
33. Tamma PD, Han JH, Rock C, et al. Carbapenem therapy is associated with improved survival compared with piperacillin-tazobactam for patients with extended-spectrum beta-lactamase bacteremia. Clin Infect Dis. 2015;60(9):1319–1325. doi:10.1093/cid/civ003
34. Vardakas KZ, Tansarli GS, Rafailidis PI, Falagas ME. Carbapenems versus alternative antibiotics for the treatment of bacteraemia due to Enterobacteriaceae producing extended-spectrum beta-lactamases: a systematic review and meta-analysis. J Antimicrob Chemother. 2012;67(12):2793–2803. doi:10.1093/jac/dks301
35. Sfeir MM, Askin G, Christos P. Beta-lactam/beta-lactamase inhibitors versus carbapenem for bloodstream infections due to extended-spectrum beta-lactamase-producing Enterobacteriaceae: systematic review and meta-analysis. Int J Antimicrob Agents. 2018;52(5):554–570. doi:10.1016/j.ijantimicag.2018.07.021
36. Pierrotti LC, Perez-Nadales E, Fernandez-Ruiz M, et al. Efficacy of beta-lactam/beta-lactamase inhibitors to treat extended-spectrum beta-lactamase-producing Enterobacterales bacteremia secondary to urinary tract infection in kidney transplant recipients (INCREMENT-SOT Project). Transpl Infect Dis. 2020;23(3)e13520.
37. Wang J, Li X, Wu X, et al. Fecal microbiota transplantation as an effective treatment for carbapenem-resistant Klebsiella pneumoniae infection in a renal transplant patient. Infect Drug Resist. 2021;14:1805–1811. doi:10.2147/IDR.S308308
38. Grosen AK, Povlsen JV, Lemming LE, Jorgensen SMD, Dahlerup JF, Hvas CL. Faecal microbiota transplantation eradicated extended-spectrum beta-lactamase-producing Klebsiella pneumoniae from a renal transplant recipient with recurrent urinary tract infections. Case Rep Nephrol Dial. 2019;9(2):102–107. doi:10.1159/000502336
39. Falagas ME, Fragoulis KN, Bliziotis IA. Oral rifampin for prevention of S. aureus carriage-related infections in patients with renal failure–a meta-analysis of randomized controlled trials. Nephrol Dial Transplant. 2006;21(9):2536–2542. doi:10.1093/ndt/gfl235
40. Lora-Tamayo J, Murillo O, Iribarren JA, et al. A large multicenter study of methicillin-susceptible and methicillin-resistant Staphylococcus aureus prosthetic joint infections managed with implant retention. Clin Infect Dis. 2013;56(2):182–194. doi:10.1093/cid/cis746
41. Schrenzel J, Harbarth S, Schockmel G, et al. A randomized clinical trial to compare fleroxacin-rifampicin with flucloxacillin or vancomycin for the treatment of staphylococcal infection. Clin Infect Dis. 2004;39(9):1285–1292. doi:10.1086/424506
42. Jung YJ, Koh Y, Hong SB, et al. Effect of vancomycin plus rifampicin in the treatment of nosocomial methicillin-resistant Staphylococcus aureus pneumonia. Crit Care Med. 2010;38(1):175–180. doi:10.1097/CCM.0b013e3181b9ecea
43. Hamid R, Javaid S, Khan M, Lal N, Luxmi S, Sarfaraz S. Multiple drug resistant urinary tract infection in kidney transplant recipients: a retrospective cohort study. Saudi J Kidney Dis Transplant. 2020;31(5):905–916. doi:10.4103/1319-2442.301197
44. Linares L, Cervera C, Cofan F, et al. Epidemiology and outcomes of multiple antibiotic-resistant bacterial infection in renal transplantation. Transplant Proc. 2007;39(7):2222–2224. doi:10.1016/j.transproceed.2007.06.061
45. Yuan X, Liu T, Wu D, Wan Q. Epidemiology, susceptibility, and risk factors for acquisition of MDR/XDR Gram-negative bacteria among kidney transplant recipients with urinary tract infections. Infect Drug Resist. 2018;11:707–715. doi:10.2147/IDR.S163979
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.