Back to Journals » Psychology Research and Behavior Management » Volume 17
Distress Symptoms of Old Age and Mild Cognitive Impairment are Two Distinct Dimensions in Older Adults Without Major Depression
Authors Tran-Chi VL, Maes M, Nantachai G , Hemrungrojn S, Solmi M , Tunvirachaisakul C
Received 1 November 2023
Accepted for publication 29 December 2023
Published 6 January 2024 Volume 2024:17 Pages 101—116
DOI https://doi.org/10.2147/PRBM.S447774
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Gabriela Topa
Vinh-Long Tran-Chi,1,2,* Michael Maes,3– 9,* Gallayaporn Nantachai,2,10 Solaphat Hemrungrojn,2,9 Marco Solmi,11– 14 Chavit Tunvirachaisakul2,8
1Ph.D. Program in Clinical Sciences, School of Global Health, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand; 2Department of Psychiatry, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand; 3Sichuan Provincial Center for Mental Health, Sichuan Provincial People’s Hospital, School of Medicine, University of Electronic Science and Technology of China, Chengdu, People’s Republic of China; 4Key Laboratory of Psychosomatic Medicine, Chinese Academy of Medical Sciences, Chengdu, People’s Republic of China; 5Research Institute, Medical University of Plovdiv, Plovdiv, Bulgaria; 6Department of Psychiatry, Medical University of Plovdiv, Plovdiv, Bulgaria; 7Kyung Hee University, Dongdaemun-gu, Seoul, South Korea; 8Cognitive Impairment and Dementia Research Unit, Department of Psychiatry, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand; 9Cognitive Fitness and Biopsychiatry Technology Research Unit, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand; 10Somdet Phra Sungharaj Nyanasumvara Geriatric Hospital, Department of Medical Services, Ministry of Public Health, Chon Buri Province, Thailand; 11Department of Psychiatry, University of Ottawa, Ontario, Canada; 12Regional Centre for the Treatment of Eating Disorders and on Track, The Champlain First Episode Psychosis Program, Department of Mental Health, The Ottawa Hospital, Ontario, Canada; 13Ottawa Hospital Research Institute (OHRI), Clinical Epidemiology Program, University of Ottawa, Ottawa, Ontario, Canada; 14Department of Child and Adolescent Psychiatry, Charité Universitätsmedizin, Berlin, Germany
*These authors contributed equally to this work
Correspondence: Michael Maes, Sichuan Provincial Center for Mental Health, Sichuan Provincial People’s Hospital, School of Medicine, University of Electronic Science and Technology of China, Chengdu, 610072, People’s Republic of China, Email [email protected] Chavit Tunvirachaisakul, Cognitive Impairment and Dementia Research Unit, Department of Psychiatry, Faculty of Medicine, Chulalongkorn University, Bangkok, 10330, Thailand, Email [email protected]
Background: Studies in old adults showed bidirectional interconnections between amnestic mild cognitive impairment (aMCI) and affective symptoms and that adverse childhood experiences (ACE) may affect both factors. Nevertheless, these associations may be confined to older adults with clinical depression.
Aim: To delineate the relationship between clinical symptoms of aMCI and affective symptoms in older adults without major depression (MDD) or dysfunctions in activities of daily living (ADL).
Methods: This case-control study recruited 61 participants with aMCI (diagnosed using Petersen’s criteria) and 59 older adults without aMCI and excluded subjects with MDD and ADL dysfunctions.
Results: We uncovered 2 distinct dimensions, namely distress symptoms of old age (DSOA), comprising affective symptoms, perceived stress and neuroticism, and mild cognitive dysfunctions, comprising episodic memory test scores, the total Mini-Mental State Examination (MMSE) and Montreal Cognitive Assessment (MoCA) scores. A large part of the variance (37.9%) in DSOA scores was explained by ACE, negative life events (health and financial problems), a subjective feeling of cognitive decline, and education (all positively). ACE and NLE have a highly significant impact on the DSOA score and are not associated with aMCI or its severity. Cluster analysis showed that the diagnosis of aMCI is overinclusive because some subjects with DSOA symptoms may be incorrectly classified as aMCI.
Conclusion: The clinical impact is that clinicians should carefully screen older adults for DSOA after excluding MDD. DSOA might be misinterpreted as aMCI.
Keywords: depression, adverse childhood experiences, mild cognitive impairments, affective disorders, negative life events, neurocognitive deficits
Introduction
Mild cognitive impairment (MCI) has been observed to have a significant prevalence among the elderly population affecting approximately 10–15% of individuals aged 65 and above.1 Mild deficits in episodic memory, executive functions, visuospatial skills, processing speed, cognitive flexibility, and problem-solving capacity are prominent characteristics of amnestic mild cognitive impairment (aMCI), while fundamental activities associated with daily living (ADL) remain unimpaired.2–4 aMCI can be seen as a cognitive phase that lies between the normal aging process and the onset of dementia.1 It is worth noting that the annual conversion rate of aMCI to Alzheimer’s disease (AD) is approximately 16.5%, although a small proportion of aMCI patients (8%) experience a recovery from this condition.5
The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) developed a neuropsychological test battery to evaluate patients with neurocognitive deficits including MCI.6 The CERAD tests indicate that individuals with MCI have deficits in verbal fluency tasks (VFT), as well as word list memory (WLM) and world list recognition (WLR).7 Age is a crucial determinant in comprehending cognitive decline due to the frequent association between MCI and the process of aging.7 Moreover, education level is frequently regarded as a potential confounding variable in cognitive decline research because it can affect cognitive performance; higher levels of education are associated with greater cognitive reserve, which may delay the onset of cognitive impairment or dementia.7,8
Previous research has indicated that adverse childhood experiences (ACE), encompassing various forms of abuse (physical, emotional, and sexual), neglect (physical and emotional), and family dysfunctions, are significantly correlated with later cognitive impairments.9–11 Negative Life Events (NLE), such as severe illness, and financial problems, may be associated with increasing cognitive decline in older adults.12–14
Late-life depression is a strong risk factor for normal subjects progressing to MCI15 and dementia.14 Several systematic reviews and meta-analyses of environmental risk factors for late-life depression have been published.16–18 MCI patients have higher rates of depression than normal adults, and those rates are independent from age, race, gender, and study type.19 A meta-analysis showed the prevalence of depression in patients with MCI is as high as 32%.20 Educational attainment is associated with depressive symptoms of aging,21 and older adults with lower education attainment showed to have higher depressive symptoms.22 Recently, Jirakran, Vasupanrajit, Tunvirachaisakul and Maes23 detected that in adult depression one factor could be extracted from increased depression, perceived stress, neuroticism, and anxiety scores, indicating that these domains are all manifestations of the underlying construct. However, there are no data whether in older adults one factor may be extracted from stress, anxiety, depression, and neuroticism domain scores.
ACE and NLE are associated with the onset of depressive symptoms24 and with higher odds of late-life depressive symptoms.25 Increased levels of depressive symptoms and ACE exposures are associated with an increased risk of depressive symptoms in the elderly.26–30 However, no research has examined whether age, educational attainment, ACE, NLE, have cumulative effects in predicting severity of mild cognitive impairments in older adults. Moreover, there are no data indicating whether in older adults, cognitive symptoms are associated with affective symptoms or whether neurocognitive and affective symptoms are independent phenomena. Furthermore, there are no data whether ACE and NLE are independently associated with the neurocognitive deficits in older adults after considering the effects of affective symptoms.
Hence, the study aims to delineate the relationship between clinical symptoms of MCI and affective symptoms, and whether in older adults without major depression, ACE and NLE predict neurocognitive deficits independently from affective and distress symptoms. In addition, we examine whether in older adults one factor may be extracted from depressive, anxiety, neuroticism, and perceived distress symptoms, and whether cognitive deficits are part of this factor.
Materials and Methods
Participants
A cross-sectional study was conducted to compare individuals with aMCI to a group of healthy control subjects, after exclusion of participants with major depression. The study sample comprised individuals of both genders, with an age range spanning from 60 to 75 years. The study recruited healthy participants from Bangkok, Thailand, whereas individuals with aMCI were recruited from the Outpatient Department of the Dementia Clinic at King Chulalongkorn Memorial Hospital in Bangkok, Thailand from May 2022 to March 2023. The clinical Petersen’s criteria were utilized to diagnose aMCI in the older adult population.31 These criteria involve the identification of subjective and objective memory impairments, together with the lack of dementia and alterations in activities of daily living (ADL). Furthermore, people with amnestic mild cognitive impairment (aMCI) complied with the Petersen criteria and exhibited a modified Clinical Dementia Rating (CDR) score of 0.5. The control group had a CDR score of 0 and did not meet Petersen’s criteria.31 The healthy older adults were recruited from the Health Check-up Clinic, members of neighbourhoods’ senior clubs, healthy elderly carers of individuals with aMCI) who were patients at the Dementia Clinic, and senior volunteers affiliated with the Red Cross.
Participants with stroke, Parkinson’s disease, any dementia subtype, and multiple sclerosis were excluded to participate. Using DSM-5 criteria, we excluded subjects with major depression, schizophrenia, bipolar disorder, autism, substance use disorders, and psycho-organic disorders. In addition, we excluded subjects with medical disorders such as metabolic disorder, malaria, HIV, chronic obstructive pulmonary disease (COPD), chronic kidney disease, and cancer. Also excluded were subjects with an inability to speak or communicate, blindness or impaired vision even with corrective lenses, hearing loss, inability to sit stably due to physical conditions such as chronic pain or low back pain, and those who had undergone cognitive training within three months prior to the study were excluded from the study. Ultimately, the entirety of the subjects was allocated to either of the two study groups, consisting of 59 individuals classified as healthy controls and 61 individuals diagnosed with aMCI.
Before taking part in the study, all volunteers, and individuals were required to submit written informed consent. The study conducted in this research adhered to ethical and privacy standards that are recognized both in Thailand and internationally. These standards are in accordance with the International Guideline for the Protection of Human Subjects, as mandated by influential documents such as the Declaration of Helsinki, the Belmont Report, the International Conference of Harmonization in Good Clinical Practice, and the CIOMS Guidelines. The present study received approval from the Institutional Review Board (IRB) of the Faculty of Medicine at Chulalongkorn University in Bangkok, Thailand (No. 0372/65).
Clinical Assessments
Neurocognition
We used a semi-structured interview to collect socio-demographic data comprising age, sex, relationship status, year of education, and marital status. The scales used to assess cognition were the Thai Mini-Mental State Examination (MMSE),32 the Thai Montreal Cognitive Assessment (MoCA),33 and three rating scales of the Thai Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) Neuropsychological Assessment Battery.7 The MMSE is a 30‐question assessment of cognitive function.34 The Thai version of the Mini-Mental State Examination was developed in 1993 and has been extensively used in Thailand to screen cognitive impairment and dementia.32 The scale consists of six subtests measuring orientation, registration, attention, word recall, language, and computation. The total score ranges from 0 to 30. The MoCA was developed by Nasreddine, Phillips, Bédirian, Charbonneau, Whitehead, Collin, Cummings and Chertkow35 as an effective and applicable screening tool for cognitive disorders. The Thai version of MoCA was used to screen and monitor cognitive impairments in the clinical practice of neurocognitive disorders validated in the Thai setting by Tangwongchai, Phanasathit, Charernboon, Akkayagorn, Hemrungrojn, Phanthumchinda and Nasreddine.33 This test measures various cognitive domains, namely: visuospatial/executive, naming, attention, language, abstraction, delayed recall, and orientation. The total sum of all individual scores (out of 30 maximum possible points) represents the severity of cognitive impairment. The CERAD was developed in 1986 by the National Institute of Aging as a standardized, validated instrument to assess Alzheimer’s dementia. In the current study, we employ the Neuropsychological Assessment Battery (CERAD-NP), in a Thai validated translation.7 In this study, we used: the Word List Memory (WLM) to assess episodic memory and learning ability for new verbal information and immediate working memory; and Word List Recall, Delayed, True Recall (WLR) recognize to probe verbal episodic memory and the ability to recall. Verbal Fluency Test (VFT), assessing semantic memory or fluency, verbal productivity, language, and cognitive flexibility.
Psychological Symptoms
Neuroticism traits were assessed with the Thai version of the 8-item subscale of the Five Factor Model standardized psychometric pool of items (IPIP-NEO; See http://ipip.ori.org).36 The neuroticism subscale measures one’s tendency to experience negative emotions (“Have frequent mood swings”), and each item was rated on a 5-point Likert scale of 1 (Strongly Disagree) to 5 (Strongly Agree).
Stress symptoms were assessed using the 10-item Perceived Stress Scale (PSS) developed by Cohen and Williamson37 and validated for use in a Thai setting by Wongpakaran and Wongpakaran.38 The scale assesses symptoms experienced during the last month preceding the survey. Each item is rated on a 5-point Likert-type scale from 0 (never) to 4 (very often). A higher score indicates greater distress. The Negative Life Events (NLE) is used to assess daily hassles.39 The NLE comprises 46 items covering the interpersonal stress in 10 domains, namely: household (6 items), problems with your work supervisor/employer (4 items), problems with parents (4 items), problems with spouse/partner (5 items), money hassles (4 items), problems with children (3 items), problems with friends (4 items), problems with other relatives (4 items), health hassles (4 items), problems with other workers (4 items), and work hassles (4 items). Participants indicate how much of a hassle each of the stressors was for them, using a 5-point Likert scale ranging from 0 (no hassle) to 4 (extreme hassle), and the scores were totalled to produce an overall hassles score. The NLE was back translated into Thai by Boonyamalik.40
Anxiety symptoms were assessed with the 20-item State-Trait Anxiety Inventory (STAI) developed by Spielberger, Gorsuch, Lushene, Vagg and Jacobs,41 and has been validated in the Thai setting by Iamsupasit and Phumivuthisarn.42 Each item is rated on a 4-point Likert scale ranging from 1 (not at all) to 4 (mostly). The total score ranges from 20–80 and higher scores are associated with greater feelings of anxiety. We also used the anxiety subscale of the Hospital Anxiety and Depression Scale (HADS-A) to assess severity of anxiety.43 The scale has been validated in the Thai setting by Nilchaikovit.44 This instrument consists of seven items rated on a four-point scale from 0 (not at all) to 3 (very often indeed), with five items reverse coded. The scores of the seven items were summed to create a scale that ranged from 0 to 21, with higher scores indicating more symptoms of anxiety. At the cutoff score ≥ 11, which was the best cutoff score, the sensitivity of the anxiety subscale of Thai HADS was 100% and the sensitivity was 86%.
The Geriatric Depression Scale (GDS) is a 30-item self-report measure designed to assess and screen depressive symptoms among older adults.45 It is a popular and widely used screening test to measure depression. The scale was validated and used with Thai elderly people for nearly three decades46 (Train the Brain Forum Committee, 1994), with each response rated on a dichotomous scale of 0 and 1. A total score greater than 12 indicates depression, with higher scores suggesting a higher level of depression. The pooled sensitivity and specificity of Thai GDS-30 are 82% and 76%.47 In addition, we used the depression subscale of the Hospital Anxiety and Depression Scale (HADS-D).43 It has been validated in the Thai setting by Nilchaikovit.44 This instrument consists of seven items rated on a four-point scale from 0 (not at all) to 3 (very often indeed), with three items reverse coded. The scores of the seven items were summed to create a scale that ranged from 0 to 21, with higher scores indicating more symptoms of depression. At the cutoff score ≥ 11, which was the best cutoff score, the sensitivity of the depression subscale of Thai HADS was 85.71% respectively, while the specificity was 91.3%.
The original Adverse Childhood Experiences (ACE) study was used to identify childhood experiences of abuse or neglect at Kaiser Permanente from 1995 to 1997 with two waves of data collection.48 ACE were assessed using the Adverse Childhood Experiences Questionnaire in a Thai translation.49 This questionnaire consists of 28 items covering the traumatic experiences in childhood in 10 domains, namely: psychological abuse (2 items), physical abuse (2 items), sexual abuse (4 items), mental neglect (5 items), physical neglect (5 items), domestic violence (4 items), substance abuse in the family (2 items), family psychiatric illness (2 items), separation or divorce in the family (1 item), and family members in criminals (1 item).49
Data Analysis
Differences in continuous variables between groups were checked using analysis of variance (ANOVA). Analysis of contingency tables (the χ2-test) was used to determine the association between nominal variables. Correlations between two variables were assessed using Pearson’s product-moment correlation coefficients. Univariate general linear model (GLM) analysis was used to examine the relationships between diagnostic classifications and clinical and cognitive data after covarying for gender, age, and education. Subsequently, the estimated marginal means (SE) were computed from the GLM model after adjusting for the gender, age, and education variables. To examine pair-wise differences in group means, we employed the protected least significant difference. We performed multiple regression analysis to determine which test scores best predicted the clinical scores using a stepwise algorithm and to compute and display partial regression analysis of clinical data. For this analysis, we always confirmed multivariate normality, homoscedasticity, and the absence of collinearity and multicollinearity. The regression analyses’ results were always bootstrapped using 5000 bootstrap samples, and the latter were reported if the findings were not concordant. Statistical tests were two-tailed and a p-value of 0.05 was used for statistical significance. IBM SPSS Windows version 29 was used for all statistical analyses.
Using the precision nomothetic approach,46 we aim to construct new phenotypes or phenotype dimensions by combining clinical data, rating scale scores and neurocognitive tests results. A two-step cluster analysis was performed considering categorical and continuous variables. The adequacy of the cluster solution was determined by evaluating the silhouette measure of cohesion and separation, which was deemed satisfactory if it exceeded a threshold of 0.3. Principal components analysis (PCA) was performed, and the Kaiser–Meyer–Olkin (KMO) sample adequacy measure was used to assess factorability (sufficient when > 0.7). Moreover, when all loadings on the first factor were > 0.66, the variance explained by the first factor was > 50%, and Cronbach’s alpha performed on the variables was > 0.7, the first PC was regarded as a valid latent construct underpinning the variables.
Partial Least Squares path analysis (SmartPLS 4.0) was employed to check the paths between ACE, NLE, CERAD, MCI, and DSOA scores. Each variable was input as a single indicator (eg, age and education; NLE health+money) or as reflective or formative constructs. We entered two reflective models: a) a first factor extracted from neuroticism, HADS-D, HADS-A, PSS, STAI, and GDS (thus reflecting the DSOA construct), and b) a second from MMSE, MOCA and Petersen item 1 (thus reflecting MCI). Moreover, we entered two formative models: a) a first based on emotional abuse, emotional neglect, and physical neglect (a composite of ACE); and b) a second formative model based on three CERAD test results, namely VFT, WLM and WLR. We conducted complete PLS analysis using 5000 bootstrap samples only when the outer and inner models complied with quality data, namely the factors have an adequate average variance extracted (AVE) > 0.5, and rho a > 0.8; all LV loadings are > 0.66 at p < 0.001; the model fit as assessed with SRMR is adequate (SRMR < 0.08); and Confirmatory Tetrad analysis (CTA) shows that the LV is not mis specified as a reflective model.
Results
Results of Factor Analyses
We employed principal component analysis (PCA) to examine whether latent vectors could be extracted from neuroticism (IPIP-NEO subdomain), PSS, HADS-A, HADS-D, STAI, TGDS. Table 1 shows the results of these PC analyses. The first PCA showed a sufficient factorability of the correlation matrix and the first factor explained 60.14% of the variance and all factor loadings were > 0.670 with an adequate Cronbach’s alpha value. This first PC, therefore, was dubbed the “Distress Symptoms of Old Age” or “DSOA”. We could also extract a single latent vector from the MoCA, TMSE, CERAD-WLR, and CERAD WLM with adequate KMO and explained variance values, and a sufficient Cronbach’s alpha value (see Table 1). Because this PC reflect a mild cognitive dysfunction, it was labelled as mCoDy. The VFT and CDR scores did not load highly on this PC (<0.5) and were, therefore, deleted from the PCA #2 model. Nevertheless, we were able to extract one validated PC from the MoCa, MMSE and CDR scores (see Table 1). Because this PC comprises the CDR score (0: control, 0.5: aMCI) we called this PC “quantitative MCI (qMCI)”. As such, we computed two different cognitive impairment scores, a first focusing on mild memory impairments and a second on a mild, more generalized cognitive decline. We were unable to combine any of the cognitive test results with the affective, stress, anxiety, or neuroticism scores. Figure 1 shows that there is no significant association between the DSOA and mCoDy PC scores.
 |
Table 1 Results of Principal Component Analyses (PCA) |
 |
Figure 1 Partial regression plot of the mild cognitive dysfunction (mCoDy) score on the distress symptoms of old age (DSOA) score (p=0.336, after adjustment for sex, age and education). |
Socio-Demographic and Clinical Data
A two-step clustering analysis with automatic determination of the number of clusters and Schwarz’s Bayesian Criterion (BIC) was conducted to determine if genuine clusters of subjects with aMCI or affective symptoms could be identified. Toward this end we performed two-step cluster analysis using the following variables: clinical diagnosis of aMCI, neuroticism, PSS, STAI, HADS-A, HAD-D, TGDS, WLM, WLR, VFT, MoCa and MMSE scores. Automatic determination of the number of clusters showed that three clusters could be retrieved from the data set with a silhouette measure of cohesion and separation of 0.4, indicating a fair cluster solution. There were 37 subjects in cluster 1, 31 in cluster 2, and 52 in cluster 3.
Table 2 shows the features of the three groups. Cluster 3 individuals were significantly older and has significantly lower education years than cluster 1 and cluster 2 individuals. Of the 59 healthy controls included in the study, the cluster analyses allocated 34 to the control group, 22 to cluster 2 and 3 to cluster 3. Of the 61 subjects with aMCI included in the study, the cluster analysis allocated 49 to cluster 3, 9 to cluster 2 and 3 to cluster 1 (see Table 2). From the demographics and clinical features of the three study groups, no significant differences were found in sex, or marital status distribution between the three groups. The DSOA score are significantly different between the three cluster and increased from cluster 1 to cluster 3 to cluster 2. The mCoDy scores were significantly greater in cluster 3 than in cluster 1 and 2; there were no significant differences between cluster 1 and 2 in the mCoDy scores. Therefore, we have labelled cluster 2 as a DSOA cluster, cluster 2 as a mCoDy cluster, and cluster 1 as the control cluster. The differences between these three clusters in DSOA (F-49.30, df=2/113, p<0.001) and mCoDy (F=78.01, df=2/113, p<0.001) score as well as the intergroup differences remained significant after covarying for age, sex, and education. Neuroticism, PSS, HADS-A, HADS-D, and STAI scores were significantly different between the three groups and increased from controls to mCoDy to DSOA. VFT, WLM, WLR and MMSE scores were significantly lower in mCoDy participants than in controls and DSOA subjects. The MoCA score was significantly different between the three clusters as decreased from controls to the DSOA to mCoDy clusters.
Differences in ACE and NLE Scores Between the Diagnostic Groups
Table 3 shows that the ACE and NLE scores in the three clusters. While there were no significant differences in the 5 ACE that were examined in this study, subjects with DSOA showed significant increased ACE scores as compared with controls. Although there was no overall significance between the three clusters in total NLE score, there was a trend towards higher NLE scores in the DSOA cluster as compared with the control cluster, while subjects in the mCoDy cluster took up an intermediate position. Examining the separate NLE items showed that the sum of money + health NLE scores was significantly higher in both the DSOA and mCoDy subjects as compared with controls.
Prediction of the DSOA and mCoDy Scores
We performed different multiple regression analyses using the DSOA and mCoDy scores as dependent variables and age, sex, education, NLE, and ACE data as explanatory variables (with and without Petersen item 1) See Table 4. Regression #1 shows that 32.8% of the variance in DSOA score could be explained by NLE health+money, ACE neglect, and education (all three positively associated). Figure 2 shows the partial regression plot (after considering the effects of age, sex, education) of the DSOA score on NLE health+money showing a significant positive association between both variables. Figure 3 shows the partial regression plot of the DSOA score on ACE neglect (after adjusting for age, sex, and education). Figure 4 shows the partial regression plot of the mCoDy score on education years. Table 4, regression #2 shows the prediction of the DSOA score was somewhat improved after considering the effects of Petersen, item 1. Thus, 37.9% of the variance in DSOA score could be explained by NLE health+money, ACE neglect, education, and Petersen_1 (all positively associated). The latter is important as it shows that subjective feelings of cognitive impairment, but not objective measurements of cognitive impairment, may increase DSOA.
 |
Table 4 Results of Multiple Regression Analyses with Distress Symptoms of Old Age (DSOA) and Mild Cognitive Dysfunction (mCoDy) Scores as Dependent Variables |
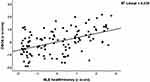 |
Figure 2 Partial regression plot of the distress symptoms of old age (DSOA) score on negative life events (health + money) (p<0.001). |
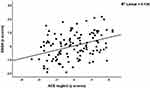 |
Figure 3 Partial regression plot of the distress symptoms of old age (DSOA) score on ACE physical + emotional neglect (p=0.002). |
 |
Figure 4 Partial regression plot of the mild cognitive dysfunction score (mCoDy) on education years (p<0.001). |
Table 4, regression #3 shows that 19.0% of the variance in the mCoDy score was explained by the combined effects of age and education. There were no significant effects of the NLE or ACE variables on the mCoDy score.
Results of PLS-Path Analysis
Figure 5 shows the final PLS model after feature reduction (eg, sex was excluded as there were no significant effects). We entered Petersen item 1, qMCI (a proxy of the MCI diagnosis), and the CERAD tests results as predictors of DSOA to examine if the latter was predicted by any of these clinical cognitive data. We entered two latent vectors, one reflecting clinical MCI symptom severity (extracted from CRC, MoCA and MMSE scores), and a second reflecting DSOA (extracted from HADS_A, HADS_D, neuroticism, PSS, STAI, and TGDS scores). ACE and CERAD were entered as two formative models using emotional abuse and neglect, and physical neglect, and VFT, WLR, and WLM, respectively. All other variables were entered as simple indicators. The model fit was more than adequate with a SRMR of 0.055. We observed adequate convergence and construct reliability validity values for (a) the DSOA latent factor with AVE = 0.598, and rho_a = 0.871, while all loadings were > 0.680 at p < 0.001; and (b) the MCI factor with AVE = 0.770, and rho_a = 0.864, while the loadings of the MCI construct were > 0.771. Both latent vectors were not mis-specified as reflective models (results of CTA). Complete PLS analysis, performed using 5000 bootstraps, showed that 40.9% of the variance in the DSOA score was explained by the clinical Petersen item 1, ACE, NLE health + money, and education, whilst 28.9% of the variance in the clinical MCI score was explained by CERAD and education years. ACE and NLE did not have any effects on CERAD or the clinical MCI scores. Total effect analyses showed that ACE (t=3.65, p<0.001), NLE health+money (t=5.50, p<0.001) were the most important predictors of the DSOA score, followed at a distance by the clinical MCI score (t=2.38, p=0.018) and education (t=2.65, p=0.008).
Discussion
Presence of a DSOA Dimension in Older Adults
The primary finding of this research is that we were able to extract one validated principal component or factor from distress, anxiety, depression, and neuroticism scores in older adults without major depression and ADL dysfunctions. The positive contributors to this principal component encompass symptoms of distress (PSS), anxiety (HADS-A and STAI), depression (HADS-D and TGDS) and neuroticism. Earlier research conducted by Jirakran, Vasupanrajit, Tunvirachaisakul and Maes23 revealed that one principal component or factor could be extracted from anxiety, depression and neuroticism scores in adults with major depression. Neuroticism has been linked with mood and anxiety symptoms in both clinical50 and non-clinical51–53 research cohorts, as well as in cross-sectional investigations.54,55 Individual with increased neuroticism suffer from mood swings, feelings of guilt, anxiety, and depression, and they cope poorly with psychological stressors.23
Distress in old age is a common phenomenon and is often linked with a range of physical and psychological symptoms such as stress, anxiety, melancholy, and neuroticism.56 However, these symptoms do not always indicate major depression, which causes significant distress and impedes daily functioning.57 Although depression is a frequent psychiatric disorder in a geriatric population and a substantial risk factor for disability and mortality, it is inaccurately diagnosed in nearly half of all instances.57 Another study showed that the levels of stress, anxiety, depression, and neuroticism are correlated with symptoms of geriatric discomfort, such as melancholy, fatigue, ambiguous physical complaints, and withdrawal from activities or social circles.58
Consequently, the principal component derived here from distress, affective, and neuroticism scores in older adults without clinical depression and ADL dysfunctions reflects more a distress or psychological strain score rather than a “clinical depression” score. Therefore, we labelled this score the “Distress Symptoms of Old Age” (DSOA) score. As such, this is a new construct indicating that mild subjective distress symptoms may occur in part of the older adults independently from major depression. The subclinical symptom domain differs significantly from the behavioral and psychological symptoms of dementia (BPSD) which may occur in patients with dementia and some subjects with MCI.56 The latter symptoms domain encompass delusions, hallucinations, agitation/aggression, depression/dysphoria, anxiety, euphoria, apathy/indifference, disinhibition, irritability/lability, aberrant motor behaviour, nocturnal behavioral disturbances, and irregularities in appetite and eating.56 It should be added that this study excluded subjects with psychosis, either hallucinations or delusions, agitation, euphoria, or aberrant behaviours and thus excluded not only patients with major depression but also subjects with BPSD. This is important as some patients with aMCI may show BPSD.56
All in all, DSOA is a subclinical symptom domain that emphasizes the presence of distress, mild affective symptoms, and increased neuroticism scores and thus reflects mild psychological strain rather than major depression or BPSD.
Presence of Mild Cognitive Dysfunction (mCoDy) in Older Adults
The second major finding of this study is that we were able to extract one cognitive dimension (principal component or factor) comprising the MoCa, MMSE, WLM, and WLR scores and indicating mild cognitive dysfunction (mCoDy). Increasing mCoDy scores suggest a decline in specific cognitive functions, particularly episodic memory, as well as a more generalized cognitive decline among older adults. Importantly, the mCoDy score contains much more information than the binary diagnosis of MCI (scored as yes or no) and allows a quantitative statistical approach. In addition, this score shows that some older adults suffer from objective dysfunctions in episodic memory, and not only from subjective feelings of cognitive impairment.
The above findings extend previous papers suggesting that MCI is often characterized by a significant impairment in episodic memory, which is the ability to recall specific events, situations, and experiences.59 Even though episodic memory impairment is a characteristic of aMCI, not everyone with episodic memory problems will develop aMCI.59 Individuals with MCI often exhibit deficits in verbal fluency tasks,7 which is the cognitive function involving the ability to retrieve and produce words quickly based on specific criteria, such as a particular letter or a category.60 Nevertheless, in our study, the VFT scores could not be combined with the MMSE, MoCa, WLM and WLR scores into one factor because the loading of VFT scores on the first factor were much too low. As expected, we found that the mCoDy score was only partially (19.0%) explained by increasing age and lower levels of education (see also introduction). This leaves a significant proportion of the variance unexplained.
Importantly, in the present study we were unable to detect any correlation between the quantitative DSOA and mCoDy scores, indicating that both dimensions are independently from each other. Consequently, the DSOA score represents a novel subclinical concept even in the absence of a major depressive episode, BPSD and ADL dysfunctions. Nevertheless, we observed that Petersen’s item 1 was correlated with the DSOA score. This suggests that the subjective perception of cognitive deterioration may be linked with heightened distress and affective scores in older adults.61 This may be explained by the theory that the subjective feeling of cognitive impairment is indeed a distress for older adults.62–64
Effects of ACEs on the DSOA and mCoDy
The third major finding of this study is that ACE and NLE (especially health and money) have a highly significant impact on the DSOA, and no effects at all on the mCoDy domain scores. ACE and NLE are significant risk factors for depression and other psychological disorders in adulthood.23,24,65 Kraaij, Arensman and Spinhoven27 reported that almost all NLE have a small but significant association with depression. The total number of negative life events and everyday problems appeared to have the strongest link with depression.27,66 Consequently, our results indicate that the DSOA dimensional score reflects in part the distress that originates from relevant NLE and that previous ACE may cause together with NLE a much stronger impact on the DSOA score. As such, the combination of ACE, NLE and subjective feelings of cognitive impairments are together major predictors of the increasing DSOA scores in older adults. This perspective acknowledges the impact of negative life experiences (ACE and NLE) on mental health and proposes that DSOA scores (comprising the neuroticism trait) may be partly determined by the individual’s resilience and coping mechanisms.
The final question is then whether DSOA should be regarded either as a psychopathological dimension or as a human response to environmental stressors, such as NLE and negative formative experiences. The first viewpoint considers that DSOA are not a normal part of aging, but rather a sign of pathology that requires treatment or clinical intervention. On the other hand, if these symptoms are seen as a response to environmental stressors only, it implies that they are a normal reaction to the challenges and hardships that an individual has encountered throughout their life. Clinicians could use the subjective DSOA complaints as a criterium whether psychotherapeutic intervention is indicated or not. Alternatively, future biomarker research should determine whether the DSOA have pathway substrates indicative of physiological stress (eg, cortisol axis, immune activation, oxidative stress) that should be targeted by novel treatments.
Regarding the mCoDy score, prior research findings may require revision, because previous suggestions that NLE may accelerate cognitive decline in older adults12,13 may not be correct after excluding subjects with clinical depression (this study). Also, studies showing that ACE are substantially correlated with cognitive impairments in older adults9–11 cannot be confirmed after excluding subjects with major depression (this study). In this respect, one study reported that ACE had a negative effect on cognitive function in middle-aged and older adults and that depression mediated this relationship.23,67 However, since we excluded individuals with major depression, no such mediating effects may be observed.
DSOA and mCoDy Clusters
This study’s fourth significant finding is that cluster analysis identified three clusters of people based on mCoDy and DSOA scores and that these classes do not align with the clinically defined distinction between aMCI and healthy controls. Thus, three cases diagnosed with aMCI were allocated to the healthy control group, while three healthy controls were assigned to the aMCI group. In addition, the cluster analysis uncovered a new cluster that was shaped by DSOA rating scales, and part of the aMCI cases were allocated to that new cluster. The DSOA cluster included healthy controls (70.96%) and individuals with aMCI (29.04%). As such, cluster analysis derived two classes of individuals, a first with DSOA, and another with mCoDy. The first cluster showed much higher scores on all DSOA rating scales (except TGDS), and the latter cluster showed lower cognitive scores on MMSE, MoCA, WLM, WLR and VFT.
Without computing the DSOA scores and without cluster analysis we would never have been able to uncover that part of aMCI individuals and “healthy controls” may be allocated to a DSOA class. Our results indicate that the clinical diagnosis based on Petersen criteria is overinclusive, as it includes subjects who belong to the DSOA group. The latter cases might be clinical misclassifications, whereby cases with DSOA may mistakenly be classified as aMCI, presumably because they also exhibit subjective cognitive symptoms (Petersen item 1), although neuropsychological testing did not show any objective signs of cognitive decline. In addition, previous papers have demonstrated that the Petersen criteria are overinclusive.7,8 As a result, we propose the term “mild cognitive dysfunction” (mCoDy) as an alternative to the overinclusive aMCI diagnosis, and the quantitative mCoDy score as a better alternative than the incorrect (as overinclusive) binary (limited information) aMCI diagnosis.
All in all, this study clarified why the diagnosis of aMCI according to the Petersen criteria is overinclusive, because this diagnosis includes subjects with DSOA who have subjective feelings of cognitive difficulties but lack objective signs of cognitive decline.
Limitations and Future Research
The current results deserve replication in individuals without clinical depression and ADL dysfunctions in other countries and cultures. Future research should examine whether the DSOA and mCoDy scores can be externally validated by any physiological stress-associated pathways that may impact affect or neurocognition, including immune, oxidative stress, neurotrophic, and neuroplasticity pathways. In addition, longitudinal studies are needed to explore long-term outcomes of DSOA.
Conclusion
The study concludes that in older adults without major depression and ADL dysfunctions, mCoDy and DSOA are two distinct dimensions. The DSOA dimensions is influenced by ACE, NLE, and the subjective feeling of cognitive decline, whereas the mCoDy score is affected by aging and lower education. This distinction is crucial for understanding and addressing mental health issues in older adults without major depression. The results also highlight the long-term effects of ACE in early life and NLE on mental health in older adults. The clinical impact of the current study is that clinicians should carefully screen older adults for DSOA after excluding a major depressive episode and BPSD. This may be important for the treatment of DSOA versus mCoDy. While the former is associated with psychological trauma and psychosocial stressors, psychotherapeutic interventions may be needed, whereas mCoDy might be treatable instead with lifestyle interventions, including physical activity68 and perhaps specific antioxidant treatments targeting the oxidative stress pathways.69
Institutional Review Board Statement
This study was approved by the Institutional Review Board (IRB) of the Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand (IRB no. 0372/65), which complies with the International Guideline for Human Research Protection as required by the Declaration of Helsinki.
Informed Consent
Before taking part in the study, all participants and/or their caregivers provided written informed consent.
Data Sharing Statement
The dataset generated during and/or analysed during the current study will be available from MM upon reasonable request and once the authors have fully exploited the dataset.
Acknowledgment
Vinh-Long Tran-Chi and Michael Maes contributed equally to this work and share first authorship.
Funding
This research is supported by the Ratchadapisek Sompoch Fund, Faculty of Medicine, Chulalongkorn University (Grant no. GA66/037); The 90th Anniversary of Chulalongkorn University Scholarship under the Ratchadapisek Somphot Endowment Fund (Grant no. GCUGR1125661006D), Thailand. MM received funding from the Thailand Science Research and Innovation Fund, Chulalongkorn University (Grant no. HEA663000016), and a Sompoch Endowment Fund, Faculty of Medicine, Chulalongkorn University (Grant no. RA66/016).
Disclosure
MS received honoraria and has been a consultant for AbbVie, Angelini, Lundbeck, Otsuka. Other authors declare that they have no known competing financial interests or personal relationships that could have influenced the work reported.
References
1. Anderson ND. State of the science on mild cognitive impairment (MCI). CNS Spectrums. 2019;24(1):78–87. doi:10.1017/S1092852918001347
2. Dwolatzky T, Whitehead V, Doniger GM, et al. Validity of the Mindstreams™ computerized cognitive battery for mild cognitive impairment. J Mol Neurosci. 2004;24:33–44. doi:10.1385/JMN:24:1:033
3. Gualtieri CT, Johnson LG. Neurocognitive testing supports a broader concept of mild cognitive impairment. Am J Alzheimers Dis Other Demen. 2005;20(6):359–366. doi:10.1177/153331750502000607
4. Hemrungrojn S, Tangwongchai S, Charoenboon T, et al. Use of the Montreal cognitive assessment Thai version to discriminate amnestic mild cognitive impairment from alzheimer’s disease and healthy controls: machine learning results. Dementia Geriatric Cognit Disord. 2021;50(2):183–194. doi:10.1159/000517822
5. Petersen RC, Aisen PS, Beckett LA, et al. Alzheimer’s Disease Neuroimaging Initiative (ADNI): clinical characterization. Neurology. 2010;74(3):201–209. doi:10.1212/WNL.0b013e3181cb3e25
6. Fillenbaum GG, van Belle G, Morris JC, et al. Consortium to Establish a Registry for Alzheimer’s Disease (CERAD): the first twenty years. Alzheimer’s Dementia. 2008;4(2):96–109. doi:10.1016/j.jalz.2007.08.005
7. Tunvirachaisakul C, Supasitthumrong T, Tangwongchai S, et al. Characteristics of mild cognitive impairment using the Thai version of the consortium to establish a registry for Alzheimer’s disease tests: a multivariate and machine learning study. Dementia Geriatric Cognit Disord. 2018;45(1):38–48. doi:10.1159/000487232
8. Tran-Chi V-L, Amrapala A, Nantachai G, Hemrungrojn S, Tunvirachaisakul C, Maes M. Cognitive features of amnestic mild cognitive impairment using specific Cambridge neuropsychological test automated battery test scores. medRxiv. 2022;1–35. doi:10.1101/2022.06.09.22276176
9. Petkus AJ, Lenze EJ, Butters MA, Twamley EW, Wetherell JL. Childhood trauma is associated with poorer cognitive performance in older adults. J Clini Psych. 2017;79(1):1–14. doi:10.4088/JCP.16m11021
10. Ritchie K, Jaussent I, Stewart R, et al. Adverse childhood environment and late‐life cognitive functioning. Int J Geriatr Psychiatry. 2011;26(5):503–510. doi:10.1002/gps.2553
11. Wang L, Yang L, Yu L, et al. Childhood physical neglect promotes development of mild cognitive impairment in old age–A case-control study. Psychiatry Res. 2016;242:13–18. doi:10.1016/j.psychres.2016.04.090
12. Rosnick CB, Small BJ, McEvoy CL, Borenstein AR, Mortimer JA. Negative life events and cognitive performance in a population of older adults. J Aging Health. 2007;19(4):612–629. doi:10.1177/0898264307300975
13. Nilaweera D, Gurvich C, Freak-Poli R, et al. Adverse events in older adults and the risk of dementia and cognitive decline. J Affect Disord. 2023;13:1–8. doi:10.1016/j.jadr.2023.100592
14. Solmi M, Veronese N, Galvano D, et al. Factors associated with loneliness: an umbrella review of observational studies. J Affective Disord. 2020;271:131–138. doi:10.1016/j.jad.2020.03.075
15. Byers AL, Yaffe K. Depression and risk of developing dementia. Nat Rev Neurol. 2011;7(6):323–331. doi:10.1038/nrneurol.2011.60
16. Arango C, Dragioti E, Solmi M, et al. Risk and protective factors for mental disorders beyond genetics: an evidence‐based atlas. World Psychiatry. 2021;20(3):417–436. doi:10.1002/wps.20894
17. de Pablo GS, Solmi M, Vaquerizo-Serrano J, et al. Primary prevention of depression: an umbrella review of controlled interventions. J Affective Disord. 2021;294:957–970. doi:10.1016/j.jad.2021.07.101
18. Dragioti E, Radua J, Solmi M, et al. Global population attributable fraction of potentially modifiable risk factors for mental disorders: a meta-umbrella systematic review. Mol Psychiatry. 2022;27(8):3510–3519. doi:10.1038/s41380-022-01586-8
19. Ma L. Depression, anxiety, and apathy in mild cognitive impairment: current perspectives. Front Aging Neurosci. 2020;12:1–8. doi:10.3389/fnagi.2020.00009
20. Ismail Z, Elbayoumi H, Fischer CE, et al. Prevalence of depression in patients with mild cognitive impairment: a systematic review and meta-analysis. JAMA Psychiatry. 2017;74(1):58–67. doi:10.1001/jamapsychiatry.2016.3162
21. Fiske A, Gatz M, Pedersen NL. Depressive symptoms and aging: the effects of illness and non-health-related events. J Gerontol B Psychol Sci Soc Sci. 2003;58(6):320–328. doi:10.1093/geronb/58.6.P320
22. Kosciuszko M, Steptoe A, Ajnakina O. Genetic propensity, socioeconomic status, and trajectories of depression over a course of 14 years in older adults. Transl Psychiatry. 2023;13(1):1–8. doi:10.1038/s41398-023-02367-9
23. Jirakran K, Vasupanrajit A, Tunvirachaisakul C, Maes M. The effects of adverse childhood experiences on depression and suicidal behaviors are partially mediated by neuroticism: a subclinical manifestation of major depression. Frontiers in Psychiatry. 2023;14:1–13. doi:10.3389/fpsyt.2023.1158036
24. Maes M, Almulla AF. Research and diagnostic algorithmic rules (radar) and radar plots for the first episode of major depressive disorder: effects of childhood and recent adverse experiences on suicidal behaviors, Neurocognition and phenome features. Brain Sci. 2023;13(5):1–20. doi:10.3390/brainsci13050714
25. Cheong EV, Sinnott C, Dahly D, Kearney PM. Adverse childhood experiences (ACEs) and later-life depression: perceived social support as a potential protective factor. BMJ Open. 2017;7(9):1–11. doi:10.1136/bmjopen-2016-013228
26. Cairney J, Krause N. Negative life events and age-related decline in mastery: are older adults more vulnerable to the control-eroding effect of stress? J Gerontol B Psychol Sci Soc Sci. 2008;63(3):162–170. doi:10.1093/geronb/63.3.S162
27. Kraaij V, Arensman E, Spinhoven P. Negative life events and depression in elderly persons: a meta-analysis. J Gerontol B Psychol Sci Soc Sci. 2002;57(1):87–94. doi:10.1093/geronb/57.1.P87
28. Maes M. Psychological stress, cytokines and the inflammatory response system. Curr Opin Psychiatry. 1999;12(6):695–700. doi:10.1097/00001504-199911000-00019
29. Maes M. Psychological stress and the inflammatory response system. Clin Sci. 2001;101(2):193–194. doi:10.1042/cs1010193
30. Li J, Lin S, Pei L. Adverse childhood experiences and depressive symptoms trajectories among middle-aged and elderly—China, 2011–2018. China CDC Weekly. 2022;4(27):588–592. doi:10.46234/ccdcw2022.129
31. Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256(3):183–194. doi:10.1111/j.1365-2796.2004.01388.x
32. Committee TTBF. Thai Mental State Examination (TMSE). Siriraj Hospital Gaz. 1993;45(6):359–374.
33. Tangwongchai S, Phanasathit M, Charernboon T, et al. The validity of Thai version of the Montreal cognitive assessment (MoCA-T). Dement Neuropsychol. 2009;3(2):172.
34. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi:10.1016/0022-3956(75)90026-6
35. Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. doi:10.1111/j.1532-5415.2005.53221.x
36. Yomaboot P, Cooper AJ. Factor structure and psychometric properties of the International Personality Item Pool-NEO (IPIP-NEO) Thai version. J Somdet Chaopraya Inst Psychiatry. 2016;10(2):36–49.
37. Cohen S, Williamson GM. Stress and infectious disease in humans. Psychol Bull. 1991;109(1):5–24. doi:10.1037/0033-2909.109.1.5
38. Wongpakaran N, Wongpakaran T. The Thai version of the PSS-10: an Investigation of its psychometric properties. Biopsychosoc Med. 2010;4:1–6. doi:10.1186/1751-0759-4-6
39. Maybery D, Neale J, Arentz A, Jones-Ellis J. The negative event scale: measuring frequency and intensity of adult hassles. Anxiety Stress Coping. 2007;20(2):163–176. doi:10.1080/10615800701217654
40. Boonyamalik P. Epidemiology of Adolescent Suicidal Ideation: Roles of Perceived Life Stress, Depressive Symptoms, and Substance Use. The Johns Hopkins University; 2005.
41. Spielberger CD, Gorsuch R, Lushene R, Vagg P, Jacobs G. Manual for the state-trait anxiety scale. Consult Psychol. 1983;1983:1.
42. Iamsupasit S, Phumivuthisarn P. A study of cognitive factors related to anxiety sensitivity.
43. Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983;67(6):361–370. doi:10.1111/j.1600-0447.1983.tb09716.x
44. Nilchaikovit T. Development of Thai version of hospital anxiety and depression scale in cancer patients. J Psychiatr Assoc Thai. 1996;4:18–30.
45. Yesavage JA. Imagery pretraining and memory training in the elderly. Gerontology. 1983;29(4):271–275. doi:10.1159/000213126
46. Poungvarin N, Committee TT. Thai geriatric depression scale-TGDS. Siriraj Hospital Gaz. 1994;46:1–9.
47. Krishnamoorthy Y, Rajaa S, Rehman T. Diagnostic accuracy of various forms of geriatric depression scale for screening of depression among older adults: systematic review and meta-analysis. Arch Gerontol Geriatr. 2020;87:2–11. doi:10.1016/j.archger.2019.104002
48. Felitti VJ, Anda RF, Nordenberg D, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults: the Adverse Childhood Experiences (ACE) Study. Am J Preventive Med. 1998;14(4):245–258. doi:10.1016/S0749-3797(98)00017-8
49. Rungmueanporn L, Buathong N, Chandarasiri P, Wittayasai W. Development of the adverse childhood experiences (ACE) questionnaire Thai version. Chula Med Bulletin. 2019;1(3):251–260. doi:10.14456/chulamedbull.2019.21
50. Malouff JM, Thorsteinsson EB, Schutte NS. The relationship between the five-factor model of personality and symptoms of clinical disorders: a meta-analysis. J Psychopathol Behav Assess. 2005;27:101–114. doi:10.1007/s10862-005-5384-y
51. Jylhä P, Isometsä E. The relationship of neuroticism and extraversion to symptoms of anxiety and depression in the general population. Depression Anxiety. 2006;23(5):281–289. doi:10.1002/da.20167
52. Saklofske D, Kelly I, Janzen B. Neuroticism, depression, and depression proneness. Pers Individ Dif. 1995;18(1):27–31. doi:10.1016/0191-8869(94)00128-F
53. Uliaszek AA, Hauner KK, Zinbarg RE, et al. An examination of content overlap and disorder-specific predictions in the associations of neuroticism with anxiety and depression. J Res Personality. 2009;43(5):785–794. doi:10.1016/j.jrp.2009.05.009
54. Griffith JW, Zinbarg RE, Craske MG, et al. Neuroticism as a common dimension in the internalizing disorders. Psychol Med. 2010;40(7):1125–1136. doi:10.1017/S0033291709991449
55. Kotov R, Gamez W, Schmidt F, Watson D. Linking “big” personality traits to anxiety, depressive, and substance use disorders: a meta-analysis. Psychol Bull. 2010;136(5):768–821. doi:10.1037/a0020327
56. Hemrungrojn S, Tangwongchai S, Charernboon T, et al. Cognitive impairments predict the behavioral and psychological symptoms of dementia. Front Neurol. 2023;14:1–9. doi:10.3389/fneur.2023.1194917
57. Zenebe Y, Akele B, Necho M, Necho M. Prevalence and determinants of depression among old age: a systematic review and meta-analysis. Ann Gen Psychiatry. 2021;20(1):1–19. doi:10.1186/s12991-021-00375-x
58. Fabbri C, Mutz J, Lewis CM, Serretti A. Depressive symptoms and neuroticism-related traits are the main factors associated with wellbeing independent of the history of lifetime depression in the UK Biobank. Psychol Med. 2023;53(7):3000–3008. doi:10.1017/S003329172100502X
59. Chatzikostopoulos A, Moraitou D, Tsolaki M, et al. Episodic Memory in Amnestic Mild Cognitive Impairment (aMCI) and Alzheimer’s Disease Dementia (ADD): using the “doors and people” tool to differentiate between early aMCI—Late aMCI—Mild ADD diagnostic groups. Diagn. 2022;12(7):1768. doi:10.3390/diagnostics12071768
60. Cottingham ME, Hawkins KA. Verbal fluency deficits co-occur with memory deficits in geriatric patients at risk for dementia: implications for the concept of mild cognitive impairment. Behav. Neurol. 2009;22(3–4):73–79. doi:10.3233/ben-2009-0246
61. Chapman S, Weiss D, Broulíková HM, et al. Examining the role of aging perceptions in subjective cognitive decline. Alzheimer Dis Associated Disord. 2022;36(4):288–294. doi:10.1097/WAD.0000000000000518
62. Sunderland M, Hobbs MJ, Anderson TM, Andrews G. Psychological distress across the lifespan: examining age-related item bias in the Kessler 6 psychological distress scale. Int Psychogeriatr. 2012;24(2):231–242. doi:10.1017/S1041610211001852
63. Gondek D, Bann D, Patalay P, et al. Psychological distress from early adulthood to early old age: evidence from the 1946, 1958 and 1970 British birth cohorts. Psychol Med. 2022;52(8):1471–1480. doi:10.1017/S003329172000327X
64. Moustaka K, Nega C, Beratis IN. Exploring the impact of age of onset of mild cognitive impairment on the profile of cognitive and psychiatric symptoms. Geriatrics. 2023;8(5):96. doi:10.3390/geriatrics8050096
65. Fang M. The Effect of Adverse Childhood Experiences on Depression Symptoms Among Older Adults in China. University of Waterloo; 2019.
66. Almulla AF, Abo Algon AA, Maes MF. Adverse childhood experiences and recent negative events activate immune and growth factor pathways, which are associated with first episode major depression and suicidal behaviours. medRxiv. 2023;1–47. doi:10.1101/2023.06.19.23291597
67. Wang G, Zhou Y, Duan J, Kan Q, Cheng Z, Tang S. Effects of adverse childhood health experiences on cognitive function in Chinese middle-aged and older adults: mediating role of depression. BMC Public Health. 2023;23(1):1–16. doi:10.1186/s12889-023-16169-7
68. Veronese N, Soysal P, Demurtas J, et al. Physical activity and exercise for the prevention and management of mild cognitive impairment and dementia: a collaborative international guideline. Eur Geriatric Med. 2023;14:1–28. doi:10.1007/s41999-022-00716-3
69. Nantachai G, Vasupanrajit A, Tunvirachaisakul C, Solmi M, Maes M. Oxidative stress and antioxidant defenses in mild cognitive impairment: a systematic review and meta-analysis. Ageing Res Rev. 2022;79:101639. doi:10.1016/j.arr.2022.101639
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.