Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Disseminated Herpes Zoster with Decreased CD4 Counts in a HIV-Infected Patient
Authors Zeng L , Feng S, Yao L, Zhao J, Zhang G
Received 10 August 2023
Accepted for publication 17 October 2023
Published 2 November 2023 Volume 2023:16 Pages 3165—3170
DOI https://doi.org/10.2147/CCID.S429308
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Anne-Claire Fougerousse
Linxi Zeng,1 Sen Feng,1 Lulu Yao,1 Jiaqing Zhao,1 Guoqiang Zhang1,2
1Department of Dermatology, The First Hospital of Hebei Medical University, Shijiazhuang City, Hebei Province, People’s Republic of China; 2Candidate Branch of National Clinical Research Center for Skin Diseases, Shijiazhuang, People’s Republic of China
Correspondence: Guoqiang Zhang, The First Hospital of Hebei Medical University, 89 Donggang Road, Yuhua District, Shijiazhuang City, Hebei Province, People’s Republic of China, Email [email protected]
Abstract: Herpes zoster is typically a blister rash involving a single skin group, caused by the reactivation of primary varicella zoster virus infection. Disseminated herpes zoster refers to the presence of more than 20 small blisters outside the primary or adjacent skin, which is rare and usually occurs in individuals with weakened immune function. This case described a patient diagnosed with disseminated herpes zoster, with a decrease in CD4 count (379 cells/mm3) and certain skin lesions. He was subsequently screened positive for HIV. Also, we summarized other studies on the CD4 value of HIV patients with herpes zoster. Overall, for herpes zoster patients with decreased CD4 levels and certain skin manifestations, such as diffuse, ulcerative, or pustular lesions, clinicians should be aware of HIV infection.
Keywords: disseminated herpes zoster, CD4, HIV infection
Introduction
Varicella zoster virus (VZV) is a double-stranded DNA virus that causes varicella, its primary infection, and herpes zoster in recurrent infection. After initial exposure, VZV will lurk in the dorsal root ganglion. When immunity decreases, varicella shingles virus (VZV) will reactivate from its dormant state, resulting in herpes zoster.1 It usually manifests as a unilateral skin distributed blister rash associated with pain. Disseminated herpes zoster is usually defined as a systemic rash with more than 20 skin vesicles within a week after the onset of typical skin lesions. Disseminated herpes zoster is rare and occurs mainly in the elderly or infirm.2
Acquired immune deficiency syndrome (AIDS) is a very harmful infectious disease, caused by human immunodeficiency virus (HIV) infection. HIV can directly attack CD4+cells that play an important role in the human immune system. After infection, it can cause complete or partial loss of immune system function.3 Today, the disease burden of HIV is high, and the late diagnosis of HIV will increase the incidence rate and mortality. Early identification can initiate appropriate treatment as early as possible, thereby reducing the risk of opportunistic infections and transmission.4
Herpes zoster, as an opportunistic infection associated with HIV infection, may be the initial or early manifestation of HIV infection.5 Here, we report a case of disseminated herpes zoster patient who was subsequently found to be HIV positive. Notably, some characteristics of this patient may serve as predictive factors for HIV infection.
Case Description
A 77-year-old male was admitted to our dermatology clinic with a 1-week history of rash on the right side of his head and face, accompanied by pain and numbness. The rash gradually spread to the chest, back and limbs and his condition worsened. The patient was diagnosed with diabetes 10 years ago and was treated with gliclazide and metformin. He underwent cataract surgery 10 years ago and was diagnosed with neurological deafness 7 years ago, with almost complete loss of hearing. In addition, the patient denied history of sexual contact. Physical examination manifested that the right side of the head and face was swollen, and a large amount of erythema and dark purple spots were along the right mandibular nerve in a zonal distribution. Above the erythema and dark purple spots were clustered vesicles, pustules, and papules ranging from millet to rice. Some of them were ulcerated and exuded, and dark red crusts were on the surface. Apart from the primary site on the right side of his head and face, there were over 20 rice grain sized blisters and pustules scattered throughout the body, including the chest, back, and limbs, with blood scabs on some surfaces. Thus, he was diagnosed with disseminated herpes zoster (Figure 1). Laboratory test showed that CD4 T cells counted 16.49%, 379 cells/mm3 (500–1500 cells/mm3), CD8 T cells counted 56.15%, 1291 cells/mm3 (320–1250 cells/mm3) and the ratio of CD4 to CD8 was 0.29 (1.4–2.0). Subsequently, it was found that his HIV antibody was positive in the serum.
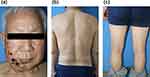 |
Figure 1 A case of disseminated herpes zoster with HIV infection in an elderly man. (a–c) are the conditions of the patient’s head/face, back, and limbs at admission, respectively. |
The patient was treated with penciclovir to fight against the virus, placental polypeptides to enhance immunity, oral mecobalamin tablets and vitamin B1 tablets to provide nutrition for nerves, gabapentin capsules and lovendaine sustained-release tablets to relieve pain. Moreover, he began receiving antiretroviral therapy (ART) and regular monitoring. On the 15th day, the patient’s symptoms of rash and pain significantly improved, and the number of CD4+T cells increased to 465/u1. It demonstrated that the right side of the head and face was slightly swollen, and there were only a small amount of erythema and dark purple spots along the nerve, with dark red scabs on the surface.
Discussion
HIV can invade the immune system and reduce the immunity of patients. These patients are more prone to opportunistic infections, with some common infections including pneumoconiosis pneumonia and candidiasis.6 Moreover, there are other manifestations of HIV infection, which may become a predictor of such patients. This case provided an HIV infected patient with herpes zoster as the first manifestation. Given his age, the patient was at a higher risk of developing herpes zoster. But we noticed that his symptoms of herpes zoster were severe, starting from the head and face, and rapidly spreading to the chest, back, and limbs, with skin lesions containing pustules and ulcers. In addition, his CD4 level was below normal, indicating deterioration of immune function. Although he denied a history of sexual contact, it was still necessary to screen for HIV. There were no other opportunistic infections in this case. And his CD4 level was greater than 200, indicating that he has not reached the advanced stage of HIV infection.7 For this patient, early detection of HIV infection was crucial. On one hand, early treatment can better control HIV infection. On the other hand, it can assist in the management of herpes zoster.
CD4+ T lymphocyte, as a key component of the immune system, are primary target cells for HIV. Generally, the number of CD4 cells will decline with the progress of AIDS, reflecting the severity of the disease.8 Furthermore, the CD4 cells count may be a clue for the onset of herpes zoster in HIV patients. Marshall et al9 found that a lower CD4 cell count is more closely related to the incidence of herpes zoster in HIV population. Among the HIV-infected women, using CD4 (750 cells/uL) as a reference, CD4 500–749 cells/uL was 1.43 (95% CI 0.86–2.37), CD4 350–499 cells/uL was 2.07 (95% CI 1.27–3.38), CD4 200–349 cells/uL was 2.72 (95% CI 1.66–4.46), and CD4 200 cells/uL was 3.16 (95% CI 1.92–5.18). Three studies from different countries have found that the average CD4 count of patients with HIV infection and herpes zoster decreased, with 152, 128 and 261, respectively.10–12 According to data from Thailand, the CD4 200–499/µL subgroup and CD4<200/µL subgroup had significantly more herpes zoster than the CD4≥500/µL subgroup in HIV population.13 A survey in China recorded 185 patients with HIV and herpes zoster, and found that there were significantly more patients with CD<200 than those with CD≥200 (P<0.05).14 A study in Turkey found that there was a significant correlation between herpes zoster and low CD4 cell count (P<0.05).15 However, a prospective cohort study from Uganda showed no significant correlation between CD4 cell count and herpes zoster in HIV patients.16 Anna et al17 reported 146 HIV infected people, 30 of whom developed herpes zoster. It showed that there was no significant difference in CD4 levels between individuals with and without herpes zoster.
Specifically, there are studies that separately investigate the incidence of disseminated herpes zoster in HIV patients. An observational study in Colombia found that the CD4 count of HIV patients with disseminated herpes zoster was between 200 and 499 cells/mm3, which was statistically significant (p=0.04). It was pointed out that the incidence of disseminated herpes zoster increased with the decrease of CD4 T cells.18 Catherine et al19 reported a middle-aged woman without other HIV risk factors. She had severe, disseminated, persistent herpes zoster with a low CD4 T cell count <200/mcL. She was later found to be HIV positive. However, Pooja et al20 reported disseminated herpes zoster with increased CD4 counts in 3 HIV-infected patients (case1=762, case2=420, case3=540). More studies and cases recording the CD4 count in patients with herpes zoster and HIV are summarized in Tables 1 and 2. Overall, most studies have shown that the count of CD4 has certain significance in the diagnosis of HIV patients with herpes zoster, promoting clinicians to screen for HIV in patients with decreased CD levels.
 |
Table 1 The Studies of the HIV-Infected Patients with Herpes Zoster |
 |
Table 2 The Cases of the HIV-Infected Patients with Herpes Zoster |
Meanwhile, the skin lesions characteristics of herpes zoster in HIV patients can also provide some clues. Herpes zoster in HIV-infected patient is usually very severe and can cause epidermal ulcers and necrosis, resulting in more severe scarring. It can diffuse and may have a prolonged course and poor prognosis.28 Philip et al29 described a 45-year-old man with HIV infection who was initially presented with herpes zoster. The patient’s characteristics are as follows: several adjacent skin blisters, multiple vesicles and pustules merging to form large blisters, extensive necrosis accompanied by scab formation, and very painful skin lesions. It should be noted that disseminated herpes zoster is more common in human immunodeficiency virus (HIV) positive patients compared to the general population. Bong et al30 reported that a 51-year-old man had segmental vesicles on his left upper trunk and arm and a varicella like rash on his whole body. He was diagnosed with disseminated herpes zoster, and then he was found to be HIV positive. John et al28 reported a case of HIV infected pregnant woman with disseminated herpes zoster as the initial manifestation.
Conclusion
In this case, the patient has disseminated herpes zoster, accompanied by ulcers, pustules, and blood crust, and the value of CD4+T cells is 379 cells/mm3 (16.49%). Subsequently, he was tested positive for HIV. In conclusion, if the CD4 level of herpes zoster patients decreases and certain skin manifestations develop, such as disseminated, ulcerative, or pustular lesions, it is necessary to screen for HIV.
Consent
We had obtained the patient’s written informed consent for the publication of this paper and the accompanying images. This case does not require institutional approval.
Disclosure
There is no conflict of interest in this work.
References
1. Kennedy PGE, Gershon AA. Clinical features of varicella-zoster virus infection. Viruses. 2018;10(11):609. doi:10.3390/v10110609
2. Ellis DL, Barsell A, Riahi RR, et al. Varicella zoster virus encephalitis in a patient with disseminated herpes zoster: report and review of the literature. Dermatol Online J. 2014;21:3.
3. Lv T, Cao W, Li T. HIV-related immune activation and inflammation: current understanding and strategies. J Immunol Res. 2021;2021:7316456. doi:10.1155/2021/7316456
4. Goldschmidt R, Chu C. HIV infection in adults: initial management. Am Fam Physician. 2021;103(7):407–416.
5. Duff P. Prevention of opportunistic infections in women with HIV infection. Clin Obstet Gynecol. 2019;62(4):816–822. doi:10.1097/grf.0000000000000483
6. Low A, Gavriilidis G, Larke N, et al. Incidence of opportunistic infections and the impact of antiretroviral therapy among HIV-infected adults in low- and middle-income countries: a systematic review and meta-analysis. Clin Infect Dis. 2016;62(12):1595–1603. doi:10.1093/cid/ciw125
7. Oboho IK, Paulin H, Corcoran C, et al. Modelling the impact of CD4 testing on mortality from TB and cryptococcal meningitis among patients with advanced HIV disease in nine countries. J Int AIDS Soc. 2023;26(3):e26070. doi:10.1002/jia2.26070
8. Sanchez-Martinez A, Perdomo-Celis F, Acevedo-Saenz L, et al. Cytotoxic CD4(+) T-cells during HIV infection: targets or weapons? J Clin Virol. 2019;119:17–23. doi:10.1016/j.jcv.2019.08.004
9. Glesby MJ, Hoover DR, Tan T, et al. Herpes zoster in women with and at risk for HIV: data from the Women’s Interagency HIV Study. J Acquir Immune Defic Syndr. 2004;37(5):1604–1609. doi:10.1097/00126334-200412150-00013
10. Gebo KA, Kalyani R, Moore RD, et al. The incidence of, risk factors for, and sequelae of herpes zoster among HIV patients in the highly active antiretroviral therapy era. J Acquir Immune Defic Syndr. 2005;40(2):169–174. doi:10.1097/01.qai.0000178408.62675.b0
11. Huang XJ, Li HY, Chen DX, et al. Clinical analysis of skin lesions in 796 Chinese HIV- positive patients. Acta Derm Venereol. 2011;91(5):552–556. doi:10.2340/00015555-1107
12. Josephine M, Issac E, George A, et al. Patterns of skin manifestations and their relationships with CD4 counts among HIV/AIDS patients in Cameroon. Int J Dermatol. 2006;45(3):280–284. doi:10.1111/j.1365-4632.2004.02529.x
13. Wiwanitkit V. Prevalence of dermatological disorders in Thai HIV-infected patients correlated with different CD4 lymphocyte count statuses: a note on 120 cases. Int J Dermatol. 2004;43(4):265–268. doi:10.1111/j.1365-4632.2004.01649.x
14. Li YY, Yang SH, Wang RR, et al. Effects of CD4 cell count and antiretroviral therapy on mucocutaneous manifestations among HIV/AIDS patients in Yunnan, China. Int J Dermatol. 2020;59(3):308–313. doi:10.1111/ijd.14725
15. Altuntaş Aydin Ö, Kumbasar Karaosmanoğlu H, Korkusuz R, et al. Mucocutaneous manifestations and the relationship to CD4 lymphocyte counts among Turkish HIV/AIDS patients in Istanbul, Turkey. Turkish J Med Sci. 2015;45(1):89–92. doi:10.3906/sag-1308-3
16. Morgan D, Mahe C, Malamba S, et al. Herpes zoster and HIV-1 infection in a rural Ugandan cohort. AIDS. 2001;15(2):223–229. doi:10.1097/00002030-200101260-00012
17. McNulty A, Li Y, Radtke U, et al. Herpes zoster and the stage and prognosis of HIV-1 infection. Genitourin Med. 1997;73(6):467–470. doi:10.1136/sti.73.6.467
18. Sanín AM, Londoño ÁM, Gil V, et al. Mucocutaneous manifestations and their relationship with CD4 T-lymphocyte count in hospitalized patients infected with the human immunodeficiency virus (HIV) in Medellín, Colombia. Biomedica. 2022;42(2):278–289. doi:10.7705/biomedica.6117
19. Brahe C, Ellis R. GOT SHINGLES? TEST FOR HIV. Severe shingles as first presenting infection in HIV/AIDS patient. IDCases. 2020;19:e00725. doi:10.1016/j.idcr.2020.e00725
20. Lidhoo P, Unemori P, Leslie KS, et al. Disseminated herpes zoster with increased CD4 counts in 3 HIV-infected patients. J Am Acad Dermatol. 2009;61(2):345–347. doi:10.1016/j.jaad.2008.11.891
21. Lameiras C, Patrocínio de Jesus R, Flor-de-lima B, et al. A case of varicella-zoster virus meningomyelitis in an HIV-1-infected patient: facing the challenges related to its management and prognosis. Cureus. 2022;14(8):e27652. doi:10.7759/cureus.27652
22. Muço E, Karruli A, Hoxha N, et al. Visceral leishmaniasis and herpes zoster as a component of syndrome of immune reconstitution inflammatory syndrome in an HIV-positive patient. Case Rep Infect Dis. 2022;2022:2784898. doi:10.1155/2022/2784898
23. Saxena R, Phuljhele S, Aalok L, et al. A rare case of orbital apex syndrome with herpes zoster ophthalmicus in a human immunodeficiency virus-positive patient. Indian J Ophthalmol. 2010;58(6):527–530. doi:10.4103/0301-4738.71708
24. Bender Ignacio RA, Ramchandani MS, Laing KJ, et al. T cell immunity to varicella-zoster virus in the setting of advanced HIV and multiple varicella-zoster virus recurrences. Viral Immunol. 2017;30(1):77–80. doi:10.1089/vim.2016.0097
25. Breton G, Bouldouyre MA, Gervais A, et al. Failure of valacyclovir for herpes zoster in a moderately immunocompromised HIV-infected patient. AIDS Patient Care STDS. 2004;18(5):255–257. doi:10.1089/108729104323075990
26. Handa S, Narang T, Wanchu A. Dermatologic immune restoration syndrome: report of five cases from a tertiary care center in north India. J Cutan Med Surg. 2008;12(3):126–132. doi:10.2310/7750.2008.07017
27. Ogoina D, Adekunle V, Obiako R, et al. Disseminated infections due to immune reconstitution inflammatory syndrome after highly active antiretroviral therapy--report of 3 cases from Nigeria. Pan Afr Med J. 2011;9:38. doi:10.4314/pamj.v9i1.71216
28. Petrozza JC, Monga M, Oshiro BT, et al. Disseminated herpes zoster in a pregnant woman positive for human immunodeficiency virus. Am J Perinatol. 1993;10(6):463–464. doi:10.1055/s-2007-994633
29. Cohen PR, Beltrani VP, Grossman ME. Disseminated herpes zoster in patients with human immunodeficiency virus infection. Am J Med. 1988;84(6):1076–1080. doi:10.1016/0002-9343(88)90315-4
30. Shin BS, Na CH, Song IG, et al. A case of human immunodeficiency virus infection initially presented with disseminated herpes zoster. Ann Dermatol. 2010;22(2):199–202. doi:10.5021/ad.2010.22.2.199
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
