Back to Journals » Infection and Drug Resistance » Volume 15
Disseminated Cryptococcal Infection of the Lumbar Spine in an Immunocompetent Man
Authors Wang R , Luo H, Xin X, Qin B, Huang W
Received 23 January 2022
Accepted for publication 21 April 2022
Published 4 August 2022 Volume 2022:15 Pages 4229—4234
DOI https://doi.org/10.2147/IDR.S359612
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Rui Wang,1,* Huating Luo,2,* Xiaojuan Xin,1 Bo Qin,1 Wenxiang Huang2
1Department of Infectious Diseases, The First Affiliated Hospital, Chongqing Medical University, Chongqing, China; 2Department of Geriatrics, The First Affiliated Hospital, Chongqing Medical University, Chongqing, China
*These authors contributed equally to this work
Correspondence: Bo Qin, Department of Infectious Diseases, The First Affiliated Hospital, Chongqing Medical University, Chongqing, China, Tel + 86 23 89012887, Email [email protected] Wenxiang Huang, Department of Geriatrics, The First Affiliated Hospital, Chongqing Medical University, Chongqing, China, Tel +86 13883533808, Email [email protected]
Abstract: Cryptococcus (C) neoformans infection mainly occurs in immunocompromised hosts, especially those with AIDS, and skeletal infection is a rare presentation of cryptococcosis. We report a rare case of disseminated cryptococcal infection of the lumbar spine in an immunocompetent man caused by Cryptococcus neoformans var. grubii. The lesion position first appeared on upper right lung and then spread to the fourth lumbar vertebra. The result of periodic acid–Schiff (PAS) and Gomori’s methenamine silver (GMS) staining of the tissue sample matched cryptococcal infection, but multiple culture was negative. Eventually, C. neoformans var. grubii was confirmed using next-generation sequencing (NGS). Current follow-up of 12 months indicated a stable condition after antifungal therapy (fluconazole 400 mg/day) combined with surgery. Our case reminds that physicians must consider the possibility of skeletal cryptococcosis in patients with bone lesions, and NGS might be an excellent option to obtain the most accurate diagnosis in clinical practice.
Keywords: cryptococcosis, vertebral infection, immunocompetent, antifungal therapy, next-generation sequencing
Introduction
Cryptococcosis is a globally widespread infectious disease in humans caused by two species of Cryptococcus, C. neoformans and C. gattii, which are mainly distributed in rotting food, soil, and avian feces, especially pigeon droppings.1 The infection can involve any body site, but the principal sites of infection are the lungs and central nervous system (CNS).2 The most common origin of cryptococcosis is inhalation of spores or yeast cells; other sites are involved through hematogenous dissemination.3,4 Cryptococcus establishes clinical disease mainly in immunocompromised individuals, rarely in immunocompetent individuals.4 Pathogens generally invade the lungs first, and then spread throughout the body. In about 5%–10% of patients with Cryptococcus, the lung disease has healed spontaneously and spread to the bones and joints through blood.5 Here, the case that we describe is a rare disseminated vertebral cryptococcosis without immunodeficiency and eventually identified to the species level as C. neoformans var. grubii. Moreover, the patient was successfully managed through antifungal therapy combined with surgery. It is difficult to distinguish on imaging examinations because of lack of specificity. Accordingly, clinicians should be vigilant for Cryptococcus infections in patients with lumbar vertebral masses to avoid a misdiagnosis, missed diagnosis, and a delay in treatment.
Case Presentation
A 41-year-old male farmer presented to our hospital complaining of waist pain and the left leg with lower limb weakness for the past 5 months. He reported weight loss of 5 kg, but no fever, cough, headache, dizziness, chills, night sweats, nausea, or vomiting. In local hospital, magnetic resonance imaging (MRI) of the lumbar spine at that time showed bone destruction of the fourth lumbar vertebral body (L4) and left side appendages and the paravertebral soft tissue. Infection with abscess formation or bone tumor was tentatively diagnosed at the local hospital. He was transferred to our hospital for further management. The patient denied a history of exposure to pigeons.
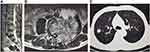
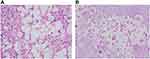
On admission, physical examination revealed obvious tenderness with pain upon percussion over the spine on the fourth lumbar vertebra, muscle atrophy of left lower extremity, and the straight leg raising test (SLRT) was suspiciously positive. MRI of lumbar spine was performed, which displayed partial cortical bone destruction of L4 that involved spinal canal and intervertebral foramen causing secondary spinal canal stenosis at that level and cauda equina compression. A chest CT scan demonstrated small round nodules with uneven density of upper right lung (Figure 1). No abnormalities were seen on MRI scan of the brain. Initial bloodwork showed the following: leukocyte count of 6.72 (normal: 4–11) × 109/L, and the erythrocyte sedimentation rate (ESR) was 62 mm/h (normal: <20 mm/h). Cryptococcus capsular polysaccharide antigen was positive. 1,3-β-D-Glucan detection and T-SPOT were negative. HbA1c was in the normal range. Tumor markers (including alpha fetoprotein, carcinoembryonic antigen, β2-microglobulin, ferroprotein, and estradiol), antinuclear antibody, human immunodeficiency virus (HIV) antibody, antineutrophil cytoplasmic antibody, CD4 count, CD8 count, and serum complement were not obviously abnormal. Blood culture was negative. Patient had no diabetes mellitus, solid-organ transplantation, malignancy, and cirrhosis. In addition, vastus lateralis by percutaneous needle biopsy under local anesthesia percutaneous puncture on L4 spine was performed, and postoperative pathology showed fungal-like structure and infiltration with chronic inflammatory cells in the tissues submitted for examination (Figure 2). Immunohistochemistry revealed CD68 (+), S100 (-), CK (-), KI67 15% (+). The cerebrospinal fluid (CSF) sample was not obtained because our patient declined lumbar puncture. We first considered the diagnosis of cryptococcal infection with results of histopathological analysis and imaging examinations. Then we started empirical therapy with flucytosine plus amphotericin B. After five days, the plan was stopped, and switched to fluconazole at the dose of 400 mg per day because of impaired renal function.
Two weeks after his admission, the localized skin on the left lower back of the patient was slightly swollen with increased localized skin temperature. B-scan ultrasonography showed a 120 mm × 92 mm abnormal echo. Local abscess formation was considered. Then we performed percutaneous biopsy under ultrasound guidance. Pus culture was negative. The patient continued the treatment with the above-mentioned therapy, but his symptoms were not relieved. Ten days later, he had left lower extremity pain with hypoesthesia. Then he underwent surgical management by posterior lumbar open-window focal debridement. Postoperative pathology reported the following: periodic acid–Schiff (PAS) (+), Gomori’s methenamine silver (GMS) (+), CK (+), CD68(+), LCA (+), KI67 (+) (Figure 2). Cryptococcus neoformans var. grubii was confirmed from NGS data (Figure 3). The final diagnosis of disseminated vertebral cryptococcosis was made on the basis of the result of NGS combined with clinical manifestation, laboratory, imaging, and pathological findings. The patient continued treatment by fluconazole at the dose of 400 mg per day and was discharged, having made an improvement, 20 days after surgery. The patient’s focal lesion was reduced, and the clinical symptoms disappeared at 12-month follow-up.
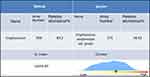 |
Figure 3 Next-generation sequencing (NGS) data. |
Discussion
We present a rare case of C. neoformans var. grubii infection of disseminated vertebral cryptococcosis in an immunocompetent patient. In our patient, Cryptococcus capsular polysaccharide antigen was positive, and CD4 count and CD8 count were in the normal range. Non-specific clinical manifestations and imaging findings caused a significant delay in the diagnosis in our patient, who eventually developed a refractory skeletal cryptococcosis that required a combination treatment of drug and surgery. Current follow-up of 12 months indicated a stable condition. Finally, spinal lesions may occur in cryptococcosis, therefore clinicians must identify the cause of these lumbar vertebral masses early to ensure accurate diagnosis.
Our patient’s final diagnosis was disseminated vertebral cryptococcosis. Disseminated skeletal cryptococcosis is defined as concurrent infection with two or more non-contiguous bone sites or one bone site associated with extra-skeletal sites.6 Most skeletal infections arise from a lung focus via the bloodstream; other possible sources include bony trauma, embolic phenomenon, contiguous skin infection, or contiguous neural infection.7 In our case, the solitary pulmonary nodule was the primary lesion, and Cryptococcus involved the L4 through blood. Disseminated skeletal cryptococcosis is a rare disease. Interrelated examination for disseminated cryptococcosis includes chest imaging; lumbar puncture for India ink staining, antigen testing, and culture; blood and urine culture; and sputum stain and culture.7 Our diagnosis is based on clinical, laboratory, imaging, biopsy findings, and NGS, particularly the histopathological analysis of lesion specimens from punch biopsy and surgical biopsy and the data of NGS. Our patient’s Cryptococcus capsular polysaccharide antigen was positive. The result of histopathological analysis of lesion specimens matched cryptococcal infection. But twice microbial cultures were negative. Negative blood culture upon admission may be associated with the sample collection and the culture conditions. Negative pus culture could be related to the specimen which was taken from the patient after the commencement of antifungal therapy. C.neoformans var.grubii was finally confirmed using NGS.
The identification of pathogenic microorganisms is a prerequisite and critical step in establishing appropriate treatment for cryptococcosis. Culture is the gold standard diagnosis, but often it is negative limited by culture conditions and techniques. The application of NGS technology allows for the detection and identification of microorganisms using a culture-independent approach. Most culture-independent methods such as PCR involve numerous individual tests for specifically targeted organisms which might enable unexpected microorganisms to evade detection, or use primers containing mismatches to the microbial strain involved thus causing false results. A main advantage of NGS is to enable broad identification of potentially known, unexpected or even the discovery of new pathogens from patient samples.8 In addition, the role of sequencing data provided by NGS includes infection classification on the basis of host response, characterization of antimicrobial resistance, and genotyping of pathogens.9 Our case is a reflection of the aid of NGS in a final diagnosis. However, NGS has not been in routine clinical microbiological diagnostics. There exist significant concerns about the lack of studies regarding the clinical utility and cost-effectiveness of NGS assays for pathogen detection, and this area requires further investigation. In summary, NGS has significant implications for diagnosis of cryptococcosis in clinical practice.
The most common symptoms of skeletal cryptococcosis were soft tissue swelling and pain, and fever was not a primary described clinical presentation,10 as was the case in our patient. These clinical presentations were not typical. In addition, the imaging test findings of vertebral cryptococcosis also resemble those of spinal tuberculosis and bone metastatic tumor; we need to combine the results of hematology examination, histopathological analysis, and microbial culture for diagnosis.11,12 A study revealed 20 of the 88 (22.7%) evaluable patients delayed in diagnosis. Misdiagnosis occurred among 13 (of 20, 65.0%) patients, and the most common diagnosis was tuberculosis (6 of 13, 46.2%) primarily in the vertebrae (5 of 6, 83.4%).6 Because of abscess formation in our case, we also need to differentiate from bacterial abscess by biopsy. Previous reports have indicated that fungal osteomyelitis presents a rare phenomenon in spinal infections, and is mostly caused by Candida and Aspergillus. General examinations are usually insufficient to distinguish these atypical vertebral infections, and an early biopsy should be performed at the site of the lesion to identify the causative fungus.13,14 Beyond that, high-demand state of biopsy techniques and low positivity rate of the pathogen test in ordinary hospitals are common reasons for missed diagnosis and misdiagnosis of skeletal cryptococcosis. We present a case which illustrates that cryptococcosis should be included in the differential diagnosis of tuberculosis and a bony lesion even in a normal host.
Disseminated cryptococcosis has previously been linked to HIV/AIDS and cell-mediated immunological disorders.15 However, the patient that we describe was an immunocompetent man, and his CD4 count was in the normal range. Based on the completed inspections, we had not found an underlying cause for impaired immunity, including diabetes mellitus, solid-organ transplantation, malignancy, sarcoidosis, systemic lupus erythematosus, and cirrhosis.16,17 This is a reminder that clinicians should also consider disseminated cryptococcosis in patients with normal immune functions. A retrospective analysis showed dissemination was a risk factor with an overall mortality rate (P = 0.041) in cryptococcosis.6 But disseminated cryptococcosis is still treatable. The guidelines of the Infectious Diseases Society of America (IDSA) recommend AmB combined with flucytosine as induction therapy for disseminated cryptococcosis, and fluconazole as consolidation and maintenance therapy.18 However, surgical treatment was performed due to the ineffectiveness of medical treatment alone in our patient. Then he was treated with fluconazole at the dose of 400 mg per day after surgery. It is noteworthy that surgery may be an important treatment for refractory skeletal cryptococcosis. Our patient’s lesion did not obviously improve due to poor compliance.
C. neoformans is predominantly reported in the immunocompromised, and C. gattii is more often found in the immunocompetent.4 The data collected by Dou et al showed that C. neoformans var. grubii (81 of 83, 97.6%) was the most common genotype in China.19 The previous study reported that most cryptococcosis patients in China were immunocompetent.
This is different in other countries.20 We consider the possibility that immunocompetent Chinese cryptococcosis patients may have genetic variation in immune function that may have predisposed them to cryptococcosis. A previous study provided evidence that the low-affinity Fc gamma receptor genes could be associated with risk for C. neoformans infection in individuals with no immunodeficiency.21 A recent study indicated that the AA genotype of PTX3 rs2305619 increased the risk of cryptococcosis in immunocompetent patients.22 But these results were based on a small size study and used historical controls. Therefore, we need some larger, prospective studies to more precisely confirm these conclusions.
Conclusion
Disseminated vertebral cryptococcosis is a rare disease, which behaves similarly to spinal tuberculosis and bone metastatic tumor. Simultaneously, dissemination is a risk factor of overall mortality rate. Clinicians need to be aware of the rare probability. In this case, surgery combined with antifungal treatment result in particularly good therapeutic effect. When a clinician suspects cryptococcosis, histopathological analysis and microbial culture must be performed immediately to facilitate prompt and appropriate therapy. Moreover, NGS has significant implications for diagnosis of cryptococcosis in clinical practice.
Data Sharing Statement
The data used and analyzed during the current study are available from the corresponding author on reasonable request. All data files mentioned in this manuscript are available.
Ethics Approval and Consent to Participate
The study was conducted according to the guidelines of the Declaration of Helsinki. The First Affiliated Hospital, Chongqing Medical University approved the publication of the case details.
Informed Consent Statement
Written informed consent was obtained from the individual for the publication of any potentially identifiable images or data included in this article.
Funding
This work was supported by General project of Chongqing Natural Science Foundation (Grant no. csct2020jcyj-msxmX0221).
Disclosure
The authors declare no conflict of interest.
References
1. Montoya MC, Magwene PM, Perfect JR. Associations between Cryptococcus genotypes, phenotypes, and clinical parameters of human disease: a review. J Fungi. 2021;7(4):260. doi:10.3390/jof7040260
2. Maziarz EK, Perfect JR. Cryptococcosis. Infect Dis Clin North Am. 2016;30(1):179–206. doi:10.1016/j.idc.2015.10.006
3. Botts MR, Hull CM. Dueling in the lung: how Cryptococcus spores race the host for survival. Curr Opin Microbiol. 2010;13(4):437–442. doi:10.1016/j.mib.2010.05.003
4. Kwon-Chung KJ, Fraser JA, Doering TL, et al. Cryptococcus neoformans and Cryptococcus gattii, the etiologic agents of cryptococcosis. Cold Spring Harb Perspect Med. 2014;4(7):a019760–a019760. doi:10.1101/cshperspect.a019760
5. Zhou HX, Ning GZ, Feng SQ, et al. Cryptococcosis of lumbar vertebra in a patient with rheumatoid arthritis and scleroderma: case report and literature review. BMC Infect Dis. 2013;13(1):128. doi:10.1186/1471-2334-13-128
6. Zhou HX, Lu L, Chu T, et al. Skeletal cryptococcosis from 1977 to 2013. Front Microbiol. 2015;5:740. doi:10.3389/fmicb.2014.00740
7. Wood L, Miedzinski L. Skeletal cryptococcosis: case report and review of the literature. Can J Infect Dis. 1996;7(2):125–132. doi:10.1155/1996/102103
8. Gu W, Miller S, Chiu CY. Clinical metagenomic next-generation sequencing for pathogen detection. Annu Rev Pathol. 2019;14(1):319–338. doi:10.1146/annurev-pathmechdis-012418-012751
9. Miller S, Chiu C, Rodino KG, Miller MB. Point-counterpoint: should we be performing metagenomic next-generation sequencing for infectious disease diagnosis in the clinical laboratory? J Clin Microbiol. 2020;58(3). doi:10.1128/JCM.01739-19
10. Behrman RE, Masci JR, Nicholas P. Cryptococcal skeletal infections: case report and review. Rev Infect Dis. 1990;12(2):181–190. doi:10.1093/clinids/12.2.181
11. Gupta SK, Chhabra R, Sharma BS, Das A, Khosla VK. Vertebral cryptococcosis simulating tuberculosis. Br J Neurosurg. 2003;17(6):556–559. doi:10.1080/02688690310001626868
12. Hickie JB, Walker T. Cryptococcosis (Torulosis): some problems in diagnosis and management. Australas Ann Med. 1964;13(3):229–239. doi:10.1111/imj.1964.13.3.229
13. Gabrielli E, Fothergill AW, Brescini L, et al. Osteomyelitis caused by Aspergillus species: a review of 310 reported cases. Clin Microbiol Infect. 2014;20(6):559–565. doi:10.1111/1469-0691.12389
14. Slenker AK, Keith SW, Horn DL. Two hundred and eleven cases of Candida osteomyelitis: 17 case reports and a review of the literature. Diagn Microbiol Infect Dis. 2012;73(1):89–93. doi:10.1016/j.diagmicrobio.2012.02.004
15. Elisabeth Gressler A, Volke D, Firacative C, et al. Identification of Disease-associated cryptococcal proteins reactive with serum IgG from cryptococcal meningitis patients. Front Immunol. 2021;12:2677.
16. Archuleta S, Gharamti AA, Sillau S, et al. Increased mortality associated with uncontrolled diabetes mellitus in patients with pulmonary cryptococcosis: a single US cohort study. Ther Adv Infect Dis. 2021;8:20499361211004367. doi:10.1177/20499361211004367
17. Motoa G, Pate A, Chastain D, et al. Increased cryptococcal meningitis mortality among HIV negative, non-transplant patients: a single US center cohort study. Ther Adv Infect Dis. 2020;7:2049936120940881. doi:10.1177/2049936120940881
18. Perfect JR, Dismukes WE, Dromer F, et al. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the infectious disease society of America. Clin Infect Dis. 2010;50(3):291–322. doi:10.1086/649858
19. Dou HT, Xu YC, Wang HZ, et al. Molecular epidemiology of Cryptococcus neoformans and Cryptococcus gattii in China between 2007 and 2013 using multilocus sequence typing and the DiversiLab system. Eur J Clin Microbiol Infect Dis. 2015;34(4):753–762. doi:10.1007/s10096-014-2289-2
20. Chen J, Varma A, Diaz MR, Litvintseva AP, Wollenberg KK, Kwon-Chung KJ. Cryptococcus neoformans strains and infection in apparently immunocompetent patients, China. Emerg Infect Dis. 2008;14(5):755–762. doi:10.3201/eid1405.071312
21. Meletiadis J, Walsh TJ, Choi EH, et al. Study of common functional genetic polymorphisms of FCGR2A, 3A and 3B genes and the risk for cryptococcosis in HIV-uninfected patients. Medl Mycol. 2007;45(6):513–518. doi:10.1080/13693780701390140
22. Zhang W, Liao Q, Liu Y, et al. PTX3 gene polymorphism associated with cryptococcosis in HIV-uninfected Chinese patients. Mycoses. 2021;64(4):405–411. doi:10.1111/myc.13228
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.