Back to Journals » Risk Management and Healthcare Policy » Volume 15
Disparities in Recommendations for Colorectal Cancer Screening Among Average-Risk Individuals: An Ecobiosocial Approach
Authors Syed Soffian SS , Mohammed Nawi A , Hod R, Abdul Manaf MR , Chan HK, Abu Hassan MR
Received 9 February 2022
Accepted for publication 1 May 2022
Published 13 May 2022 Volume 2022:15 Pages 1025—1043
DOI https://doi.org/10.2147/RMHP.S359450
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Mecit Can Emre Simsekler
Sharifah Saffinas Syed Soffian,1 Azmawati Mohammed Nawi,1 Rozita Hod,1 Mohd Rizal Abdul Manaf,1 Huan-Keat Chan,2 Muhammad Radzi Abu Hassan2
1Department of Community Health, Faculty of Medicine, Universiti Kebangsaan Malaysia, Kuala Lumpur, 56000, Malaysia; 2Clinical Research Center, Sultanah Bahiyah Hospital, Alor Setar, 05400, Kedah, Malaysia
Correspondence: Azmawati Mohammed Nawi, Department of Community Health, Faculty of Medicine, Universiti Kebangsaan Malaysia, Kuala Lumpur, 56000, Malaysia, Tel +60 3 9145 8408, Email [email protected]
Abstract: Regardless of the high global burden of colorectal cancer (CRC), the uptake of CRC screening varies across countries. This systematic review aimed to provide a picture of the disparities in recommendations for CRC screening in average-risk individuals using an ecobiosocial approach. It was reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The literature search was conducted through Scopus, Web of Science, PubMed, and EBSCOHost. Full-text guidelines which were published between 2011 and 2021, along with guidelines which provided recommendations on CRC screening in average-risk individuals, were included in the review. However, guidelines focusing only on a single screening modality were excluded. Fourteen guidelines fulfilling the eligibility criteria were retained for the final review and analysis. Quality assessment of each guideline was performed using the AGREE II instrument. Disparities in guidelines identified in this review were classified into ecological (screening modalities and strategies), biological (recommended age, gender and ethnicities), and social (smoking history, socioeconomic status, and behavior) factors. In general, unstandardized practices in CRC screening for average-risk individuals are likely attributable to the inconsistent and non-specific recommendations in the literature. This review calls on stakeholders and policymakers to review the existing colorectal cancer screening practices and pursue standardization.
Keywords: colorectal cancer, screening, guidelines, ecology, biology, social, disparities
Introduction
Colorectal cancer (CRC) is the second leading cause of death in the cancer population worldwide. The age-standardized incidence rate of CRC in high-income countries is 29.0 per 100,000 population, four times higher compared with low- and middle-income countries with 7.4 per 100,000 population.1 Nevertheless, the reported CRC mortality rates in high-income countries were 45%, which is generally lower than in low- and middle-income countries with 64%, possibly due to the availability of advanced treatment and screening services.1–3 CRC also increasingly becomes a public health concern in countries with a middle to high human development index (HDI) following the adoption of a westernized lifestyle as the consequence of economic growth.4,5
While the CRC incidence of the elderly population shows a gradual decrease, the burden of CRC in the younger age groups is conversely on the rise.5–7 Previous studies consistently demonstrated an uptrend in young-onset CRC over the past 20 years, particularly in high-HDI countries from North America and Oceania.6,8–10 In the US alone, at least seven population-based studies based on different data sources ranging from surveillance to patient registries confirmed the increasing trend in the incidence of young-onset CRC between 2017 and 2021.9,11–16 A recent multinational cohort study in Asia also identified a similar increasing trend in young-onset CRC, in line with the trend shown in other regions.6 The alarming increase of young-onset CRC can be partly explained by the exemption from recommended screening besides the influence of sedentary lifestyle changes and westernized dietary patterns as traditionally studied by many among the older generation. Thus, future studies that explore the underlying factors related to young-onset CRC are recommended to better understand the progress of disease and preventive strategies.
However, the current uptake of CRC screening widely varies across countries, even among those with strong support from their health systems. The US reported that 67.3% of the adults aged between 50 and 75 turned up for CRC screening yearly.17 On the other hand, the screening uptake in average-risk individuals was 39% lower among those living in rural areas and with a lower socioeconomic status, as reported in Australia.18 Since the introduction of the national CRC screening program in 1992, Japan only achieved a screening uptake of 41.4% in men and 34.5% in women.19 Similarly, approximately one-third of the average-risk individuals in Korea took up CRC screening despite the availability of national guidelines.20 At the same time, other countries without a nationwide CRC screening program in the Asia Pacific region reported a much lower CRC screening uptake, generally below 10%.21 Overall, the global CRC screening uptake in average-risk individuals is only approximately 65%,26 much lower than the 80% targeted by the US Preventive Task Force.27
In fact, screening has long been recognized as an effective primary preventive strategy to lower the incidence and mortality of CRC.17,28,29 Whereas CRC screening is mainly recommended only for average-risk individuals, who are above 50 years of age,22–24 the younger population has been relatively neglected. The health policy, capacity of the health system and public awareness are all likely to have a great impact on the uptake of CRC screening, particularly of the older age group.23,25,28,30 These seem to arise as cross-cutting issues throughout the high-, middle-, and low-income countries.
Current literature has broadly classified the individual-level risk factors associated with CRC into modifiable and non-modifiable.31,32 The expansion of studies on the ecological influence against carcinogenesis illustrates the extent to which the availability of a healthy environment, accessibility to healthcare facilities, and the existence of effective screening programs contributed to the CRC incidence.28,33,34 Spatial studies demonstrated a possible link between neighborhood influence and geographic pattern of CRC distribution across different socioeconomic backgrounds.35,36 The epidemiological characteristics of CRC incidence were leveraged in those aged above 50 years, family history of CRC, male preponderance, higher among Whites, and presence of comorbidities such as inflammatory bowel disease and type 2 diabetes, hence the priority for screening.37 Notwithstanding that, social factors as determined by sedentary lifestyles, cigarette smoking, unhealthy dietary pattern, and poor health-seeking behavior, in the long term have an indirect contribution to the occurrence of CRC. The overlap between the ecological, biological and social factors emphasizes the complex interaction and equal need for intervention.
The introduction of the ecobiosocial concept in CRC research to address the interdependency between ecological, biological, and social factors can potentially produce strong evidence to guide future preventive and control management strategies.38 While such a concept has long been adopted in the management of vector-borne diseases such as dengue,39,40 its use in chronic non-communicable diseases is limited. In the context of CRC screening, the ecobiosocial concept could be valuable to help identify disparities in ecological factors, including screening modalities and strategies; biological factors, including in the recommended age, gender and ethnicity; and social factors, including smoking history, socioeconomic status, and health-seeking behaviors. As all these factors are important, equal attention must be given to them to ensure the success of a CRC screening program. Therefore, this review was performed to provide a picture of the ecological, biological, and social disparities in recommendations for CRC screening in average-risk individuals.
Materials and Methods
This systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Adopting the PRISMA guidelines enabled the systematic retrieval of literature and synthesis of evidence.41 A specific research question was formulated, followed by a systematic search, identification, screening, quality appraisal, and data extraction of the literature obtained from databases (Figure 1).
 |
Figure 1 PRISMA flow diagram. Notes: Adapted from: Page MJ, McKenzie JE, Bossuyt PM et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372. doi:10.1136/bmj.n71.41 Creative Commons Attribution (CC BY 4.0) license (http://creativecommons.org/licenses/by/4.0/). |
Formulation of the Research Question
The research question was built on the PICo concept that identifies the average-risk individuals (population), CRC screening guidelines (interest), and screening strategies (context). The PICo concept has been extensively used in the evidence-based medical research area for developing clinical questions.42,43 The application of the PICo process facilitates well-built questions, a fundamental need in a thorough search of the scientific literature.42 Based on the systematic process, the research question was framed as “How do disparities in guidelines affect CRC screening strategies for the average-risk individuals?”.
Literature Search and Identification
The literature search started with deciding on the keywords. The search strings were created for each database, combined with the Boolean operators (Table 1). The literature search was performed between November 1–4, 2021 through four databases (Scopus, Web of Science, PubMed, and EBSCOHost) accessible to the authors, yielding 3779 records. A total of 111 duplicate records were found and removed. The records were then exported from the databases and organized using a Microsoft Excel sheet.
 |
Table 1 Keyword Search Used in the Identification Process |
Screening
The title and abstract of each article were examined to evaluate their relevance. To be selected for the review, the articles must be (1) published between 2011 and 2021, (2) full original text, and (3) guidelines, recommendations by consensus, or position statements providing recommendations for CRC screening in average-risk individuals. Articles reporting on national CRC screening programs, focused only on single CRC screening modalities, and expert opinions were excluded from the review.
Eligibility
A total of 79 articles were retrieved for eligibility confirmation. All of them were thoroughly examined and the reason to exclude any of them was recorded. Sixty-five articles were excluded at this stage as they were either not in the form of guidelines (n = 14), expert opinions (n = 10), focusing only on a single screening modality (n = 18), review articles (n = 6), focusing on financial issues related to CRC screening (n = 6), community trials (n = 6), or studies on instrument validation (n = 5).
Quality Appraisal
The selected 14 guidelines went through the risk of bias assessment. The degrees of methodological rigor and transparency of the guidelines were evaluated using the Appraisal of Guidelines for Research and Evaluation (AGREE) II tool. AGREE II is a validated tool, which has been widely used to assess the methodological quality of guidelines.44 It consists of 23 items, which are further categorized into six domains: scope and purpose, stakeholder involvement, the rigor of development, clarity of presentation, applicability, and editorial independence.
The authors rated each item on a seven-point scale between 1 (strongly disagree) and 7 (strongly agree) for each set of guidelines. One point was given if the information was poorly reported or unavailable for an item, while seven points were given if the information provided was sufficient. The domain scores were then converted to percentages using the following formula: (Obtained score – Minimum possible score)/(Maximum possible score – Minimum possible score) x 100%. The results of the quality appraisal are presented in Table 2. The authors also independently recommended the inclusion of each set of guidelines for the review.
 |
Table 2 Scaled AGREE II Domain Scores for Each Guideline and Overall Assessment |
The selected guidelines encompassed CRC screening using both stool-based tests and visualization techniques. The variations in quality across the guidelines were mostly due to the stakeholder involvement, rigor of development, and applicability. Nine22,24–26,45–49 out of 14 guidelines scored more than 6 points out of 7; whereas another five guidelines scored between 3 and 5 points. Of the six domains assessed, both the clarity of presentation and editorial independence had mean domain scores as high as 98% on average. This indicated a high clarity of recommendations and the absence of potential influences or conflicts of interest. The domain with the lowest mean score was applicability (77%), which implies insufficient consideration of the practicality of recommendations.
Data Abstraction and Analysis
The two authors independently extracted information from each selected guideline, including the authors, year of publication, organization, country of origin, recommended age range for CRC screening, screening modality, screening interval, screening strategy, and other relevant information (Table 3). Subsequently, they independently suggested the categorization of findings under three themes: ecology, biology, and social factors. Any disagreements in the data extraction analysis were resolved by consensus between the authors.
 |  |  |
Table 3 Characteristics of Included Guidelines |
Results
The systematic searching strategy has finalized 14 guidelines to be analyzed in the review. Descriptive summary of included guidelines concerning organization and countries or regions involved is shown in Figure 2. The CRC screening guidelines published spanned six countries with two regions that include the United States (6),22,24–26,49,50 Canada (1),51 China (1),52 Saudi Arabia (1),53 Korea (1),47 Spain (1),54 the European Union member state countries (2)48,55 and the Asia Pacific region (1).45 Comparing the location of selected guidelines based on the WHO regions, seven guidelines accounted for the Region of the Americas (AMR), three guidelines from the European Region (EUR), three guidelines from the Western Pacific Region (WPR), and one from the Eastern Mediterranean Region (EMR). The guidelines were published between 2012 and 2021.
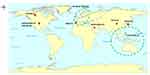 |
Figure 2 Summary of guidelines included based on countries and regions. Notes: () number of guidelines included, |
Screening Modalities
Three guidelines by the United States Preventive Services Task Force (USPSTF), Saudi Arabia, and the National Comprehensive Cancer Network (NCCN)49,50,53 recommended colonoscopy as the primary CRC screening modalities. The USPSTF guideline specifically indicates that individuals who underwent colonoscopy screening are not required to perform additional iFOBT screening. This demonstrates the strong system-based support held in the US that is able to cope with the colonoscopy demand for the population. Similarly in Saudi Arabia where the population at large prefers colonoscopy compared with the stool-based test for CRC screening. Besides the 1-tier approach of using colonoscopy as the screening basis, other guidelines recommended for 2-tier approach whereby FIT is performed first then followed by colonoscopy if a positive result.
Previous studies showed the sensitivity and specificity of FIT superseded gFOBT, thus it was chosen as the primary screening test by many.26,46 Apart from that, only the ACS guideline acknowledged high-sensitivity gFOBT and multi-targeted DNA stool blood test for baseline CRC screening for the average-risk group which can be repeated every year for the former and three yearly for the latter.25 The expansion of screening modalities suggested by the ACS that includes FIT, HSgFOBT, mt-sDNA, colonoscopy, CT colonography, and flexible sigmoidoscopy may underscore the importance of patient preferences and choices for particular testing to encourage screening uptake.
Age at Screening
Generally, the recommended starting age for screening is at 50 years and above. However, the ACG guideline26 recommended the earliest age for screening to begin at 45–49 years (conditional recommendation) specifically for African Americans in view of the highest mortality rates recorded among the ethnicities. On the other hand, the ACS guideline25 allowed for early CRC screening at 45–50 years (qualified recommendation) while emphasizing the importance of patient preferences for timing and screening options. The guidelines published in Saudi Arabia53 preferred early screening as early as 45 years with strong recommendations, owing to the epidemiologic trend of CRC incidence recorded in the country.
There has been no clear consensus on the age to stop screening.25,45,54 Most of the guidelines recommends the healthcare provider to proceed for individualized risk assessment for those beyond age 75, considering the comorbidities and ten years life expectancy. However, few guidelines allowed for continuous screening as long as the potential complications following screening procedures are clearly explained to the patient. In resource-limited settings, average-risk individuals of more than 75 years were excluded from the CRC screening program to prevent unnecessary harm following the invasive procedures of colonoscopy.
Screening Strategy
Six24,26,48,52,54,55 of the 14 guidelines studied mentioned the preferable screening program strategies for implementation at country level. Four guidelines24,26,48,55 supported the organized screening as a way to achieve higher screening uptake, hence significant reduction of CRC incidence and mortality. However, it requires a high-resource setting for an affordable population-based screening. On the other hand, opportunistic screening offers a great window for early detection by utilizing the optimal resources via recommendation by the healthcare providers. This strategy is more suitable in countries with limited resources for colonoscopy services and experts. In fact, two guidelines in the Asian region45,52 had adapted the risk stratification approach to selectively perform preliminary screening to gauge the targeted group for intervention.
Compared with organized screening, opportunistic screening depends highly on regular physician visits.56 Thus, a key advantage of opportunistic screening is the integration with other existing health services offered at primary care facilities. It also helps to minimize the chance of over screening to reduce unnecessary harmful risks following positive stool testing. Besides, other preventive measures such as a quit smoking program and weight reduction activities readily available at the primary care settings provide additional value to the primary prevention of CRC.
Discussion
All the 14 CRC screening guidelines included in this review were of good quality. The need for well-structured organized screening programs at the national and regional level was highlighted to boost screening adherence.57 Even with the varying range of age recommendations for starting screening, the benefits and harm of screening were justified in many of the guidelines.26,52,53,55 However, low screening participation remains a concern in many countries and requires a holistic approach by the stakeholders. Much attention has been given to investigate factors influencing CRC screening participation in average-risk individuals. Literature explored the reasons for the lack of screening uptake including limited access to screening, patient preferences, and lack of physician knowledge on screening guidelines.58–60
Based on the ecobiosocial approach, the disparities in ecology discussed in the review refer to the healthcare system, screening program strategy, and institution involved in offering CRC screening to the average-risk population. The biological factors explain the diversity of age, sex, and ethnicity preference within the screening guidelines. Meanwhile, the social factors focus more on the patient-level background that includes the socioeconomic status, smoking status, and screening behavior which are of no less importance to be considered in planning effective intervention strategies.
Ecological Disparities
The expansion of screening modalities has been considered in many CRC screening guidelines. In addition to the usual practice of stool test (gFOBT, FIT, FIT-fecal DNA) some guidelines endorsed CRC screening via structural visualized methods (colonoscopy, sigmoidoscopy), imaging technique (CT colonography, barium enema), and the Septin9 serum assay.24 Considering the cost-effectiveness and reliability of the testing, the most common primary screening modality recommended is the stool-based test.61–63 With a sensitivity of 93.9% and specificity of 100% for CRC detection, iFOBT outperformed other types of fecal occult blood tests.64 In high-resource countries, colonoscopy is regarded as the first-tier screening24,53 following the population preference. Thus, understanding the pattern of utilization of primary screening modalities within certain culture-specific populations or level of socioeconomic status is critical to inform the effectiveness of the screening program.
Comparison studies on CRC screening have concluded that implementation of a population-based screening program provides more benefits and is more cost-effective when compared with no screening, even in countries with limited financial resources.65–67 Notwithstanding that, the availability of treatment for CRC must be prioritized to ensure successful screening.23 Concerted efforts by multidisciplinary teams are needed to identify the most effective strategies that suit the country’s background, considering the life-years gained relative to the cost of the screening strategy.
Even though organized screening offers higher screening outcomes compared with opportunistic screening, the prerequisites demand conscientious components as outlined by the IARC.26 One of the crucial elements is to have an appropriate quality assurance structure for each of the process flows.65,68,69 In regions where considerable CRC screening guidelines are published, the approach to screening is largely opportunistic. It is generally understood that countries with an opportunistic program face huge challenges of low screening uptake.21,70,71 However, the involvement of multiple professional organizations in quality assurance of the CRC opportunistic screening program in the US highlighted the significant impact on the high uptake of the screening.72,73 Therefore, the review highly recommends the formation of a National Colorectal Cancer Roundtable consisting of multidisciplinary representatives and experts to specifically monitor the quality of the CRC screening program throughout each stage.
In general, utilization of any CRC screening test (USD 2428 per person) was proven to be more cost-effective compared with no screening (USD 3580 per person) per lifetime.74 Based on cost-evaluation studies, the fecal immunochemical test (FIT) was more effective when performed annually rather than 2-yearly with reported incremental cost-effectiveness ratio (ICER) below the acceptance threshold, among the average-risk population.75 Regarded as the gold standard for CRC screening methods, colonoscopy every 10years appears to be cost-effective when compared with annual FOBT despite the requirement for highly skilled personnel, instruments, and designated infrastructure.74 The sensitivity for colonoscopy is the highest with 91% and 94% specificity (Table 4) and outweighs alternatives due to the ability to detect and remove precursor cancer lesions as well as tumor cells upon examination.76 Comparison studies made between FOBT yearly and sigmoidoscopy once showed that FOBT method was consistently less costly and more effective.67,75,77 Thus, the feasibility of a selected screening test must take into account the availability of resources, acceptance by the population, and effectiveness of the screening program.
 |
Table 4 Comparison of CRC Screening Methods |
Biological Disparities
The incidence and mortality of young-onset CRC have been rising in many countries across all continents,9,83,84 indicating an alarming epidemiological shift towards the younger age group of less than 50 years. The low effectiveness of CRC screening in the young has partly been driven by the screening policy that recommends the earliest age to screen at 50 years and above of the average-risk individuals,45,52 thus missing out on the younger group. The impending health complications of the working-age group population require an upscale of screening efforts and the need to consider lowering the recommended age for CRC screening in the future.
While the CRC occurrence in men was predominantly higher than in women,30,85,86 less is seen in the screening policy that stratifies sex as the eligible individuals. Only two guidelines proposed the risk score consisting of age, sex, family history, and smoking as an effort to prioritize screening among average-risk individuals.45,52 Even though the approach is only relevant to resource-limited countries, targeted awareness activities and health promotion may benefit the population in the long term, considering the relatively lower screening participation among men.30
Screening provides an opportunity for an earlier stage at diagnosis across all ethnicities. Although notable differences by ethnicity have been reported in the CRC incidence and prognostic values, the screening eligibility has failed to underscore the racial inequalities. Of the 14 guidelines included, only the ACG guidelines confer screening to African Americans at 45 years,26 while the Asia Pacific Consensus recommended individual countries in Asia to devise respective screening policies accounting for the ethnic difference.45 Previous studies linked the high CRC incidence in black individuals with a lower rate of screening participation, largely influenced by access to screening.14,87 In a more diverse screening population, CRC screening uptake was highest among whites and Hispanics compared with blacks by more than 10-fold.29 Although CRC mortality was recorded as highest in black individuals, the current screening guidelines do not align similarly for black and white individuals. Thus, it is time for the stakeholders to revise the quality screening program to reduce the overall burden.
Additionally, biological factors found to relate with CRC also include genetic predisposition in the family and comorbidities such as inflammatory bowel disease, Lynch syndrome, and type 2 diabetes.7,78 Studies showed that young-onset CRC is linked with high degree of familial history.7 While patients with chronic diseases had regular follow-up with health clinics, this provides a great window of opportunity for CRC screening of those at risk.
Despite the extensive discussion on the promoting factors for CRC, several studies highlighted protective factors likely to reduce the rate of cancer. The ingestion of non-steroidal anti-inflammatory drugs (NSAIDs) has been linked with reduction of risk for CRC via inhibition mechanism against cyclooxygenase-2 (COX 2) activity known to trigger the tumorigenesis.79 In addition, some evidence supported the protective role of high fiber dietary intake and high consumption of fruits and vegetables which relate to a healthy gut environment.80 Therefore, further review on the need of a standardized dietary guideline pertaining to CRC is highly desirable to advise on the primary prevention strategy.
Social Disparities
In a large population-based case control study, a strong association were recorded between smoking and pre-cancerous lesions for CRC.78,87 The inflammatory pathway found was mediated through a series of gene-environment interactions initiated by the cigarette smoking.88,89 An overall increase of more than 20% in CRC risk among smokers87,90 highlighted the importance of risk stratification for screening of the total average-risk population. Nonetheless, only the WPRO region published guidelines45,52 truly consider smoking in the risk score for screening selection purposes. Smoking cessation programs coupled with CRC screening may benefit to reduce further the CRC incidence as well as mortality.
The social disparities identified within the guidelines also involved the issue of low socioeconomic status group whereby studies had associated this with a lower CRC screening participation rate.91 This is hampered by the low awareness and lack of health-seeking behavior that collectively contributed to the low screening uptake, both of which were likely influenced by the level of socioeconomic status.92–94 Substantial studies demonstrated the great impact of patient-level barriers towards CRC screening uptake,95–97 indicating extensive efforts are needed to educate the public on CRC and the importance of early detection.
The use of the ecobiosocial approach helps to elucidate the disparities in a systematic manner. The ecological, biological, and social factors are equally important to ensure continuous effective preventive measures within a population. The interdependent relationship between these factors should be emphasized in bridging the gap in the cancer continuum.38 In addition, a diversified ethnicity population compounded with culturally specific lifestyles may impede a universal CRC screening strategy when there is a lack of planning, as there is no one screening structure that can fit all settings.
Against the background of industrialization and drastic economic development, the shift towards a westernized lifestyle is inevitable. The proliferation of the food retailing industry likely exposes people to easy access of fast-food outlets, which already known for energy-dense and nutrient-poor foods.81 Neighborhoods with a high proportion of fast-food outlets may be significantly influential as they are perceived as an easier choice, thus frequently consumed more than other, healthier food. Studies showed that people living in the vicinity of greater access to fast-food outlets opted for an unhealthy diet, which has been linked to excessive weight gain and obesity over time.68,81 Compounded with lack of access to healthcare facilities offering CRC screening services, this not only dilutes the preventive strategies focus, but limits the opportunity for screening among those at risk and who eventually presented late for treatment. A well-organized CRC screening program as practiced in Canada and the US witnessed the success of increased screening uptake by nearly half, from 38.9% in 2000 to 82.7% in 2015 and this was reflected in the reduction of yearly CRC incidence by 25% and 52.4% of CRC mortality.82 Readily available and responsive CRC screening services within community reach, support the best practice of early detection and intervention. Besides, being receptive to the consumer preference on the options of screening modalities may provide additional value to encourage participation. The ecobiosocial approach serves to review the disparities in a holistic manner whereby it incorporates the influence of surrounding environments reflecting the real situation within a living community and not just merely the individual-level determinants.38
The approach helps to explain the role of each component towards an efficient and effective CRC screening program. In order to overcome the issue of lack of adherence to CRC screening, multiple measures must be taken including the healthcare screening services with either opportunistic or organized systems, availability of facilities offering CRC screening, coverage of the targeted population to screen, incorporation of risk stratification scoring, and patient preference for screening methods.38,71
Examining the recent updated international CRC screening guidelines, the review identified the need to develop a specific CRC screening policy at the national or regional level tailored to the respective population to improve the screening uptake. This is particularly concerning in Asian regions where the incidence of CRC is the highest but there is a limited number of established guidelines, supported by low CRC screening uptake. Concerted efforts must be given to increase awareness and there is the need to be equipped with experts and up-to-date facilities. Furthermore, the translation of the ecobiosocial approach as proposed in the review provides insights to the stakeholders in planning and evaluation of a comprehensive accredited screening program of the country.
Countries that do not publish their guidelines were not included, leading to publication bias. Certain guidelines such as the European Guidelines consist of more than one volume to explain each component of the screening program in detail, limiting the extent of clarification on the disparities addressed. With wide arrays of screening options and approaches, the current guidelines on average-risk individuals are broad and non-specific, leading to dilution of organizational focus. Revision to the screening policy should consider the overall level of risk underlying the average-risk individuals to yield better screening outcomes.
Conclusion
Considering the identification of ecobiosocial disparities in CRC screening guidelines and the epidemiological shift towards young-onset CRC, further reviews of the status quo recommendations are warranted on the matter. The disparities addressed call for a need to revise the current guidelines to reach consensual recommendations for a standardized universal CRC screening program.
Acknowledgments
The authors would like to thank The Ministry of Higher Education Malaysia and The Universiti Kebangsaan Malaysia (UKM) for funding this study under the Fundamental Research Grant Scheme-(FRGS/1/2021/SKK05/UKM/02/1) and (FF-2021-121). We also thank the team for their commitment and tireless efforts in ensuring that the manuscript was well executed.
Disclosure
The authors report no conflicts of interest in relation to this work.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Schreuders EH, Ruco A, Rabeneck L, et al. Colorectal cancer screening: a global overview of existing programmes. Gut. 2015;64(10):1637–1649. doi:10.1136/gutjnl-2014-309086
3. Sur D, Colceriu M, Sur G, Floca E, Dascal L, Irimie A. Colorectal cancer: evolution of screening strategies. Med Pharm Rep. 2019;92(1):21–24. doi:10.15386/cjmed-1104
4. Nguyen B, Cranney L, Bellew B, Thomas M. Implementing food environment policies at scale: what helps? what hinders? a systematic review of barriers and enablers. Int J Environ Res Public Health. 2021;18(19):10346. doi:10.3390/ijerph181910346
5. Chiu HM, Hsu WF, Chang LC, Wu MH. Colorectal cancer screening in Asia. Curr Gastroenterol Rep. 2017;19(10). doi:10.1007/s11894-017-0587-4
6. Sung JJ, Chiu HM, Jung KW, et al. Increasing trend in young-onset colorectal cancer in Asia: more cancers in men and more rectal cancers. Am J Gastroenterol. 2019;114(2):322–329. doi:10.14309/ajg.0000000000000133
7. Ahnen DJ, Wade SW, Jones WF, et al. The increasing incidence of young-onset colorectal cancer: a call to action. Mayo Clin Proc. 2014;89(2):216–224.
8. Cancer Council Australia. Clinical practice guidelines for the prevention, early detection and management of colorectal cancer; 2018.
9. El Din KS, Loree JM, Sayre EC, et al. Trends in the epidemiology of young-onset colorectal cancer: a worldwide systematic review. BMC Cancer. 2020;20(1):1–4.
10. Ibrahim NRW, Chan HK, Soelar SA, Azmi AN, Said RM, Hassan MRA. Incidence, clinico-demographic profiles and survival rates of colorectal cancer in Northern Malaysia: comparing patients above and below 50 years of age. Asian Pacific J Cancer Prev. 2020;21(4):1057–1061. doi:10.31557/APJCP.2020.21.4.1057
11. Wang W, Chen W, Lin J, Shen Q, Zhou X, Lin C. Incidence and characteristics of young-onset colorectal cancer in the United States: an analysis of SEER data collected from 1988 to 2013. Clin Res Hepatol Gastroenterol. 2019;43(2):208–215. doi:10.1016/j.clinre.2018.09.003
12. Aloysius MM, Goyal H, Shah NJ, et al. Impact of race and socioeconomics disparities on survival in young-onset colorectal adenocarcinoma- A SEER registry analysis. Cancers. 2021;13:3262. doi:10.3390/cancers13133262
13. Bhandari A, Woodhouse M, Gupta S. Colorectal cancer is a leading cause of cancer incidence and mortality among adults younger than 50 years in the USA: a SEER-based analysis with comparison to other young-onset cancers. J Investig Med. 2017;65(2):311–315. doi:10.1136/jim-2016-000229
14. Murphy C, Wallace K, Sandler RS, Baron JA. Racial disparities in incidence of young-onset colorectal cancer and patient survival. Gastroenterology. 2019;156(4):958–965. doi:10.1053/j.gastro.2018.11.060
15. He X, Wu W, Ding Y, Li Y, Si J, Sun L. Excessive risk of second primary cancers in young-onset colorectal cancer survivors. Cancer Med. 2018;7(4):1201–1210. doi:10.1002/cam4.1437
16. Koblinski J, Jandova J, Nfonsam V. Disparities in incidence of early- and late-onset colorectal cancer between Hispanics and Whites: a 10-year SEER database study. Am J Surg. 2018;215(4):581–585. doi:10.1016/j.amjsurg.2017.03.035
17. Joseph DA, King JB, Richards TB, Thomas CC, Richardson LC. Use of colorectal cancer screening tests by state. Prev Chronic Dis. 2018;15:170535. doi:10.5888/pcd15.170535
18. Jideh B, Bourke MJ. Colorectal cancer screening reduces incidence, mortality and morbidity. Med J Aust. 2018;208(11):483–484. doi:10.5694/mja18.00279
19. Sano Y, Byeon JS, Li XB, et al. Colorectal cancer screening of the general population in East Asia. Dig Endosc. 2016;28(3):243–249. doi:10.1111/den.12579
20. Rim JH, Youk T, Kang JG, et al. Fecal occult blood test results of the national colorectal cancer screening program in South Korea (2006–2013). Sci Rep. 2017;7. doi:10.1038/s41598-017-03134-9
21. Arunah C, Feisul IM, Nor Saleha IT, Muhammad Radzi AH. Overview of colorectal cancer screening programme in Malaysia. Med J Malaysia. 2020;75(3):235–239.
22. Qaseem A, Crandall CJ, Mustafa RA, et al. Screening for colorectal cancer in asymptomatic average-risk adults: a guidance statement from the American College of Physicians. Ann Intern Med. 2019;171(9):643–654. doi:10.7326/M19-0642
23. Schliemann D, Ramanathan K, Matovu N, et al. The implementation of colorectal cancer screening interventions in low- and middle-income countries: a scoping review. BMC Cancer. 2021;21. doi:10.1186/s12885-021-08809-1
24. Rex DK, Boland CR, Dominitz JA, et al. Colorectal cancer screening: recommendations for physicians and patients from the U.S. multi-society task force on colorectal cancer. Gastroenterology. 2017;153(1):307–323. doi:10.1053/j.gastro.2017.05.013
25. Wolf AMD, Fontham ETH, Church TR, et al. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA Cancer J Clin. 2018;68(4):250–281. doi:10.3322/caac.21457
26. Shaukat A, Kahi CJ, Burke CA, Rabeneck L, Sauer BG, Rex DK. ACG clinical guidelines: colorectal cancer screening 2021. Am J Gastroenterol. 2021;116(3):458–479. doi:10.14309/ajg.0000000000001122
27. Davidson KW, Barry MJ, Mangione CM, et al. Screening for colorectal cancer: US preventive services task force recommendation statement. JAMA - J Am Med Assoc. 2021;325(19):1965–1977. doi:10.1001/jama.2021.6238
28. Chuang E, Pourat N, Chen X, et al. Organizational factors associated with disparities in cervical and colorectal cancer screening rates in community health centers. J Health Care Poor Underserved. 2019;30(1):161–181.
29. Viramontes O, Bastani R, Yang L, Glenn BA, Herrmann AK, May FP. Colorectal cancer screening among Hispanics in the United States: disparities, modalities, predictors, and regional variation. Prev Med. 2020;138.
30. White A, Ironmonger L, Steele RJC, Ormiston-smith N, Crawford C, Seims A. A review of sex-related differences in colorectal cancer incidence, screening uptake, routes to diagnosis, cancer stage and survival in the UK. BMC Cancer. 2018;18(906):1–11. doi:10.1186/s12885-018-4786-7
31. Khil H, Kim SM, Hong SE, et al. Time trends of colorectal cancer incidence and associated lifestyle factors in South Korea. Sci Rep. 2021;11(1):1–12. doi:10.1038/s41598-021-81877-2
32. Cho YA, Lee J, Oh JH, et al. Genetic risk score, combined lifestyle factors and risk of colorectal cancer. Cancer Res Treat off J Korean Cancer Assoc. 2019;51(3):1033–1040.
33. Kuo TM, Meyer AM, Baggett CD, Olshan AF. Examining determinants of geographic variation in colorectal cancer mortality in North Carolina: a spatial analysis approach. Cancer Epidemiol. 2019;59(August2018):8–14. doi:10.1016/j.canep.2019.01.002
34. Kurani SS, McCoy RG, Lampman MA, et al. Association of neighborhood measures of social determinants of health with breast, cervical, and colorectal cancer screening rates in the US midwest. JAMA Netw Open. 2020;3(3):e200618. doi:10.1001/jamanetworkopen.2020.0618
35. Anderson AE, Henry KA, Samadder NJ, Merrill RM, Kinney AY. Rural vs urban residence affects risk-appropriate colorectal cancer screening. Clin. Gastroenterol. Hepatol. 2013;11:526–533. doi:10.1016/j.cgh.2012.11.025
36. Mansori K, Mosavi-Jarrahi A, Motlagh AG, et al. Exploring spatial patterns of colorectal cancer in Tehran City, Iran. Asian Pac J Cancer Prev. 2018;19(4):1099–1104. doi:10.22034/APJCP.2018.19.4.1099
37. Johnson CM, Wei C, Ensor JE, et al. Meta-Analyses of colorectal cancer risk factors. Cancer Causes Control. 2013;24(6):1207–1222. doi:10.1007/s10552-013-0201-5
38. Soffian SSS, Nawi AM, Hod R, Chan HK, Hassan MRA. Area-level determinants in colorectal cancer spatial clustering studies: a systematic review. Int J Environ Res Public Health. 2021;18(19):1–26. doi:10.3390/ijerph181910486
39. Sommerfeld J, Kroeger A. Eco-bio-social research on dengue in Asia: a multicountry study on ecosystem and community-based approaches for the control of dengue vectors in urban and peri-urban Asia. Pathog Glob Health. 2012;106(8):428–435. doi:10.1179/2047773212Y.0000000055
40. Quintero J, Pulido NR, Logan J, Ant T, Bruce J, Carrasquilla G. Effectiveness of an intervention for Aedes aegypti control scaled-up under an intersectoral approach in a Colombian city hyperendemic for dengue virus. PLoS One. 2020;15(4):1–16. doi:10.1371/journal.pone.0230486
41. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;88:105906.
42. Eriksen MB, Frandsen TF. The impact of patient, intervention, comparison, outcome (Pico) as a search strategy tool on literature search quality: a systematic review. J Med Libr Assoc. 2018;106(4):420–431. doi:10.5195/jmla.2018.345
43. Miller SA, Forrest JL. Enhancing your practice through evidence-based decision making: PICO, learning how to ask good questions. J Evid Based Dent Pract. 2001;1(2):136–141. doi:10.1016/S1532-3382(01)70024-3
44. Brouwers MC, Kho ME, Browman GP, et al. AGREE II: advancing guideline development, reporting, and evaluation in health care. Prev Med. 2010;51(5):421–424. doi:10.1016/j.ypmed.2010.08.005
45. Sung JJY, Ng SC, Chan FKL, et al. An updated Asia Pacific consensus recommendations on colorectal cancer screening. Gut. 2015;64(1):121–132. doi:10.1136/gutjnl-2013-306503
46. Canadian Task Force on Preventive Health Care. Recommendations on screening for colorectal cancer in primary care. Can Med Assoc J. 2016;188(5):340–348. doi:10.1503/cmaj.151125
47. Lee BI, Hong SP, Kim SE, et al. Korean guidelines for colorectal cancer screening and polyp detection. Clin Endosc. 2012;45(1):25–43. doi:10.5946/ce.2012.45.1.25
48. Von Karsa L, von Karsa L, Patnick J, et al. European guidelines for quality assurance in colorectal cancer screening and diagnosis: overview and introduction to the full Supplement publication. Endoscopy. 2013;45(1):51–59. doi:10.1055/s-0032-1325997
49. Lin JS, Perdue LA, Henrikson N, Bean S, Blasi P. Screening for colorectal cancer: an evidence update for the U. S. preventive services task force. Evidence Synthesis No. 202; 2021:202.
50. Provenzale D, Ness RM, Llor X, et al. Colorectal cancer screening, version 2.2020 featured updates to the NCCN guidelines. JNCCN J Natl Compr Cancer Netw. 2020;18(10):1312–1320. doi:10.6004/jnccn.2020.0048
51. Canadian. Task Force on Preventive Health Care. Recommendations on screening for colorectal cancer in primary care. Can Med Assoc J. 2016;188(5):1–9.
52. Fang J-Y, Zheng S, Jiang B, et al. Consensus on the prevention, screening, early diagnosis and treatment of colorectal tumors in China: Chinese society of gastroenterology, October 14–15,2011, Shanghai, China. Gastrointest Tumors. 2014;1(2):53–75. doi:10.1159/000362585
53. Alsanea N, Almadi MA, Abduljabbar AS, et al. National guidelines for colorectal cancer screening in Saudi Arabia with strength of recommendations and quality of evidence. Ann Saudi Med. 2015;35(3):189–195. doi:10.5144/0256-4947.2015.189
54. Segura PP, Fombella JPB, Lorenzo BP, Martín MR, Lopez PG. SEOM guide to primary and secondary prevention of cancer: 2014. Clin Transl Oncol. 2014;16(12):1072–1078. doi:10.1007/s12094-014-1215-5
55. Labianca R, Nordlinger B, Beretta GD, et al. Clinical practice guidelines early colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up † clinical practice guidelines. ESMO Updat Clin Pract Guidel. 2013;24:vi64–vi72.
56. Bryant J, Patterson K, Vaska M, et al. Cancer screening interventions in indigenous populations: a rapid review. Curr Oncol. 2021;28(3):1728–1743. doi:10.3390/curroncol28030161
57. Barre S, Leleu H, Taleb S, Vimont A. Estimation of the epidemiological impact of the organized screening program for colorectal cancer. Eur J Public Health. 2019;29(4). doi:10.1093/eurpub/ckz185.053
58. Syed Soffian SS, Safian N, Nawi AM, et al. Rate and associated factors of refusal to perform immunochemical Faecal Occult Blood Test (iFOBT) among semi-urban communities. PLoS One. 2021;16(10):1–11. doi:10.1371/journal.pone.0258129
59. Valent F, Sammartano F, Degano S, et al. Reasons for non-participation in public oncological screening programs in the Italian region Friuli Venezia Giulia. Public Health. 2020;181:80–85. doi:10.1016/j.puhe.2019.12.005
60. He E, Bin LJ, Egger S, et al. Factors associated with participation in colorectal cancer screening in Australia: results from the 45 and Up Study cohort. Prev Med. 2018;106(July2017):185–193. doi:10.1016/j.ypmed.2017.10.032
61. Millien VO, Levine P, Suarez MG. Colorectal cancer screening in African Americans: are we following the guidelines? Cancer Causes Control. 2021;32(9):943–951. doi:10.1007/s10552-021-01448-8
62. Kisiel JB, Eckmann JD, Limburg PJ. Multitarget stool DNA for average risk colorectal cancer screening: major achievements and future directions. Gastrointest Endosc Clin N Am. 2020;30(3):553–568. doi:10.1016/j.giec.2020.02.008
63. Stracci F, Zorzi M, Grazzini G. Colorectal cancer screening: tests, strategies, and perspectives. Front Public Heal. 2014;2(October):1–9.
64. Kalantari H, Khodadoostan M, Yaran M, Tavakoli A. Diagnostic value of pyruvate kinase isoenzyme type M2 in colon cancer proven with colonoscopy. Adv Biomed Res. 2020;9(76). doi:10.4103/abr.abr_91_20
65. Onyoh EF, Hsu W-F, Chang L-C, Lee Y-C, Wu M-S, Chiu H-M. The rise of colorectal cancer in Asia: epidemiology, screening, and management. Curr Gastroenterol Rep. 2019;21(36). doi:10.1007/s11894-019-0703-8
66. Melnitchouk N, Soeteman DI, Davids JS, et al. Cost-effectiveness of colorectal cancer screening in Ukraine. Cost Eff Resour Alloc. 2018;16(20). doi:10.1186/s12962-018-0104-0
67. Naber SK, Almadi MA, Guyatt G, Xie F, Lansdorp-Vogelaar I. Cost-effectiveness analysis of colorectal cancer screening in a low incidence country: the case of Saudi Arabia. Saudi J Gastroenterol. 2021;27(4):208–216. doi:10.4103/sjg.sjg_526_20
68. Dominitz JA, Levin TR. What is organized screening and what is its value? Gastrointest Endosc Clin N Am. 2020;30(3):393–411.
69. Kalyta A, De Vera MA, Peacock S, et al. Canadian colorectal cancer screening guidelines: do they need an update given changing incidence and global practice patterns? Curr Oncol. 2021;28(3):1558–1570. doi:10.3390/curroncol28030147
70. Turnbull E, Priaulx J, de Kok IM, et al. Results of a health systems approach to identify barriers to population-based cervical and colorectal cancer screening programmes in six European countries. Health Policy (New York). 2018;122(11):1206–1211. doi:10.1016/j.healthpol.2018.08.005
71. Bujang -N-N-A, Lee Y-J, S-a-s M-Z, et al. Factors associated with colorectal cancer screening via immunochemical fecal occult blood test in an average-risk population from a multiethnic, middle-income setting. JCO Glob Oncol. 2021;7:333–341. doi:10.1200/GO.20.00460
72. Bravo RL, Kietzman KG, Toy P, Duru OK, Wallace SP. Linking primary care and community organizations to increase colorectal cancer screening rates: the HAPPI project. Salud Publica Mex. 2020;61:427–435. doi:10.21149/9450
73. Hall IJ, Tangka FK, Sabatino SA, Thompson TD, Graubard BI, Breen N. Peer reviewed: patterns and trends in cancer screening in the United States. Prev Chronic Dis. 2018;15. doi:10.5888/pcd15.170465
74. Khalili F, Najafi B, Mansour-Ghanaei F, Yousefi M, Abdollahzad H, Motlagh A. Cost-effectiveness analysis of colorectal cancer screening: a systematic review. Risk Manag Healthc Policy. 2020;13:1499–1512. doi:10.2147/RMHP.S262171
75. Sharaf Ravi N, Ladabaum U. Comparative effectiveness and cost-effectiveness of screening colonoscopy vs. sigmoidoscopy and alternative strategies. Am J Gastroenterol. 2013;108(1):120–132. doi:10.1038/ajg.2012.380
76. Issa IA, NouredDine M. Colorectal cancer screening: an updated review of the available options. World J Gastroenterol. 2017;23(28):5086–5096. doi:10.3748/wjg.v23.i28.5086
77. Senore C, Hassan C, Regge D, et al. Cost-effectiveness of colorectal cancer screening programmes using sigmoidoscopy and immunochemical faecal occult blood test. J Med Screen. 2019;26(2):76–83. doi:10.1177/0969141318789710
78. Bhatia D, Lega IC, Wu W, Lipscombe LL. Breast, cervical and colorectal cancer screening in adults with diabetes: a systematic review and meta-analysis. Diabetologia. 2020;63(1):34–48. doi:10.1007/s00125-019-04995-7
79. Hullings AG, Sinha R, Liao LM, Freedman ND, Graubard BI, Loftfield E. Whole grain and dietary fiber intake and risk of colorectal cancer in the NIH-AARP diet and health study cohort. Am J Clin Nutr. 2020;112(3):603–912. doi:10.1093/ajcn/nqaa161
80. Burgoine T, Sarkar C, Webster CJ, Monsivais P. Examining the interaction of fast-food outlet exposure and income on diet and obesity: evidence from 51,361 UK Biobank participants. Int J Behav Nutr Phys Act. 2018;15(1):1–12. doi:10.1186/s12966-017-0635-3
81. Gibson DC, Prochaska JD, Yu X, Kaul S. An examination between census tract unhealthy food availability and colorectal cancer incidence. Cancer Epidemiol. 2020;67(January):101761. doi:10.1016/j.canep.2020.101761
82. da Silva FMM, Duarte RP, Leao CC, et al. Colorectal cancer in patients under age 50: a five-year experience. Rev Col Bras Cir. 2020;47(1):1–10. doi:10.1590/0100-6991e-20202406
83. Siegel RL, Torre LA, Soerjomataram I, et al. Global patterns and trends in colorectal cancer incidence in young adults. Gut. 2019;68(12):2179–2185. doi:10.1136/gutjnl-2019-319511
84. Hultcrantz R. Aspects of colorectal cancer screening, methods, age and gender. J Intern Med. 2020;289(4):493–507. doi:10.1111/joim.13171
85. Kang Y, Son H. Gender differences in factors associated with colorectal cancer screening: a national cross- sectional study in Korea. Asia Pacific J Public Heal. 2017;29:495–505. doi:10.1177/1010539517718336
86. Rutter CM, Knudsen AB, Lin JS, Bouskill KE. Black and white differences in colorectal cancer screening and screening outcomes: a narrative review. Cancer Epidemiol Biomarkers Prev. 2021;30(1):3–12. doi:10.1158/1055-9965.EPI-19-1537
87. Amitay EL, Carr PR, Jansen L, et al. Smoking, alcohol consumption and colorectal cancer risk by molecular pathological subtypes and pathways. Br J Cancer. 2020;122:1604–1610. doi:10.1038/s41416-020-0803-0
88. Fu Y, Zhang Y, Cui J, et al. SNP rs 12982687 affects binding capacity of lncRNA UCA1 with miR-873-5p: involvement in smoking-triggered colorectal cancer progression. Cell Commun Signal. 2020;18(1):1–18. doi:10.1186/s12964-020-0518-0
89. Chen X, Jansen L, Guo F, Hoffmeister M, Chang-Claude J, Brenner H. Smoking, genetic predisposition, and colorectal cancer risk. Clin Transl Gastroenterol. 2021;12(3):e45.
90. Gram IT, Park SY, Wilkens LR, Haiman CA, Le Marchand L. Smoking-related risks of colorectal cancer by anatomical subsite and sex. Am J Epidemiol. 2020;189(6):543–553. doi:10.1093/aje/kwaa005
91. Yamashita H, Takahashi Y, Ishizaki T, Imura H, Nakayama T. Associations of multimorbidity with breast, cervical and colorectal cancer screening delivery: a cross-sectional study of a nationally representative Japanese sample. Cancer Epidemiol. 2020;69:101798. doi:10.1016/j.canep.2020.101798
92. Deding U, Henig AS, Salling A, Torp-Pedersen C, Bøggild H. Sociodemographic predictors of participation in colorectal cancer screening. Int J Colorectal Dis. 2017;32(8):1117–1124. doi:10.1007/s00384-017-2832-6
93. Venturelli F, Sampaolo L, Carrozzi G, Zappa M, Giorgi Rossi P. Associations between cervical, breast and colorectal cancer screening uptake, chronic diseases and health-related behaviours: data from the Italian PASSI nationwide surveillance. Prev Med. 2019;120(April2018):60–70. doi:10.1016/j.ypmed.2019.01.007
94. Zhang D, Matthews CE, Powell-Wiley TM, Xiao Q. Ten-year change in neighborhood socioeconomic status and colorectal cancer. Cancer. 2019;125(4):610–617. doi:10.1002/cncr.31832
95. Al Abdouli L, Dalmook H, Abdo MA, Carrick FR, Rahman MA. Colorectal cancer risk awareness and screening uptake among adults in the United Arab Emirates. Asian Pacific J Cancer Prev. 2018;19(8):2343–2349.
96. Lee SJ, O’leary MC, Umble KE, Wheeler SB. Eliciting vulnerable patients’ preferences regarding colorectal cancer screening: a systematic review. Patient Prefer Adherence. 2018;12:2267–2282. doi:10.2147/PPA.S156552
97. Lau J, Lim TZ, Jianlin Wong G, Tan KK. The health belief model and colorectal cancer screening in the general population: a systematic review. Prev Med Rep. 2020;20:101223. doi:10.1016/j.pmedr.2020.101223
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

