Back to Journals » Clinical Interventions in Aging » Volume 18
Discordance in Frailty Measures in Old Community Dwelling People with Multimorbidity – A Cross-Sectional Study
Authors Lindh Mazya A , Axmon A , Sandberg M , Boström AM , W Ekdahl A
Received 8 March 2023
Accepted for publication 5 September 2023
Published 26 September 2023 Volume 2023:18 Pages 1607—1618
DOI https://doi.org/10.2147/CIA.S411470
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Maddalena Illario
Amelie Lindh Mazya,1,2 Anna Axmon,3 Magnus Sandberg,4 Anne-Marie Boström,5– 7 Anne W Ekdahl1,8
1Division of Clinical Geriatrics, Department of Neurobiology, Care Sciences and Society, Karolinska Institutet, Stockholm, Sweden; 2Department of Geriatric Medicine of Danderyd Hospital, Stockholm, Sweden; 3EPI@LUND (Epidemiology, Population Studies, and Infrastructures at Lund University), Division of Occupational and Environmental Medicine, Lund University, Lund, Sweden; 4Department of Health Sciences, Lund University, Lund, Sweden; 5Theme Inflammation and Aging, Nursing Unit Aging, Karolinska University Hospital, Huddinge, Sweden; 6Division of Nursing, Department of Neurobiology, Care Sciences and Society, Karolinska Institutet, Stockholm, Sweden; 7R&D unit, Stockholms Sjukhem, Stockholm, Sweden; 8Department of Clinical Sciences Helsingborg, Lund University, Helsingborg, Sweden
Correspondence: Amelie Lindh Mazya, Danderyd Hospital, Entrévägen 10, Danderyd, Stockholm, 182 88, Sweden, Tel + 46 736 22 45 29, Email [email protected]
Purpose: Assessment of frailty is a key method to identify older people in need of holistic care. However, agreement between different frailty instrument varies. Thus, groups classified as frail by different instruments are not completely overlapping. This study evaluated differences in sociodemographic factors, cognition, functional status, and quality of life between older persons with multimorbidity who were discordantly classified by five different frailty instruments, with focus on the Clinical Frailty Scale (CFS) and Fried’s Frailty Phenotype (FP).
Participants and Methods: This was a cross-sectional study in a community-dwelling setting. Inclusion criteria were as follows: ≥ 75 years old, ≥ 3 visits to the emergency department the past 18 months, and ≥ 3 diagnoses according to ICD-10. 450 participants were included. Frailty was assessed by CFS, FP, Short Physical Performance Battery (SPPB), Grip Strength and Walking Speed.
Results: 385 participants had data on all frailty instruments. Prevalence of frailty ranged from 34% (CFS) to 75% (SPPB). Nine percent of participants were non-frail by all instruments, 20% were frail by all instruments and 71% had discordant frailty classifications. Those who were frail according to CFS but not by the other instruments had lower cognition and functional status. Those who were frail according to FP but not CFS were, to a larger extent, women, lived alone, had higher cognitive ability and functional status.
Conclusion: The CFS might not identify physically frail women in older community-dwelling people with multimorbidity. They could thus be at risk of not be given the attention their frail condition need.
Keywords: geriatrics, frailty phenotype, clinical frailty scale, outpatient assessment
Introduction
Frailty is an age-related syndrome of declining function and low reserves in several organs resulting in an increased vulnerability and reduction in one’s ability to cope with acute stressors.1 Depending on setting and method of measurement, the prevalence of frailty ranges from 4% to 59%.2 The prevalence increases with age and is higher in women.2 The frailty syndrome is clinically recognizable and there are several promising interventions for reducing frailty, such as physical activity programs, nutritional supplementation and reduction of medications.3,4 There is no international consensus regarding the definition of frailty and no gold standard for assessment of frailty. Researchers are however unified in the definition of frailty as
a medical syndrome with multiple causes and contributors characterized by diminished strength, endurance, and reduced physiologic function that increases an individual’s vulnerability for developing increased dependency and/or death.5
Several definitions of multimorbidity are present in the literature but it is often defined as the presence of two or more long-term diseases.6 The prevalence of multimorbidity depends on the definition but usually ranges between 55% and 98% in those older than 65 years.7 Frailty and multimorbidity are sometimes used interchangeably but earlier studies show that the majority of older people with multimorbidity (two or more diseases) are not frail, and that not all frail people have multimorbidity.8,9 In a population with multimorbidity (three or more diseases), aged over 75 years and with a high health care utilization, the prevalence of frailty was almost 50% and an additional 40% were pre-frail.10 The causality regarding frailty and multimorbidity is not fully understood; either frailty predisposes for development of multimorbidity, or frailty develops from multimorbidity.8,9,11 Assessment of frailty is however considered a key method to identify older people, including those with multimorbidity, who may benefit from a tailored approach to treatment and care.12 In the coming decades there is an expected increase of older people in the population thus the number of people affected by frailty and/or multimorbidity will increase.13 Identification and management of these conditions are highly relevant not only for the affected individuals themselves but for health care, social care and policy makers.14–16
There are numerous frailty instrument and the agreement between them varies.17,18 Instruments are also used with different purposes and in different settings, such as in research, population screening and diagnosis of frailty.19,20 Most instruments to measure frailty are based on one of three models: Frailty as a biological phenotype by Fried et al,21 frailty as acquired deficits in multiple organ system by Mitnitski et al,22 or the more holistic model, frailty as a loss of functioning in one or more domains of function (physical, psychological or social by Gobbens et al).23 As a biological phenotype, frailty is operationalized into five measurable criteria, the Fried Frailty Phenotype (FP), which is one of the most widely used frailty measures.21 Assessment of frailty using the FP requires equipment since you need to measure grip strength, walking speed and weight. It is time-consuming and therefore mainly used in research and not clinical practice. The operationalization of acquired deficits is a frailty index (FI) calculated by dividing existing deficits in a patient, by a predefined set of deficits (symptoms, signs, diseases, disabilities or laboratory, abnormalities).22 The Clinical Frailty Scale (CFS) is theoretically rooted in the acquired deficits theory but was developed as a frailty instrument based on clinical judgement. It was first used to summarize a Comprehensive Geriatric Assessment (CGA).24 The CFS assesses comorbidity, function and cognition, and uses pictures and written descriptions to stratify patients into one of nine categories ranging from 1 (very fit) to 9 (terminally ill) where the cut-off for frailty is at 5 (mildly frail).24 The CFS has gained wide acceptance as a frailty measure and is used in clinical practice in emergency, cardiology and geriatric departments.25
Physical performance tests have been used as alternative measures of frailty since they are associated with, or predictive of, frailty.18,20,26 The Short Physical Performance Battery (SPPB) was originally developed as a test for function of lower limbs but has in previous studies been used as a measure of physical frailty.27,28 Walking speed and grip strength have been used as single item measures for frailty in several studies and are feasible to perform in a clinical setting.18,29
Since many frailty instruments are based on one of the three different models of frailty described above, the variation in prevalence and heterogeneity in agreement between them is not surprising. The lack of a unifying model and a gold standard of measurement hinders both advances in research and the implementation of frailty assessment in clinical practice. The groups identified as frail by different instruments are not identical and at the same time frailty is considered one construct, albeit with several theoretical definitions. To increase the knowledge of clinical usefulness of different frailty measures, it is important to explore differences between groups with discordant frailty classifications using different measures of frailty.
One study found similar prevalence of frailty for both the FP and the FI, but substantial discordance in individuals classified as frail by the two instruments.30 Another study found moderate agreement between the FP and the FI in finding the same frail persons.31 Furthermore, authors discuss that discordant identification could be related to the measures’ capacity in capturing sensory, motor and psychological impairment.31 A recent study of the CFS and an electronic FI found substantial heterogeneity between the measures and low support for convergent validity.32 Assessment of frailty increased during the COVID-19 pandemic since it was found to be a useful way to determine risk for adverse outcomes and prognosis of older patients with COVID-19 in the acute care setting.33 The CFS was endorsed by the UK National Institute for Health and Care Excellence for this purpose.34 The use of the CFS thus increased during the COVID-19 pandemic and it is now the most commonly used frailty instrument at emergency departments (ED).35 Since the FP and CFS are the most used frailty instrument in research and the clinical setting, respectively, it is relevant to investigate discordant identifications between the FP and the CFS and other instruments in clinical use such as SPPB, walking speed and grip strength.
Aim
The primary aim of this study was to explore differences between groups of community dwelling older people with multimorbidity discordantly classified by five frailty instruments: The CFS, the FP, the SPPB, walking speed and grip strength. The secondary aim was to investigate the sensitivity and the specificity of these instruments using the CFS and the FP, respectively, as reference instrument.
Materials and Methods
This is a cross-sectional study and a secondary analysis of baseline data from the randomized controlled study The Comprehensive Geriatric Assessment with Mobile Teams (GerMoT) Trial. The study was conducted in one municipality in Region Skåne, in the south of Sweden. The study protocol has been published and the study is registered at ClinicalTrials.Gov (NCT02923843).36
Participants
Subjects eligible for the GerMoT Trial had to 1. Be 75 years or older. 2. Have had three or more visits to the ED in the past 18 months (with or without admission). 3. Have diagnoses in at least three different diagnostic chapters according to the International Statistical Classification of Diseases and Related Health Problems, 10th revision (ICD-10) 4. Not live in a nursing home and live in or close to the municipality where the study hospital was situated. These criteria have been used by the Swedish National Board of Health and Welfare to describe a population affected by multimorbidity and high health care consumption.37
Data Collection
Lists of eligible participants were obtained from the patient administrative system in Region Skåne (PASIS). All eligible participants received an invitation letter by post that included information about of the study. They were later contacted by telephone and asked to provide verbal informed consent for participation. Upon that, home visits were made to obtain written informed consent at the first study visit. Data were collected by research nurses between October 2016 and June 2018 in the participants’ homes. Background characteristics and health-related measures were sex, age, body mass index (BMI), living situation, education level, cognition, functional status, and health-related quality-of-life (QoL). Cognition was measured with the Montreal Cognitive Assessment (MoCA).38 Although there is no validation study of the Swedish version of the MoCA, normative data for the Swedish version of the MoCA has been presented.39 Functional status was measured with the Activities of Daily Living (ADL) staircase, developed from the Katz’ index of daily living.40 This consists of five personal ADLs (PADL); feeding, transfer, going to the toilet, dressing, and bathing, combined with four instrumental ADLs (IADL); cooking, transportation, shopping, and cleaning. Participants were classified as dependent or independent for each activity. Health-related QoL was measured with the EuroQol five-dimension scale (EQ-5D-5L).41 The Swedish version of EQ-5D-5L has been approved by EuroQol. An index was calculated using the Danish reference value set, as no reference values for the Swedish population were available at the time of the analyses.41,42
Measurements of Frailty
Five measurements of frailty were included: The CFS, the FP, grip strength, walking speed and the SPPB.
The CFS has been translated and validated into Swedish.43 CFS was scored as the final instrument in the data collection procedure, when information important for assessment of the CFS (cognition, chronic diseases, physical performance, and PADL/IADL) were available for the research nurse. The cut-off for frailty was a score of five (mildly frail) or more.
The FP was measured by the five-criteria from the Cardiovascular Health Study.21 Exhaustion was assessed by two questions from the Centre for Epidemiological Studies Depression Scale (“I felt that everything I did was an effort” and “I could not get going”).44 Weakness was measured with grip strength using a Jamar handheld dynamometer, two attempts, the best values were used and adjusted for sex and BMI, using the same cut-offs as presented by Fried in 2001.21 For women the cut-offs for the weakness criteria for frailty was (BMI/grip strength in Kg): ≤23/≤17, 23.1–26/≤17.3, 26.1–29/≤18, >29/≤21. For men the corresponding cut-offs; ≤24/≤29, 24.1–26/≤30, 26.1–28/≤30, > 28/≤32. Slowness was measured with walking speed. This was assessed by measuring the walking speed over 4 or 5 m at usual pace and with walking aids if needed. A walking speed under 0.8 m/s was considered a cut-off for frailty.45 Those chronically bedridden or in a wheelchair, ie, where walking speed could not be measured, were considered frail for the walking speed criteria. Both grip strength and walking speed have been used as single item measures for frailty in several studies.18,29,46 For assessment of low physical activity, the International Physical Activity Questionnaire–Short Form (IPAQ-SF) was used.47 It is a validated self-report instrument that summates the duration and frequency of different kinds of activity, leading to the categorization of activity level as high, moderate, or low. A low activity level was considered frail per this criterion. Weight loss was determined by self-reported unintentional weight loss of more than five percent in the past year. Participants meeting three or more of the five criteria for the FP were classified as frail and those meeting zero to two criteria were classified as non-frail.
The SPPB was originally developed as a test for function of lower limbs but has also been used as measure of frailty.27,28,48 It includes measurements of balance, walking speed and the time it takes to stand up five times from a chair. Points are given according to predetermined time limits to a maximum score of 12. A score of nine or lower has in previous studies been used to identify frail older adults and was also used in the present study.28,49
Data Analysis
All frailty assessments were dichotomized (frail/non-frail). All available data were used in the pair-wise comparisons but only those with data on all five instruments were included in the analyses of the prevalence of all five instrument (n = 385). Sensitivity and specificity were calculated for the four instruments in relation to the two chosen reference instruments, CFS and FP. To determine differences between the groups that were discordantly classified as frail, student’s independent sample’s t-test, Mann–Whitney U-test, chi-square test and Fisher’s exact test were used depending on data level and distribution. For these analyses all participants of the 450 that had data on the instruments that were compared were included. The number of individuals in each analysis are declared in the results. This is a secondary analysis of pre-existing data from an RCT and thus the power calculation was made for the primary outcome of the RCT, not the present cross-sectional study. The primary outcome variable was mean number of days in the hospital and the assumed difference between the Intervention Group and the Control Group was 4.1 days (SD 15). The significance level was set to 0.05 for all analyses. Data were stored and analyzed using IBM SPSS Statistics for Windows, Version 22.0, 26.0 and 27.0 (Armonk, NY: IBM Corp.).
Results
There were 450 participants in the GerMoT Trial, the mean age was 82.6 years and 54% were women. Out of all participants, 385 had complete data on all frailty instruments. Proportions of missing data for the individual instruments were for SPPB 0%, for FP 9%, for walking speed 4%, for grip strength 7.5%, for CFS 3%. The prevalence of frailty for these 385 ranged from 34% (CFS) to 75% (SPPB) (Figure 1). Nine percent were non-frail by all five instruments and 20% were frail by all instruments, thus 71% were discordantly classified by at least two of the five frailty instruments.
FP as Reference Instrument
The discordant classifications between the FP and the other four instruments ranged from 22% to 32%. The differences between the groups who were discordantly classified by the FP and the other instruments are shown in Table 1. There were differences in sex and cognitive ability in all four instrument pairs, but for those classified as frail by FP and non-frail by SPPB, walking speed and CFS, respectively, the majority were women and had higher cognitive ability whereas there were only men and people with lower cognitive ability in the FP-frail/grip strength -non-frail group. All groups classified as FP-frail/non-frail by either of the other instruments had lower QoL than the comparison group, except for the FP-frail/CFS-non-frail group. When using the FP as reference instrument, the sensitivity of the different instruments ranged from 54% to 92%, and the specificity from 43% to 87% (Table 2). CFS was the only one of the four instruments that had a higher specificity than sensitivity in relation to the FP.
 |
Table 1 Discordant Classifications with the Frailty Phenotype |
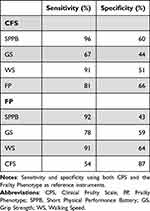 |
Table 2 Sensitivity and Specificity of the Clinical Frailty Scale and the Frailty Phenotype, Respectively |
CFS as Reference Instrument
The discordant classifications between the CFS and the other four instruments ranged from 28% to 48%. The differences between the groups who were discordantly classified by the CFS and the four other instruments are presented in Table 3. There were statistically significant differences in all groups showing a lower cognitive ability in those classified as frail by the CFS and non-frail by either of the four instruments. They also had a higher dependency in either PADL, IADL or both. The sensitivity and specificity of the four compared instruments in relation to the CFS ranged respectively from 67% to 96% and from 44% to 66%, as shown in Table 2.
 |
Table 3 Discordant Classifications with the Clinical Frailty Scale |
Discussion
Groups of older adults with multimorbidity who were discordantly identified as frail by the five frailty instruments CFS, FP, SPPB, walking speed and grip strength differed in several aspects. Those identified as frail by CFS but not by the other instruments had lower cognitive function and were more likely to be dependent in PADL or IADL than those identified as frail by the other instruments but not by the CFS. Thus, the CFS was better at capturing people with low cognitive function and high dependence in ADL. This is also a reflection of the underlying concept of the CFS as a more global assessment of frailty. The finding of lower cognitive ability in those identified as frail by the CFS but not by the physical instruments highlights the ability of the CFS to include cognitive decline in the frailty assessment. Those classified as frail by the FP but not by the CFS were to a larger extent, women, lived alone, had higher cognitive ability and lower dependency in IADL, compared to those classified as frail by the CFS but not by the FP. A consequence of this may be that older women with multimorbidity and physical frailty will not be considered eligible for certain care paths (for example geriatric care) if the CFS rather than the FP is used as an instrument for decisions regarding level of care. They may also be at risk of receiving potentially harmful treatments usually not given to frail people.
In the present study, only nine percent of the participants were considered non-frail by all five instruments and many individuals were classified discordantly by at least two instruments. Since two of the instruments (FP, CFS) are based on different theoretical backgrounds, and the other three (SPPB, walking speed, grip strength) considered alternative measures for frailty, we did not find the discordance between the instruments surprising. Moreover, it is congruent with previous studies stating that frailty measures cannot be used interchangeably.21,24,50 Frailty is however often regarded as a single concept and it is important to highlight that different frailty instruments indeed identify different subpopulations, to continue the discussion on what frailty is.17 Our findings contribute to knowledge about limitations of both the CFS and the FP in identifying certain vulnerable groups. However, longitudinal studies of groups that are discordantly identified by different frailty measures are needed to further understand the implications and prognostic importance of discordant frailty measures.
In the present study, no women were identified as non-frail by grip strength but frail by the CFS or the FP. Moreover, the proportions of women classified as frail by grip strength but not by the CFS or the FP were high, 97% and 95%, respectively. Since grip strength weakens with age, and is associated with being female and with multimorbidity, the original cut-offs from Fried et al21 might not be suitable in an older population with multimorbidity.51 There are few age- and sex stratified data for hand grip strength, but a systemic review suggest a cut-off of 16 kg for women, based on British data, which is lower than the lowest BMI-related, cut-off for frailty in present study and in the original Fried study (17 kg).52 Moreover, a cut-off of 16 kg for women is also recommended for diagnosing sarcopenia, when measured by hand grip strength.53 Perhaps an even lower cut-off would be more accurate for older persons highly affected by multimorbidity, future studies of normative values in this population could explore this. For the present study a lower cut-off would have identified fewer as frail by hand grip strength and the prevalence of frailty would have been closer to the FP and the CFS, resulting in a larger overlap of people considered frail.
There is no gold standard for assessment of frailty, but we conducted analyses of sensitivity and specificity using the CFS and the FP as reference measurements, respectively. When using the FP as the reference, the CFS was only able to identify 54% of those classified as frail by the FP (sensitivity). However, the ability of the CFS to classify the non-frail FP as non-frail CFS (specificity) was 87%, meaning that those classified as non-frail indeed were non-frail. In a study comparing three frailty instruments at an ED, the CFS had similar values (sensitivity and specificity) for frailty diagnosed with a CGA.54 We therefore suggest that a combination of the CFS and a measure with high sensitivity for identifying physical frailty, may lead to a more holistic assessment of frailty.54 In the present study both WS and the SPPB had a sensitivity of more than 90% in relation to FP, and either may thus be suitable for use in conjunction with the CFS to detect physical frailty. For those unable to walk, grip strength could be an alternative. The predictive ability on mortality, hospitalization, and other important outcomes of these instruments separately and in combination must however be taken into consideration and studied more before any recommendations can be made.
The present study has several strengths. Data were collected by research nurses trained in Good Clinical Practice, the measurements were all made using validated instruments and the time for testing was rather short, a mean time of 50 minutes, thus limiting the risk of exhausting the participants. Some limitations need however to be taken into consideration. Since this was a secondary analysis of data from an RCT, there was no power calculation for this cross-sectional study. This limits the statistical power of the study and reduces the generalizability of the results. Some participants were tired and could not participate in all physical measurements, 65 individuals had therefore not data on all frailty instruments. If all these individuals were classified as frail by the frailty instruments based on physical measurements, the prevalence of frailty for the instruments would have been higher and most likely less discordant with the other instruments. A complementary subgroup analysis was made and there were significant differences in dependence in PADL and IADL, with the 65 being more dependent than the other group, thus probably more frail than the other group. There were however no differences between the groups in age, BMI, education and civil state. Another limitation is that the GerMoT Trial was not designed to evaluate frailty and we only could use the already existing measurements included in the GerMoT Trial. This study thus includes four instruments of physical frailty and the CFS. Four of the instruments, walking speed, CFS, SPPB and the FP were however, in that order, the most used frailty assessments methods in daily practice by European practitioners according to a survey a few years ago.48 Future studies should perhaps include frailty measurements based on a frailty index (FI) and other multidimensional models of frailty for example, the Tilburg Frailty Indicator, since these measures also are commonly used.22,23,55
The frailty assessments in this study were dichotomized, since the main interest was to explore those identified as frail or non-frail by the pre-defined cut-offs that has been used in earlier studies. However, in the clinical reality a grading of frailty may be more relevant when it comes to a global clinical judgement of a patient.56
Conclusion
There are high proportions of discordant frailty measures and significant differences between the subgroups with discordant classifications. Specifically, the CFS might not identify physical frail women or/and persons with high cognitive ability in a cohort of old community dwelling people with multimorbidity. Combining frailty instruments might improve routine frailty assessment.
Data Sharing Statement
The dataset used during the current study is available from the corresponding author on reasonable request and insofar that it is in accordance with Swedish law.
Ethics Approval and Informed Consent
Informed consent was mandatory for participating in the study. Ethical approval was obtained from Lund Regional Ethical Committee (Dnr: 2016/630). The research was carried out in accordance with the Declaration of Helsinki.
Acknowledgments
This study was accomplished within the context of the Swedish National Graduate School for Competitive Science on Ageing and Health (SWEAH) funded by the Swedish Research Council.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by Region Skåne and the Kamprad Foundation paid to Dr Anne W Ekdahl (Grant number 20170077).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381(9868):752–762. doi:10.1016/S0140-6736(12)62167-9
2. Collard RM, Boter H, Schoevers RA, Oude Voshaar RC. Prevalence of frailty in community-dwelling older persons: a systematic review. J Am Geriatr Soc. 2012;60(8):1487–1492. doi:10.1111/j.1532-5415.2012.04054.x
3. Walston J, Buta B, Xue QL. Frailty screening and interventions: considerations for clinical practice. Clin Geriatr Med. 2018;34(1):25–38. doi:10.1016/j.cger.2017.09.004
4. Dent E, Martin FC, Bergman H, Woo J, Romero-Ortuno R, Walston JD. Management of frailty: opportunities, challenges, and future directions. Lancet. 2019;394(10206):1376–1386. doi:10.1016/S0140-6736(19)31785-4
5. Morley JE, Vellas B, van Kan GA, et al. Frailty consensus: a call to action. J Am Med Dir Assoc. 2013;14(6):392–397. doi:10.1016/j.jamda.2013.03.022
6. Vetrano DL, Calderon-Larranaga A, Marengoni A, et al. An international perspective on chronic multimorbidity: approaching the elephant in the room. J Gerontol a Biol Sci Med Sci. 2018;73(10):1350–1356. doi:10.1093/gerona/glx178
7. Marengoni A, Angleman S, Melis R, et al. Aging with multimorbidity: a systematic review of the literature. Ageing Res Rev. 2011;10(4):430–439. doi:10.1016/j.arr.2011.03.003
8. Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci. 2004;59(3):255–263. doi:10.1093/gerona/59.3.M255
9. Vetrano DL, Palmer K, Marengoni A, et al. Frailty and multimorbidity: a systematic review and meta-analysis. J Gerontol A Biol Sci Med Sci. 2018;74(5):659–666.
10. Mazya AL, Garvin P, Ekdahl AW. Outpatient comprehensive geriatric assessment: effects on frailty and mortality in old people with multimorbidity and high health care utilization. Aging Clin Exp Res. 2019;31(4):519–525. doi:10.1007/s40520-018-1004-z
11. Cesari M, Perez-Zepeda MU, Marzetti E. Frailty and multimorbidity: different ways of thinking about geriatrics. J Am Med Dir Assoc. 2017;18(4):361–364. doi:10.1016/j.jamda.2016.12.086
12. Yarnall AJ, Sayer AA, Clegg A, Rockwood K, Parker S, Hindle JV. New horizons in multimorbidity in older adults. Age Ageing. 2017;46(6):882–888. doi:10.1093/ageing/afx150
13. United Nations. World Population Ageing 2019: highlights; New York,; 2019. ST/ESA/SER.A/430.
14. Ilinca S, Calciolari S. The patterns of health care utilization by elderly Europeans: frailty and its implications for health systems. Health Serv Res. 2015;50(1):305–320. doi:10.1111/1475-6773.12211
15. Ensrud KE, Kats AM, Schousboe JT, et al. Frailty phenotype and healthcare costs and utilization in older women. J Am Geriatr Soc. 2018;66(7):1276–1283. doi:10.1111/jgs.15381
16. Wise J. Number of older people with four or more diseases will double by 2035, study warns. BMJ. 2018;360:k371. doi:10.1136/bmj.k371
17. Aguayo GA, Donneau AF, Vaillant MT, et al. Agreement between 35 published frailty scores in the general population. Am J Epidemiol. 2017;186(4):420–434. doi:10.1093/aje/kwx061
18. Clegg A, Rogers L, Young J. Diagnostic test accuracy of simple instruments for identifying frailty in community-dwelling older people: a systematic review. Age Ageing. 2015;44(1):148–152. doi:10.1093/ageing/afu157
19. Buta BJ, Walston JD, Godino JG, et al. Frailty assessment instruments: systematic characterization of the uses and contexts of highly-cited instruments. Ageing Res Rev. 2016;26:53–61. doi:10.1016/j.arr.2015.12.003
20. Dent E, Kowal P, Hoogendijk EO. Frailty measurement in research and clinical practice: a review. Eur J Intern Med. 2016;31:3–10. doi:10.1016/j.ejim.2016.03.007
21. Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–M156. doi:10.1093/gerona/56.3.M146
22. Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. TheScientificWorldJOURNAL. 2001;1:323–336.
23. Gobbens RJ, Luijkx KG, Wijnen-Sponselee MT, Schols JM. Towards an integral conceptual model of frailty. J Nutr Health Aging. 2010;14(3):175–181. doi:10.1007/s12603-010-0045-6
24. Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. Can Med Assoc J. 2005;173(5):489–495. doi:10.1503/cmaj.050051
25. Church S, Rogers E, Rockwood K, Theou O. A scoping review of the Clinical Frailty Scale. BMC Geriatr. 2020;20(1):393. doi:10.1186/s12877-020-01801-7
26. Abizanda P, Romero L, Sanchez-Jurado PM, Atienzar-Nunez P, Esquinas-Requena JL, Garcia-Nogueras I. Association between functional assessment instruments and frailty in older adults: the FRADEA study. J Frailty Aging. 2012;1(4):162–168.
27. Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85–94. doi:10.1093/geronj/49.2.M85
28. Pritchard JM, Kennedy CC, Karampatos S, et al. Measuring frailty in clinical practice: a comparison of physical frailty assessment methods in a geriatric out-patient clinic. BMC Geriatr. 2017;17(1):264. doi:10.1186/s12877-017-0623-0
29. Sousa-Santos AR, Amaral TF. Differences in handgrip strength protocols to identify sarcopenia and frailty - a systematic review. BMC Geriatr. 2017;17(1):238. doi:10.1186/s12877-017-0625-y
30. Xue QL, Tian J, Walston JD, Chaves PHM, Newman AB, Bandeen-Roche K. Discrepancy in frailty identification: move beyond predictive validity. J Gerontol A Biol Sci Med Sci. 2019;75(2):387–393.
31. Beier F, Löffler M, Nees F, Hausner L, Frölich L, Flor H. Sensory and motor correlates of frailty: dissociation between frailty phenotype and frailty index. BMC Geriatr. 2022;22(1):755. doi:10.1186/s12877-022-03416-6
32. Broad A, Carter B, McKelvie S, Hewitt J. The convergent validity of the electronic Frailty Index (eFI) with the Clinical Frailty Scale (CFS). Geriatrics. 2020;5(4):88. doi:10.3390/geriatrics5040088
33. Lee C, Frishman WH. Implications of frailty in COVID-19. Cardiol Rev. 2021;29(6):285–288. doi:10.1097/CRD.0000000000000409
34. National Institute for Health and Care Excellence (NICE). COVID-19 Rapid Guideline: Managing COVID-19. National Institute for Health and Care Excellence (NICE); 2021.
35. Fehlmann CA, Nickel CH, Cino E, Al-Najjar Z, Langlois N, Eagles D. Frailty assessment in emergency medicine using the Clinical Frailty Scale: a scoping review. Intern Emerg Med. 2022;17(8):2407–2418. doi:10.1007/s11739-022-03042-5
36. Ekdahl AW, Axmon A, Sandberg M, Steen Carlsson K. Is care based on comprehensive geriatric assessment with mobile teams better than usual care? A study protocol of a randomised controlled trial (The GerMoT study). BMJ open. 2018;8(10):e023969. doi:10.1136/bmjopen-2018-023969
37. Gurner U, Thorslund M. Helhetssyn behövs i vården av multisviktande aldre. Förslag till förandring av vård- och omsorgsstrukturen i Stockholms lan [Holistic perspective is needed in care of multiimpaired elderly. A proposal for structural change of the organization of care in the county of Stockholm]. Lakartidningen. 2001;98(21):2596–2602. Swedish.
38. Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. doi:10.1111/j.1532-5415.2005.53221.x
39. Borland E, Nägga K, Nilsson PM, Minthon L, Nilsson ED, Palmqvist S. The Montreal cognitive assessment: normative data from a large Swedish population-based cohort. J Alzheimers Dis. 2017;59(3):893–901. doi:10.3233/JAD-170203
40. Asberg KH, Sonn U. The cumulative structure of personal and instrumental ADL. A study of elderly people in a health service district. Scand J Rehabil Med. 1989;21(4):171–177. doi:10.2340/165019778921171177
41. Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. 2011;20(10):1727–1736. doi:10.1007/s11136-011-9903-x
42. Group TE. EuroQol--a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199–208. doi:10.1016/0168-8510(90)90421-9
43. Ekerstad N, Swahn E, Janzon M, et al. Frailty is independently associated with short-term outcomes for elderly patients with non-ST-segment elevation myocardial infarction. Circulation. 2011;124(22):2397–2404. doi:10.1161/CIRCULATIONAHA.111.025452
44. Radloff LS. The CES-D Scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401. doi:10.1177/014662167700100306
45. Abellan van Kan G, Rolland Y, Andrieu S, et al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) task force. J Nutr Health Aging. 2009;13(10):881–889. doi:10.1007/s12603-009-0246-z
46. Syddall H, Cooper C, Martin F, Briggs R, Aihie Sayer A. Is grip strength a useful single marker of frailty? Age Ageing. 2003;32(6):650–656. doi:10.1093/ageing/afg111
47. Ekelund U, Sepp H, Brage S, et al. Criterion-related validity of the last 7-day, short form of the international physical activity questionnaire in Swedish adults. Public Health Nutr. 2006;9(2):258–265. doi:10.1079/PHN2005840
48. Bruyere O, Buckinx F, Beaudart C, et al. How clinical practitioners assess frailty in their daily practice: an international survey. Aging Clin Exp Res. 2017;29(5):905–912. doi:10.1007/s40520-017-0806-8
49. da Câmara SM, Alvarado BE, Guralnik JM, Guerra RO, Maciel AC. Using the Short Physical Performance Battery to screen for frailty in young-old adults with distinct socioeconomic conditions. Geriatr Gerontol Int. 2013;13(2):421–428. doi:10.1111/j.1447-0594.2012.00920.x
50. Perracini MR, Mello M, de Oliveira Maximo R, et al. Diagnostic accuracy of the short physical performance battery for detecting frailty in older people. Phys Ther. 2020;100(1):90–98. doi:10.1093/ptj/pzz154
51. Cheung CL, Nguyen US, Au E, Tan KC, Kung AW. Association of handgrip strength with chronic diseases and multimorbidity: a cross-sectional study. Age. 2013;35(3):929–941. doi:10.1007/s11357-012-9385-y
52. Dodds RM, Syddall HE, Cooper R, Kuh D, Cooper C, Sayer AA. Global variation in grip strength: a systematic review and meta-analysis of normative data. Age Ageing. 2016;45(2):209–216. doi:10.1093/ageing/afv192
53. Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48(1):16–31. doi:10.1093/ageing/afy169
54. O’Caoimh R, Costello M, Small C, et al. Comparison of Frailty screening instruments in the emergency department. Int J Environ Res Public Health. 2019;16(19):3626. doi:10.3390/ijerph16193626
55. Gobbens RJ, Van assen MA, Luijkx KG, Wijnen-Sponselee MT, Schols JM. The Tilburg frailty indicator: psychometric properties. J Am Med Dir Assoc. 2010;11(5):344–355. doi:10.1016/j.jamda.2009.11.003
56. Rockwood K, Theou O, Mitnitski A. What are frailty instruments for? Age Ageing. 2015;44(4):545–547. doi:10.1093/ageing/afv043
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

