Back to Journals » Infection and Drug Resistance » Volume 15
Direct Identification, Antimicrobial Susceptibility Testing, and Extended-Spectrum β-Lactamase and Carbapenemase Detection in Gram-Negative Bacteria Isolated from Blood Cultures
Authors Wen H, Xie S, Liang Y, Liu Y, Wei H, Sun Q, Wang W, Wen BJ, Zhao J
Received 5 January 2022
Accepted for publication 23 March 2022
Published 6 April 2022 Volume 2022:15 Pages 1587—1599
DOI https://doi.org/10.2147/IDR.S350612
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Hainan Wen,1 Shoujun Xie,2 Yueyi Liang,2 Yanchao Liu,2 Honglian Wei,1 Qian Sun,1 Weigang Wang,1,3 Baojiang Wen,1,3 Jianhong Zhao1,3
1The Second Hospital of Hebei Medical University, Shijiazhuang, Hebei, People’s Republic of China; 2Clinical Laboratory, the Affiliated Hospital of Chengde Medical University, Chengde, Hebei, People’s Republic of China; 3Hebei Provincial Center for Clinical Laboratories, Shijiazhuang, Hebei, People’s Republic of China
Correspondence: Jianhong Zhao, The Second Hospital of Hebei Medical University, 215 West Heping Road, Shijiazhuang, Hebei Province, 050051, People’s Republic of China, Tel +86 158 03210790, Fax +86 311 66002851, Email [email protected]
Purpose: To shorten the turnaround time for blood culture (BC) analyses, a rapid method was developed for the direct identification, antimicrobial susceptibility testing (AST), and multidrug resistance testing of bacteria-positive BCs.
Materials and Methods: The mixtures in BC bottles were treated with the multistep centrifugation method developed here and the conventional culture-based method. The bacterial sediment obtained after centrifugation was analyzed directly with MALDI-TOF MS and Vitek 2 Compact, and AST was performed directly with the Kirby–Bauer (K–B) disk diffusion, VITEK 2 Compact, and E-test methods. Extended spectrum lactamases (ESBLs) were detected with discs containing cefotaxime, cefotaxime/clavulanate, ceftazidime, and ceftazidime/clavulanate, and carbapenemase was detected with the modified carbapenem inactivation method (mCIM) and EDTA-mCIM (eCIM).
Results: All the results of direct testing were compared to those of the conventional methods, to evaluate the accuracy of the direct methods. The accuracies of the direct Vitek 2 Compact and MALDI-TOF MS methods were 95.5% (214/224) and 90.2% (202/224), respectively. Direct AST with K–B, Vitek 2, and E-test showed category agreement of 96.0% (2611/2721), 96.1% (2614/2721), and 97.4% (2650/2721), respectively, and the major errors and very major errors were < 2% for all three methods. In the direct determination of ESBLs, the results for cefotaxime combined with cefotaxime/clavulanate were completely consistent with those after the standard isolation method. The carbapenemase detection rate with direct mCIM and eCIM was exactly the same as that with the standard method.
Conclusion: These direct procedures based on multistep centrifugation are not only highly accurate but are appropriate for clinical laboratory use because the turnaround time is shorter.
Keywords: blood culture, direct identification, direct antimicrobial susceptibility testing, extended-spectrum β-lactamase, carbapenemase, multistep centrifugation
Introduction
Bloodstream infections (BSIs) are life-threatening diseases that occur widely throughout the world.1,2 Gram-negative bacteremia has attracted widespread attention because the antimicrobial resistance of the organisms involved is continuously increasing, with increased mortality in patients.3 The earlier the relevant information on pathogenic bacteria is known, the easier it is to treat patients effectively. Clinical microbiology laboratories often play an important role in detecting the causative pathogens of BSIs. However, the information cannot be obtained rapidly with conventional blood culture (BC) procedures because time is required to grow colonies of bacteria from positive BCs, and then identify the bacteria involved and determine their antimicrobial susceptibility. Clinicians cannot identify the etiological agent for 48–72 h after a BC is deemed positive. Therefore, the standard procedure does not allow the early development of precise therapeutic strategies for patients. The study by Kadri et al showed that approximately one in five patients with BSIs in US hospitals received discordant empiric antibiotic therapy, which was strongly associated with infections with drug-resistant pathogens. Furthermore, the receipt of discordant empiric antibiotic therapy was associated with increased overall mortality.4 Consequently, a new method is required that allows laboratories to identify bacteria and their drug resistance as soon as possible after a BC is shown to be positive.
The rapid identification of causative bacteria of BSIs provides a preliminary basis for the clinical empirical treatment of patients with bacteremia. The prognoses of patients may improve if the microorganism is identified within 1–2 h after a BC is deemed positive.5 To shorten the time required to identify pathogenic bacteria, some laboratories use molecular methods to identify those in BC bottles within 2 h. However, special equipment and skills are required for a molecular approach, and not all clinical laboratories can meet these requirements.6 Therefore, other methods that can be used in most laboratories are under investigation, such as incubating bacteria for a short time to form colonies before their identification, or rapid identification after the direct treatment of the mixtures in a bacterium-positive BC bottle.7–11 However, the results are not consistent when different processing methods are used.12–14
The classical method of detecting drug resistance in a positive BC requires overnight subculture of the organism on agar medium. Clinicians sometimes have to initiate empirical treatment before the results are available. This increases the likelihood of an unreasonable treatment. Furthermore, the increases in multidrug-resistant Gram-negative bacteria (MDR-GNB) have increased the failure of empirical treatments. This demonstrates the clinical importance of the early-stage diagnosis of drug resistance. Such early results can be obtained with various methods. PCR can be used to directly detect certain antibiotic resistance genes in positive BCs, but a major limitation is that negative amplification does not necessarily indicate susceptibility.15,16 For instance, Gram-negative bacteria that lack carbapenemase can still be resistant to carbapenem.17–20 Reducing the incubation time for AST improves the total time required, but some strains do not display antimicrobial resistance in the initial stage of culture.21,22 Studies have shown that neither molecular nor phenotypic rapid antibiotic susceptibility testing has directly improved mortality relative to standard methods. However, phenotypic rapid methods may improve the time to appropriate antibiotic therapy compared with conventional methods, whereas molecular methods will not.23 Therefore, the resistance phenotype of the bacteria in bacterium-positive BC bottles must be directly and accurately determined.
The treatment of severe infections caused by MDR-GNB is one of the most important challenges for clinicians worldwide, partly because bacterial resistance may not be identified until it is determined with AST.24 The therapy regimens differ for MDR bacteria with different resistance mechanisms.25,26 Therefore, the rapid detection of resistance mechanisms allows clinicians to select the appropriate drugs to treat MDR bacteria. The production of carbapenemases by Gram-negative bacteria is the most important mechanism of carbapenem resistance. The modified carbapenem inactivation method (mCIM) is recommended by the Clinical Laboratory Standards Institute (CLSI) guidelines to detect the carbapenemases of Enterobacteriaceae and Pseudomonas aeruginosa with high sensitivity and specificity.27 However, the test requires protracted culture, and little research has examined the direct testing of bacteria in BC bottles.
Recently, both the CLSI and the European Committee on Antimicrobial Susceptibility Testing have published guidelines for direct AST of positive BCs, which reduce the turnaround time of BC analyses to some extent. However, the rapid methods published in both guidelines target a limited number of bacteria, and only a subset of drugs are tested with AST, and carbapenems are not addressed by the CLSI guidelines.28,29 The diameters of the zones of inhibition must be read at 4, 6, and 8 h during AST, which increases the workload of laboratory staff. Moreover, some results cannot be classified because there are areas of technical uncertainty.28 Consequently, there is an urgent need for a method that allows the accurate, rapid and comprehensive AST of BCs, which can be readily used in most clinical microbiology laboratories.
Materials and Methods
Samples
This study was performed between July 2019 and January 2020 at two tertiary university hospitals in Hebei Province, China. Blood samples were collected from patients with suspected BSIs and injected into charcoal-based BC bottles (BacT/ALERT® FA and FN; bioMérieux, Paris, France) at the Affiliated Hospital of Chengde Medical University (Chengde, China) and the Second Hospital of Hebei Medical University (Shijiazhuang, China). The BC bottles were incubated in an automatic BacT/ALERT® 3D Microbial Detection System instrument (bioMérieux, Paris, France) until they were flagged positive or for up to 7 days. Once the bottles were designated positive, Gram staining of the fluid was performed immediately. The study was approved by the Institutional Review Board of the Affiliated Hospital of Chengde Medical University and the Second Hospital of Hebei Medical University. This study was performed in line with the principles of the Declaration of Helsinki. Before the commencement of study, the samples informed consent was obtained for the use of samples from all patients. The results obtained have only been used for scientific research, and the authors have no conflicts of interest.
Standard Methods
Culture-based identification of bacteria using matrix assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS; bioMérieux) was defined as the standard method of microorganism identification. Blood agar, MacConkey agar, and chocolate agar plates (Biokont, Wenzhou, China) were inoculated with the mixture from each positive BC bottle, and incubated at 36 °C for 18–24 h. The pure bacterial colonies were used for identification, according to the manufacturer’s instructions (MALDI-TOF MS; bioMérieux).
Agar dilution is considered the standard method for determining the minimum inhibitory concentration (MIC) for AST. Specified solvents and diluents were used to prepare the dilutions of antimicrobial agents (National Institutes for Food and Drug Control, China) according to CLSI Table 6A.27 The antibacterial agent was mixed with Mueller–Hinton (MH) agar (Oxoid; Thermo Fisher Scientific, Ely, UK) in a ratio of 1:10 and poured into an empty plate. AST was performed within 24 h of agar solidification. The bacterial colonies were picked from the plate and suspended in saline, adjusted to a turbidity of 0.5 McFarland and diluted 10-fold for AST using a multipoint inoculator (MIT-P, Sakuma, Japan). The plates were incubated at 36 °C for 16–20 h.
After bacterial culture, the standard method of detecting ESBLs was performed using cefotaxime (30 μg) and cefotaxime/clavulanate (CTL) (30/10 μg), and ceftazidime (30 μg) and ceftazidime/clavulanate (CAL) (30/10 μg) (Liofilchem S.R.L., Italy), according to CLSI Table 3A.27 The medium was incubated in air at 36 °C for 16–18 h. Similarly, the pure bacterial colonies were used for standard mCIM and EDTA-mCIM (eCIM) detection. The procedures were strictly in accordance with CLSI Table 3C.27
Testing with all standard methods was repeated to resolve any inconsistency.
Direct Methods
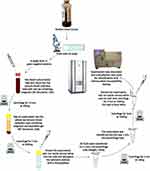
Bacteria were prepared for analysis with the direct methods as follows. The positive BCs were immediately subjected to Gram-staining. The BCs containing Gram-negative rods were included in the study, whereas Gram-positive organisms were excluded. However, Gram-negative rods with the appearance of “fine sand” were excluded as suspected Brucella. Gram-variable organisms were also treated them for testing with the direct methods. If colonies of these Gram-variable organisms were Gram-positive bacteria after overnight culture, when identified with MALDI-TOF MS, they were not be included in the study. The workflow is shown in Figure 1.
Direct identification: In this study, two instruments were used for direct bacterial identification. For MALDI-TOF MS, 1–2 µL of BC sediment prepared with multistep centrifugation was spotted onto a steel target plate, dried in air, and immediately mixed with 0.5 µL of formic acid solution (bioMérieux), combined with 1 µL of α-cyano-4-hydroxycinnamic acid (CHCA) matrix solution (bioMérieux). Direct identification was performed with MALDI-TOF MS. For Vitek 2 Compact (bioMérieux) identification, the density of the bacterial sediment was adjusted to 0.6 McFarland by dilution with 0.45% saline. Vitek 2 Compact GN cards (bioMérieux) were used for the direct identification of the bacteria.
Direct AST: For the direct K–B and E-test methods, the bacterial sediment was adjusted to 0.5–0.6 McFarland with normal saline. The bacterial suspension was spread on an MH plate with a sterile swab. The plate was placed at room temperature for 3 min and the disks (Oxoid; Thermo Fisher Scientific, Ely, UK) or E-test strips (Biokont) were placed on it. The plate was then incubated in air for 16–18 h. For the Vitek 2 Compact test, the density of the bacterial sediment was adjusted to 0.6–0.7 McFarland by dilution with 0.45% saline. Vitek 2 Compact GN-09 cards (bioMérieux) were used for the direct AST of the bacteria.
Direct detection of MDR-GNB: To detect ESBLs, the bacterial sediment was adjusted to 0.5–0.6 McFarland with normal saline. The bacterial suspension was spread on an MH plate with a sterile swab. The remaining steps were the same as those for the standard method of ESBL detection. For the mCIM and eCIM analyses, 2 µL Enterobacteriaceae sediment or 10 µL of P. aeruginosa sediment was emulsified in 2 mL of tryptic soy broth. The remaining operational steps were the same as those described for standard mCIM and eCIM detection.
Data Analysis and Quality Control
All antimicrobial results were interpreted as indicating antimicrobial susceptible, intermediate, or resistant bacteria, according to the CLSI guidelines.27 All the results obtained with the direct methods were compared with those obtained with the standard corresponding culture-based methods. Category agreement (CA) was calculated and discrepant results were categorized as minor errors (MinE), major errors (ME; false resistance), or very major errors (VME; false susceptibility). MinE was calculated by dividing the number of minor errors by the total number of isolates detected; ME was calculated by dividing the number of major errors by the number of susceptible isolates detected; and VME was calculated by dividing the number of VME by the number of resistant isolates detected. Escherichia coli ATCC 25922, Pseudomonas aeruginosa ATCC 27853 and K. pneumoniae ATCC BAA-1705 (American Type Culture Collection) were used as the quality control strains to ensure the quality of the medium.27
Results
A total of 253 Gram-negative organisms were grown from positive BCs during the study period. Five positive BCs were found to be in fine shape when smears were Gram-stained, and were therefore, suspected of being Brucella and were not treated with the direct methods. Six Gram-variable organisms were detected with Gram staining, and two of them were excluded because they were identified as Bacillus cereus. The remaining four were included in this study as Acinetobacter baumannii. Twenty-two of the BCs analyzed with the direct methods were shown to contain anaerobes (n = 17) or polymicrobials (n = 5), so these 22 isolates were not included in subsequent experiments. Therefore, a total of 224 BCs containing mono-aerobic bacteria were included in this study (Table 1).
 |
Table 1 Results of Direct Bacterial Identification with VITEK 2 Compact and MALDI-TOF MS Using the Developed Method |
Direct Identification of Microorganisms
The results of direct identification with VITEK 2 Compact and MALDI-TOF MS were compared to those acquired with standard laboratory culture-based methods. Overall, the accuracy of identification achieved with direct Vitek 2 Compact and MALDI-TOF MS was 95.5% (214/224) and 90.2% (202/224), respectively (Table 1).
Direct VITEK 2 Compact misidentified 2.7% of the isolates. In detail, Acinetobacter baumannii was incorrectly identified once as P. luteola; Burkholderia cepacia was incorrectly identified once as B. pseudomallei; Stenotrophomonas maltophilia was incorrectly identified once as Sphingomonas paucimobilis; Ralstonia mannitolilytica was incorrectly identified twice as P. fluorescens; Pantoea agglomerans was incorrectly identified once as Escherichia coli. Direct MALDI-TOF MS did not incorrectly identify any bacterium. However, MALDI-TOF MS failed to identify 22 strains (9.8%), and of these, half the Enterobacter cloacae (9/18) could not be identified (Table 1).
Results of Direct AST
All 224 bacterial strains examined in this study were analyzed with direct AST. Each isolate was tested simultaneously with the K–B disk diffusion, Vitek 2 Compact, and E-test AST methods. For each AST method, a total of 2721 microorganism–antimicrobial combinations were detected, and compared with the results of culture-based AST with agar dilution. The K–B, Vitek 2 Compact, and E-test direct AST methods showed CAs of 96.0% (2611/2721), 96.1% (2614/2721), and 97.4% (2650/2721), respectively. The lowest CA observed was for levofloxacin detected with direct Vitek 2 Compact t AST (87.5%). In contrast, the AST CA values for all other antibiotics were very high (> 90%). Most VMEs were detected for the antibiotics tobramycin and aztreonam. The CAs and errors for all bacterium–antibiotic combinations are listed in Table 2. No ME or VME occurred in the analysis of 11 microorganisms (Table 3).
 |
Table 2 Agreement and Errors in Antimicrobial Susceptibility Testing When Different Direct Methods Were Used |
 |
Table 3 Microorganism–Antimicrobial Combinations That Differed from Those Identified with the Standard Method |
Comparison of ESBL Detection with Direct Methods and Standard Culture-Based Methods
During the study period, the E. coli, Klebsiella pneumonia, and K. oxytoca isolates from positive BC bottles were directly tested for ESBLs. The results of the direct method based on cefotaxime combined with cefotaxime/clavulanate (CTX+CTL) were completely consistent with those of the standard method. There was a discordant result between the direct method that combined ceftazidime and ceftazidime/clavulanate (CAZ+CAL) and the standard culture-based method. One ESBL-producing E. coli strain was missed when the direct CAZ+CAL method was used; when detected with the direct method, the inhibition zone of ceftazidime for this strain was 28 mm, but 30 mm when clavulanate was added. The difference in the zone diameter between the two discs was less than 5 mm, and the strain was judged to be ESBL negative. However, with the standard method, the ceftazidime inhibition zone was 23 mm, but 28 mm with ceftazidime/clavulanate, so the strain was judged to be ESBL positive. With the exception of this isolate, the ESBL results for all organisms were consistent when either the direct CAZ+CAL method or the standard method was used (Table 4).
 |
Table 4 Results of Extended-Spectrum β-Lactamase (ESBL) Testing with the Direct Method and the Standard Culture-Based Method |
Comparison of mCIM/eCIM Detection with Direct Methods and Standard Culture-Based Methods
In this study, the carbapenemases of Enterobacteriaceae and P. aeruginosa were detected with direct mCIM and eCIM. To reflect the effect of direct method, we conducted mCIM and eCIM simultaneously. The eCIM results were considered valid when the mCIM results were positive. In this study, one strain of Enterobacter cloacae was carbapenemase positive, but only according to eCIM, and this measurement was excluded from the analysis. A total of 18 mCIM results were positive when determined with the standard culture-based mCIM method, including K. pneumoniae (n = 12), E. coli (n = 4), Enterobacter cloacae (n = 1), and Raoultella planticola (n = 1). Four strains of E. coli had positive eCIM results. Overall, the carbapenemase detection rates with direct mCIM and eCIM were exactly the same with the detection rates of the standard methods (Table 5).
 |
Table 5 Comparison of Direct and Standard Methods in Detecting Carbapenemases in Bacterial Strains |
Discussion
Immediate effective antimicrobial treatment is necessary to ensure the survival of patients with Gram-negative bacteremia. The rapid analysis of BCs can provide a strong basis for clinically accurate treatment. Clinical microbiology laboratories have made sustained efforts to develop ways to accelerate BC analysis.30,31 In this study, a multistep centrifugation method was used to isolate bacteria from bacteria-positive BC bottles. These were then identified, subjected to AST with several methods, their expression of ESBLs confirmed directly, and their carbapenemase expression tested with direct mCIM/eCIM. We effectively removed the blood cells, charcoal, and impurities from the blood with the multistep centrifugation method to obtain pure bacterial isolates from the bacteria-positive BC bottles. Our results show that the method developed here is not only highly accurate, but reduced the time required for pathogen identification to 1 h and reduced the time of AST by about 24h relative to that required for the standard culture-based method. Therefore, microbiology laboratories can identify bacterial pathogens, determine their antimicrobial susceptibility, and detect MDR strains responsible for BSIs on the day that a BC is deemed positive.
The use of MALDI-TOF MS is a major innovation in microbial identification.32 After BCs are treated with specific methods, pathogenic bacteria can be rapidly identified directly with MALDI-TOF MS.10,33,34 Pan et al used a lysis–centrifugation–wash procedure to prepare microorganisms from positive BCs, and then directly identified them with MALDI-TOF MS, with 96.49% accuracy, consistent with our results.35 However, not all microbiology laboratories have access to MALDI-TOF MS for bacterial identification, and must identify bacteria with conventional biochemical-reaction-based instruments. In this study, we not only identified the bacteria rapidly with MALDI-TOF MS, but also evaluated the accuracy of their direct identification using VITEK 2 Compact. Both instruments highly accurately identified Gram-negative bacteria in BSIs. However, the accuracy of MALDI-TOF MS was slightly lower than that of VITEK 2 Compact (90.2% and 96.4%, respectively). This may have been because 22 strains (22/224, 9.8%) produced peaks too small to be identified with MALDI-TOF MS because too little sample was spotted onto the target plate. Among these, half of the Enterobacter cloacae (9/18) strains were not identified with direct MALDI-TOF MS, although the reason is unclear. We will examine possible reasons in further research. However, there was no misidentification, and the results detected with MALDI-TOF MS were all correct. Wu et al reported that five of 93 strains could not be identified directly with MALDI-TOF MS, whereas 88 strains were identified correctly.36 These results suggest that rapid MALDI-TOF MS is reliable. However, exceptions have been observed with rapid MALDI-TOF MS. Gu et al identified to pathogens from bacteria-positive BC bottles with a separation gel–adsorption method.37 One strain was incorrectly identified with MALDI-TOF MS, although the specific reason was not determined in that article. In our study, some erroneous identification results occurred when nonfermentative bacteria from BSIs were identified with the VITEK 2 Compact instrument. Therefore, microbiology laboratory staff should carefully consider the results of direct Vitek 2 Compact for the identification of these bacteria.
The multistep centrifugation method we used to prepare the strains from bacteria-positive BCs is suitable for direct AST. This bacterial preparation process takes about only 45 min, 10 h less than the conventional culture-based method, and is more likely to generate early AST results. An advantage of our method is that direct AST of the bacteria can be conducted simultaneously with three different methods (direct K–B AST, direct VITEK 2 Compact AST, and direct E-test AST), which better evaluates the feasibility of the direct AST methods, but has seldom been analyzed in previous studies. Our results show that the CAs of the three direct AST methods were > 90%. However, there were discrepancies between the different direct AST methods. Direct K–B AST and direct E-test AST had similar MEs and VMEs (both < 2%), whereas the ME of direct VITEK 2 Compact AST was slightly higher than those of the other two methods. Kavipriya et al showed direct susceptibility test by VITEK-2 from positively flagged BC broth for Gram-negative bacilli had a better CA of 97%.38 MEs or VMEs were detected for gentamicin, piperacillin–tazobactam, aztreonam, and cefepime with all three direct methods, suggesting that the results for these antimicrobial agents may not be credible when determined with direct methods. Errors for meropenem were also detected with direct K-B AST and direct E-test AST. Gabriele et al also found VMEs for meropenem when bacteria-positive BCs were analyzed with the direct rapid E-test.39 However, the analysis of meropenem with direct VITEK 2 Compact AST showed a high degree of concordance with the standard method. Hogan found 4.8% VME for piperacillin–tazobactam when it was analyzed directly from BCs with VITEK 2, which is consistent with our results.3 However, they also detected 3.8% ME for meropenem, which is not in agreement with our results. This may be attributable to the different treatments applied to the bacteria-positive BCs. Our results indicate that when clinicians require rapid results for meropenem in BC analyses, the direct VITEK 2 Compact method is more likely to produce accurate results.
Confirmatory ESBL testing and mCIM/eCIM testing to determine whether a strain is multidrug resistant are recommended by the CLSI guideline.27 The strains must be cultured overnight, so it takes at least 48 h for clinicians to obtain these results after a BC is deemed positive. In this study, we developed a multistep centrifugation method to prepare the organisms from positive BCs for confirmation with direct ESBL testing and mCIM/eCIM testing within 24 h. As far as we know, this is the first study to apply direct mCIM/eCIM testing to the bacteria in BCs. Although only 18 strains were tested for carbapenemase in this study, it is heartening to note that the results of the direct mCIM and eCIM tests were 100% accurate. This suggests that Enterobacteriaceae and P. aeruginosa causing BSIs can be directly tested for carbapenemase without waiting for the AST results on the second or third day. A positive ESBL test indicates that the isolate is resistant to moxalactam, cefonicid, cefamandole, and cefoperazone.27 CAZ combined with clavulanate and CTX combined with clavulanate were used for the direct detection of ESBLs in the present study. The results of the direct CTX+CTL method were completely consistent with the results of the standard method. De Gheldre et al also reported that CTX+CTL discs showed the greatest reliability in detecting ESBL-producing E. coli, Proteus spp., Klebsiella spp., and Enterobacter spp.40 However, among the 164 results, we found one discrepancy between the results of the direct CAZ+CAL method and the standard culture-based method. This may be attributable to specific enzymes produced by that isolate, but further research is required to clarify this issue. Consequently, highly accurate direct ESBL tests can be performed in combination with direct AST, which should provide more accurate assessments of bacterial resistance.
This study had several limitations. First, our direct methods were only tested on monomicrobial infections in BCs, and we did not evaluate the applicability of these methods to polymicrobial infections. Second, the sample size was relatively small, and a larger number of samples is required in future research.
Conclusion
In summary, we have developed a new method for preparing Gram-negative bacilli from bacteria-positive BCs, based on a multistep centrifugation strategy. The accuracy of direct pathogen identification with two instruments, direct AST with three methods, and the direct detection of MDR bacteria was high. These direct methods not only effectively reduce the time required for clinicians to obtain BC results, but no additional reagents or staff are required by the laboratory. These methods are appropriate for clinical laboratories because they are inexpensive and simple, and can be used as the routine workflow for daily BC analysis in any microbiology laboratory.
Acknowledgments
We thank International Science Editing (http://www.internationalscienceediting.com) for editing this manuscript. We would like to express our sincere gratitude to Professor Yongzhong Ning (Department of Laboratory Medicine of Beijing Chuiyangliu Hospital) for his valuable comments, suggestions, and revisions of this manuscript.
Funding
This study was financially supported by the Special Foundation for National Science and Technology Basic Research Program of China (grant nos. 2019FY101200 and 2019FY101204), the Foundation of Hebei Provincial Department of Finance (grant no. 361004), and the International Scientific and Technology Corporation Program of Hebei Provincial Department of Science and Technology of China (grant no. 183977118D).
Disclosure
The authors report no conflicts of interest associated with this work.
References
1. McNamara JF, Righi E, Wright H, Hartel GF, Harris P, Paterson DL. Long-term morbidity and mortality following bloodstream infection: a systematic literature review. J Infect. 2018;77:1–8. doi:10.1016/j.jinf.2018.03.005
2. Kern WV, Rieg S. Burden of bacterial bloodstream infection-a brief update on epidemiology and significance of multidrug-resistant pathogens. Clin Microbiol Infect. 2020;26:151–157. doi:10.1016/j.cmi.2019.10.031
3. Hogan CA, Watz N, Budvytiene I, Banaei N. Rapid antimicrobial susceptibility testing by VITEK®2 directly from blood cultures in patients with Gram-negative rod bacteremia. Diagn Microbiol Infect Dis. 2019;94:116–121. doi:10.1016/j.diagmicrobio.2019.01.001
4. Kadri SS, Lai YL, Warner S, et al. Inappropriate empirical antibiotic therapy for bloodstream infections based on discordant in-vitro susceptibilities: a retrospective cohort analysis of prevalence, predictors, and mortality risk in US hospitals. Lancet Infect Dis. 2021;21(2):241–251. doi:10.1016/S1473-3099(20)30477-1
5. Magarifuchi H, Hamada Y, Oho M, Kusaba K, Urakami T, Aoki Y. Clinical utility of direct application of matrix-assisted laser desorption ionization time-of-flight mass spectrometry and rapid disk diffusion test in presumptive antimicrobial therapy for bacteremia. J Infect Chemother. 2018;24:881–886. doi:10.1016/j.jiac.2018.07.014
6. Peri AM, Harris PNA, Paterson DL. Culture-independent detection systems for bloodstream infection. Clin Microbiol Infect. 2022;28(2):195–201. doi:10.1016/j.cmi.2021.09.039
7. Johnsson A, Wong A, Özenci V. The impact of delayed analysis of positive blood cultures on the performance of short-term culture followed by MALDI-TOF MS. J Microbiol Methods. 2020;177:106027. doi:10.1016/j.mimet.2020.106027
8. Machen A, Drake T, Wang YF. Same day identification and full panel antimicrobial susceptibility testing of bacteria from positive blood culture bottles made possible by a combined lysis-filtration method with MALDI-TOF VITEK mass spectrometry and the VITEK2 system. PLoS One. 2014;9:e87870. doi:10.1371/journal.pone.0087870
9. Carretero O, Rivas G, Loras C, Orellana MA. Rapid identification of bacteria directly from positive blood cultures by a modified method using a serum separator tube and matrix-assisted laser desorption ionization- time of flight MS. J Med Microbiol. 2020;69:1373–1380. doi:10.1099/jmm.0.001270
10. Dai Y, Xu X, Yan X, et al. Evaluation of a rapid and simplified protocol for direct identification of microorganisms from positive blood cultures by using Matrix Assisted Laser Desorption Ionization Time-Of-Flight Mass Spectrometry (MALDI-TOF MS). Front Cell Infect Microbiol. 2021;11:632679. doi:10.3389/fcimb.2021.632679
11. Romero-Gómez MP, Gómez-Gil R, Paño-Pardo JR, Mingorance J. Identification and susceptibility testing of microorganism by direct inoculation from positive blood culture bottles by combining MALDI-TOF and Vitek-2 compact is rapid and effective. J Infect. 2012;65:513–520. doi:10.1016/j.jinf.2012.08.013
12. Idelevich EA, Hoy M, Knaack D, et al. Direct determination of carbapenem-resistant Enterobacteriaceae and Pseudomonas aeruginosa from positive blood cultures using laser scattering technology. Int J Antimicrob Agents. 2018;51:221–226. doi:10.1016/j.ijantimicag.2017.10.009
13. Idelevich EA, Storck LM, Sparbier K, Drews O, Kostrzewa M, Becker K. Rapid direct susceptibility testing from positive blood cultures by the matrix-assisted laser desorption ionization-time of flight mass spectrometry-based direct-on-target microdroplet growth assay. J Clin Microbiol. 2018;56. doi:10.1128/JCM.00913-18
14. Prod’Hom G, Durussel C, Greub G. A simple blood-culture bacterial pellet preparation for faster accurate direct bacterial identification and antibiotic susceptibility testing with the VITEK 2 system. J Med Microbiol. 2013;62:773–777. doi:10.1099/jmm.0.049361-0
15. Idelevich EA, Becker K. How to accelerate antimicrobial susceptibility testing. Clin Microbiol Infect. 2019;25:1347–1355. doi:10.1016/j.cmi.2019.04.025
16. Shin J, Shina S, Jung SH, et al. Duplex dPCR system for rapid identification of Gram-negative pathogens in the blood of patients with bloodstream infection: a culture-independent approach. J Microbiol Biotechnol. 2021;31(11):1481–1489. doi:10.4014/jmb.2103.03044
17. Chalhoub H, Sáenz Y, Rodriguez-Villalobos H, et al. High-level resistance to meropenem in clinical isolates of Pseudomonas aeruginosa in the absence of carbapenemases: role of active efflux and porin alterations. Int J Antimicrob Agents. 2016;48:740–743. doi:10.1016/j.ijantimicag.2016.09.012
18. Culbreath K, Petti CA. Balancing enthusiasm for innovative technologies with optimizing value: an approach to adopt new laboratory tests for infectious diseases using bloodstream infections as exemplar. Open Forum Infect Dis. 2015;2:v75. doi:10.1093/ofid/ofv075
19. Idelevich EA, Becker K. Identification and susceptibility testing from shortly incubated cultures accelerate blood culture diagnostics at no cost. Clin Infect Dis. 2016;62:268–269. doi:10.1093/cid/civ824
20. Bard JD, Lee F. Why can’t we just use PCR? the role of genotypic versus phenotypic testing for antimicrobial resistance testing. Clin Microbiol Newsl. 2018;40:87–95. doi:10.1016/j.clinmicnews.2018.05.003
21. Adams-Sapper S, Nolen S, Donzelli GF, et al. Rapid induction of high-level carbapenem resistance in heteroresistant KPC-producing Klebsiella pneumoniae. Antimicrob Agents Chemother. 2015;59:3281–3289. doi:10.1128/AAC.05100-14
22. Jett B, Free L, Sahm DF. Factors influencing the Vitek Gram-positive susceptibility system’s detection of vanB-encoded vancomycin resistance among enterococci. J Clin Microbiol. 1996;34:701–706. doi:10.1128/jcm.34.3.701-706.1996
23. Anton-Vazquez V, Hine P, Krishna S, Chaplin M, Planche T. Rapid versus standard antimicrobial susceptibility testing to guide treatment of bloodstream infection. Cochrane Database Syst Rev. 2021;5(5):CD013235. doi:10.1002/14651858.CD013235.pub2
24. Giacobbe DR, Giani T, Bassetti M, Marchese A, Viscoli C, Rossolini GM. Rapid microbiological tests for bloodstream infections due to multidrug resistant Gram-negative bacteria: therapeutic implications. Clin Microbiol Infect. 2020;26:713–722. doi:10.1016/j.cmi.2019.09.023
25. Harris P, Tambyah PA, Lye DC, et al. Effect of piperacillin-tazobactam vs meropenem on 30-day mortality for patients with E. coli or Klebsiella pneumoniae bloodstream infection and ceftriaxone resistance: a randomized clinical trial. JAMA. 2018;320:984–994. doi:10.1001/jama.2018.12163
26. Bush K, Bradford PA. Epidemiology of β-lactamase-producing pathogens. Clin Microbiol Rev. 2020;33. doi:10.1128/CMR.00047-19
27. Clinical and Laboratory Standards Institute. M100 Performance Standards for Antimicrobial Susceptibility Testing.
28. The European Committee on Antimicrobial Susceptibility Testing. Rapid AST directly from blood culture bottles. Version 10.0, 2020; Available from: http://www.eucast.org.
29. Clinical and Laboratory Standards Institute. M100 Performance Standards for Antimicrobial Susceptibility Testing.
30. Sze DTT, Lau CCY, Chan TM, Ma ESK, Tang BSF. Comparison of novel rapid diagnostic of blood culture identification and antimicrobial susceptibility testing by accelerate pheno system and biofire filmarray blood culture identification and biofire filmarray blood culture identification 2 panels. BMC Microbiol. 2021;21(1):350. doi:10.1186/s12866-021-02403-y
31. Keshta AS, Elamin N, Hasan MR, et al. Evaluation of rapid immunochromatographic tests for the direct detection of extended spectrum beta-lactamases and carbapenemases in Enterobacterales isolated from positive blood cultures. Microbiol Spectr. 2021;9(3):e0078521. doi:10.1128/Spectrum.00785-21
32. Bizzini A, Greub G. Matrix-assisted laser desorption ionization time-of-flight mass spectrometry, a revolution in clinical microbial identification. Clin Microbiol Infect. 2010;16:1614–1619. doi:10.1111/j.1469-0691.2010.03311.x
33. Tanner H, Evans JT, Gossain S, Hussain A. Evaluation of three sample preparation methods for the direct identification of bacteria in positive blood cultures by MALDI-TOF. BMC Res Notes. 2017;10:48. doi:10.1186/s13104-016-2366-y
34. Tian Y, Zheng B, Wang B, Lin Y, Li M. Rapid identification and multiple susceptibility testing of pathogens from positive-culture sterile body fluids by a combined MALDI-TOF mass spectrometry and Vitek susceptibility system. Front Microbiol. 2016;7:523. doi:10.3389/fmicb.2016.00523
35. Pan HW, Li W, Li RG, Li Y, Zhang Y, Sun EH. Simple sample preparation method for direct microbial identification and susceptibility testing from positive blood cultures. Front Microbiol. 2018;9:481. doi:10.3389/fmicb.2018.00481
36. Wu S, Xu J, Qiu C, Xu L, Chen Q, Wang X. Direct antimicrobial susceptibility tests of bacteria and yeasts from positive blood cultures by using serum separator gel tubes and MALDI-TOF MS. J Microbiol Methods. 2019;157:16–20. doi:10.1016/j.mimet.2018.12.011
37. Gu YF, Li Y, Zhang XL, Yu LM, Huang BH, Sun CM. A new method aimed to quickly identify pathogen and drug susceptibility test based on matrix-assisted laser desorption/ionization time of flight mass spectrometry combined with flow cytometry. Surg Infect (Larchmt). 2019;20:219–224. doi:10.1089/sur.2018.145
38. Kavipriya D, Prakash SS, Dhandapani S, Rajshekar D, Sastry AS. Evaluation of the performance of direct susceptibility test by VITEK-2 from positively flagged blood culture broth for Gram-negative bacilli. J Lab Physicians. 2021;13(4):374–379. doi:10.1055/s-0041-1732489
39. Gabriele B, Iannaccone M, Boattini M, Cavallo R, Costa C. Assessment of rapid direct E-test on positive blood culture for same-day antimicrobial susceptibility. Braz J Microbiol. 2019;50:953–959. doi:10.1007/s42770-019-00139-6
40. De Gheldre Y, Avesani V, Berhin C, Delmée M, Glupczynski Y. Evaluation of oxoid combination discs for detection of extended-spectrum beta-lactamases. J Antimicrob Chemother. 2003;52:591–597. doi:10.1093/jac/dkg415
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.