Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 15
Diosgenin Modulates Oxidative Stress and Inflammation in High-Fat Diet-Induced Obesity in Mice
Authors Khateeb S, Albalawi A, Alkhedaide A
Received 24 December 2021
Accepted for publication 17 May 2022
Published 24 May 2022 Volume 2022:15 Pages 1589—1596
DOI https://doi.org/10.2147/DMSO.S355677
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Antonio Brunetti
Sahar Khateeb,1 Aishah Albalawi,2 Adel Alkhedaide3
1Biochemistry Division, Department of Chemistry, Faculty of Science, Fayoum University, Fayoum, Egypt; 2Biology Department, Faculty of Science, University of Tabuk, Tabuk, Saudi Arabia; 3Department of Medical Laboratory, Turabah University College, Taif University, Taif, 21944, Saudi Arabia
Correspondence: Adel Alkhedaide, Department of Medical Laboratory, Turabah University College, Taif University, P. O. Box 11099, Taif, 21944, Saudi Arabia, Tel +966540490404, Fax +966128224366, Email [email protected]
Introduction: Obesity is a chronic metabolic disorder that results in excessive energy accumulated in adipose tissue causing dysfunction of adipocytes, inflammation, and oxidative stress. Diosgenin (DG), a steroidal saponin produced by several plants, has been reported to have antioxidant activity. This study aimed to evaluate the effects of diosgenin on oxidative stress and inflammation in mice fed with a high-fat diet (HFD).
Methods: Thirty adult male mice were divided into three groups including the control group, mice fed with a normal diet; the HFD group, mice fed with a high-fat diet for 6 weeks; and the HFD+DG group, mice fed with a high-fat diet and diosgenin daily for 6 weeks. Interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α), malondialdehyde (MDA), and total antioxidant capacity (TAC) activities were evaluated. Histopathological changes in the adipose tissues have been investigated.
Results: Data showed that diosgenin increased TAC activities with a concomitant decrease in MDA levels. As well, DG reduces the TNF and IL-6 levels. The histopathological changes in the adipose tissues due to high-fat consumption were restored upon DG supplementation.
Conclusion: Our results suggested that diosgenin is a promising agent for regulating obesity by increasing the levels of antioxidants, modifying oxidative stress and pro-inflammatory cytokines, which might prevent the onset of many diseases.
Keywords: obesity, diosgenin, adipose tissue, oxidative stress, proinflammatory cytokines
Introduction
Obesity is one of the world’s leading causes of death that increases the morbidity rate of certain metabolic disorders such as type 2 diabetes, heart diseases, metabolic syndrome, and some other chronic diseases including cancer and inflammation.1–3 Excessive energy intake, poor physical exercise, and genetics are instrumental in the development of obesity.4 Obesity is the reason for increased adipose tissue; studies have shown that adipose tissue has a role in the generation of some bioactive substances called adipokines.4 There are certain inflammatory cytokines among adipokines including interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α). These cytokines are potent stimulators for the production of reactive oxygen; thus, an increase in cytokine concentration may be responsible for increased oxidative stress.4
Oxidative stress can be defined as an imbalance between ROS production and antioxidant defenses.5,6 It is well known that increased oxidative stress is causal to the high metabolic load of fats in obesity.7 In fact, there are some studies have reported that the oxidative stress caused by obesity may underlie some chronic conditions such as cardiovascular disease and metabolic syndrome.8,9 On the other hand, antioxidants are considered to be protective agents that scavenge the excess free radicals caused by the oxidative stress. Therefore, these agents can reduce the metabolic-inflammatory status associated with the increase in body weight and might be helpful in treating multiple obesity-induced diseases. Nowadays, there are some synthetic pharmaceutical drugs available for treating obesity. However, they are not preferred for long-term usage due to adverse side effects.10
These days over the worldwide serious attempts are made to produce safe and effective medicines based on natural products for the treatment of obesity.11 Amongst these natural compounds, diosgenin (DG), a steroidal saponin, derived from different plants, for example, Dioscorea, Trigonella, Costus, is found to have many biological benefits. It is highly effective against cancer, diabetes, hyperlipidemia, cardiovascular disease, oxidative stress, and inflammation.12–14 Due to its structure diosgenin acts in different ways as either a precursor of some steroid hormones such as progesterone and testosterone and both have an anti-inflammatory effect or as a genetic regulator, which upregulates the antiapoptotic and antioxidant genes15–18. Our lab has reported that diosgenin controlled lipid profile and prevented liver toxicity caused by high-fat diet in experimental animals.19 Wang et al have shown that diosgenin exerted anti-inflammatory effect in human osteoarthritis chondrocytes.20 Another study published by Cai et al reported that diosgenin has a potential effect against some neurological disorders.21 Therefore, the current study is to examine the protective effects of diosgenin on oxidative stress and inflammation caused by a high-fat diet that induces obesity in mice.
Materials and Methods
Ethics Approval and Consent to Participate
This study was approved by the National Hepatology and Tropical Medicine Research Institute Office for IRB, Egypt. The work complied with relevant guidelines for animal handling and welfare. (Approval no: 14-2018, Dated: 5 July 2018).
Chemicals
Diosgenin, 98% was purchased from Sigma Chemical Co. (St. Louis, MO). It was dissolved in 0.5% DMSO in saline before use. Biochemical kits were purchased from Life Span Biosciences. Chemicals used in this study were of high-quality analytical grade.
Animals
Thirty adult male C57BL/6J mice weighing 25–27 g (7 weeks old) were used in this study. They were picked from the animal center of the research unit of pharmacology and chemistry, Misr University Science and Technology, Cairo, Egypt. All animal experiments and methods were carried out in accordance with Local Animal Ethics Committee guidelines.
Experimental Design
Mice were subdivided randomly into three groups (n=10/each group). In all the experimental groups there was no statistically significant difference in body weights. In the control group, mice were fed with normal diet (12% dietary fat) and ingested orally by gavage for 6 weeks with the vehicle solution, 0.5% DMSO in saline. In the HFD group, mice were fed with high-fat diet (60% fat) for 6 weeks. In the HFD+DG group, mice were fed with a high-fat diet (60% fat) and supplemented in parallel with diosgenin (80 mg/kg b.w/day) orally by gavage for 6 weeks.22 After 6 weeks, blood samples were collected, and serum was separated by centrifugation at 3000 rpm for 15 min and kept at −20°C for estimation of TNF-alpha and IL-6. Mice then were killed under anesthesia; adipose tissues of each animal were excised and preserved in formalin 10% until the histopathological examination.
Body Weight Monitoring
Using an electronic weighing balance, the fasted mice were weighed individually. At the beginning of the experiment and the end of the experiment, the weights of all mice of each group were recorded.
Biochemical Analysis
Determination of Pro-Inflammatory Marker
The pro-inflammatory cytokines TNF-alpha and IL-6 levels were determined using CUSABIO (Baltimore, USA) mouse tumor necrosis factor α (TNF-α) ELISA Kit (Cat. No. CSB-E04741m) and mouse interleukin 6 (IL-6) ELISA Kit (Cat. No. CSB-E04639m).
Measurement of Oxidative Stress Markers
Oxidative stress induced by the fat accumulation as well as the potent antioxidant impact of DG was evaluated by the determination of both MDA and TAC levels. The MDA and TAC ELISA kits were purchased from Life Span Biosciences (LS BIO, North America, Cat. No. LS-F28018) and My BioSource (San Diego, USA, Cat. No. MBS733414_48T), respectively.
Histopathological Investigation
Adipose tissue from different groups was embedded in paraffin cubes, sliced into sections. Then, the tissue parts were collected and stained with hematoxylin and eosin (H&E), examined, and photographed under a light microscope.23
Statistical Analysis
Data were expressed as mean ± standard deviation (SD). SPSS-23 software was used for all statistical analyses. P values (p<0.05) were considered significant.
Results
Effect of Diosgenin on Body Weight
As demonstrated in Table 1, the HFD group reported a significant body weight gain compared to the control group with percentage changes 12.9%. In contrast, animals received DG along with the HFD showed a significant reduction in their body weight (by −5.6%) compared with the HFD group, which almost restored to the normal control weight (Table 1).
 |
Table 1 Effect of Diosgenin on the Body Weight, at the Start and the End of the Experiment |
Regulatory Effect of Diosgenin on the Selected Pro-Inflammatory Cytokines (TNF-Alpha and IL-6)
Data presented in Table 2 indicate that the TNF-alpha and IL-6 levels were significantly increased by approximately four folds in the HFD group after the 6 consecutive weeks (242.6% and 219.7% respectively) with p values <0.05 corresponding to the control group. Nevertheless, DG supplementation in combination with HFD ingestion significantly lowers these elevated levels, in which p values were < 0.05 in both cytokines corresponding to the HFD group as shown in Table 2.
 |
Table 2 Effect of Diosgenin on Pro-Inflammatory Cytokines TNF-Alpha and IL-6 in Mice |
Effect of Diosgenin on Oxidative Stress Markers
The protective effect of DG on the HFD-induced oxidative stress in mice was evaluated via the determination of MDA and TAC levels. As shown in Table 3, the level of MDA was significantly increased among the HFD group (by 380.28%) compared to the normal control group. Alongside, a significant reduction in the level of TAC was detected in the same group (by −64.72%), when compared to the normal control group. Obviously, the DG ingestion in parallel with the HFD significantly suppressed the elevation of MDA (by −55.3%) and almost returned the TAC (by 101.6%) close to the control levels, as compared to the HFD group. These present results indicate the capacity of DG in promoting the cellular antioxidant defenses and affirmed its potent antioxidant impact against fat accumulation-induced oxidative stress.
 |
Table 3 Effect of Diosgenin on Malondialdehyde (MDA) and Total Antioxidant Capacity (TAC) in Mice |
Histopathological Examination of Adipose Tissue
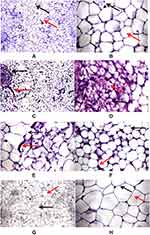
Histopathological examination of the control group’s adipose tissue showed average univacuolated adult fat cells with a compact nucleus at one side and an intact cell membrane with an average extracellular matrix (Figure 1A). Conversely, adipose tissue of the HFD group revealed many histological changes, including closely packed overcrowded variable-sized fat cells with fibrous connective tissue bands and few ruptured fat cells (Figure 1B). On the other hand, in DG ingestion in parallel with the HFD, univacuolated adult fat cells were seen with one side compressed nucleus and an intact cell membrane with an average extracellular matrix (Figure 1C). These findings visually indicate the impact of DG on improving the HFD-induced histopathological alterations. Figure 1D and F shows closely packed overcrowded small-sized fat cells with multiple vacuoles and centrally located nuclei. In addition, adipose tissue shows ruptured fat cells with fibrous connective tissue bands as shown in Figure 1E. Figure 1G and H show adipose tissue sections from HFD + DG group. In Figure 1G the adipose tissue shows average uni-vacuolated adult fat cells with an average extracellular matrix, while Figure 1H was a high-power view showing uninoculated fat cells with a compressed nucleus at one side and intact cell membrane with centrally located nuclei.
Discussion
Obesity is a metabolic condition characterized by unhealthy or unnecessary accumulation of fat, which is a significant risk factor for various diseases. Additionally, there is a lot of evidence that obesity is implicated in a sort of a condition of chronic oxidative stress due to the oxidation processes that are required for fatty acid degradation, which in turn increases the reactive oxygen species ROS particularly H2O2.24,25 DG is a versatile bioactive molecule with many therapeutic benefits that include antioxidant and anti-inflammatory activities. Hence, it is a potential molecule of interest in multiple disease prevention.26
The findings of many studies included the possible use of DG against a variety of pathologies including obesity, dyslipidemia, inflammatory diseases, and cancer indicated that DG possesses anti-inflammatory and antioxidant properties.12,26,27 This study attempted to evaluate the beneficial effects of DG on oxidative stress and inflammation on mice fed with a high-fat diet. To investigate the effects of DG on oxidative stress and inflammation in mice fed with a high-fat diet, serum levels of IL-6, TNF-α, MDA, and TAC were estimated in all experimental groups, as well as the possible histopathological changes in the adipose tissues have been investigated.
Our results showed a significant increase was detected in the body weight in the HFD group, by the end of the sixth week of HFD consumption. This weight gained reflected the excessive anabolic rate of synthesis of triglycerides over catabolism. These results are consistent with a previous study reported that consumption of high levels of dietary fat can cause obesity.28 Nonetheless, DG’s co-administration with HFD decreases this weight gain. This demonstrates the DG ‘s effect on fat accumulation by enhancing body weight control during HFD intake.
Normal levels of ROS are important for different cellular functions. However, excessive levels of ROS that surpass the capacity of the antioxidant system can cause oxidative stress leading to membrane lipid peroxidation and ultimately to MDA production.29 In terms of the role of fat accumulation in oxidative stress, the present findings show that fat accumulation due to HFD consumption significantly elevated the MDA with a concomitant decrease in the TAC levels. Obesity has been previously shown to induce systemic oxidative stress due to the generation of ROS in accumulated fat, which was the key cause of adipocytokine dysregulation and subsequent metabolic syndrome development.8 Notably, DG ingestion along with HFD returned the MDA levels near to the normal ones and boosted the levels of TAC, indicating that the protective effect of DG might be due to its ability to defend biomembranes against lipid peroxidation and to enhance the cellular antioxidant defenses. These data are consistent with a previous study illustrating the potent antioxidant activity of DG via its influence in the inhibition of lipid peroxidation and significant free radical scavenging ability.30
Inflammation was one of the fat accumulation consequences; TNF-alpha and IL-6 are among the large number of pro-inflammatory cytokines known to be common in both inflammation and obesity.31 The current study revealed three= to fourfold increase in the TNF-α and IL-6 as a consequence of HFD for 6 consecutive weeks. IL-6 controls the appetite and energy intake due to the abundance of its receptor in several regions of the brain. Furthermore, it plays a crucial role in regulating the energy homeostasis via suppressing the lipoprotein lipase activity.32
Ellulu et al affirmed the association between obesity and inflammation by describing the positive link between obesity and plasma IL-6 levels.33 Unfortunately, the increased level of IL-6 stimulates the liver to synthesize and release the C-reactive protein, accelerates fibrinogen production, and hence mediates the inflammatory response.34 Similarly, TNF-α, a multifunctional cytokine, exerts a crucial regulatory influence on the lipid metabolism, including suppression of free fatty acid (FFA) uptake and promoting lipogenesis, regulating cholesterol metabolism, in addition to its effect on regulating other adipocyte-derived adipokines.35 Furthermore, both TNF-α and IL-6 can influence each other’s secretion levels leading to inflammatory condition persistence.36,37 However, DG co-administration with HFD was shown to alleviate the inflammation accompanied by obesity via reducing these inflammatory cytokines and restoring their serum levels to the normal levels. These results illuminate the potent protective impact of DG against oxidative stress and inflammation on HFD-induced obesity in mice via its anti-inflammatory and antioxidant activity.
Conclusion
In conclusion, oxidative stress and inflammation are two interdependent pathophysiological processes that could be induced by high-fat consumption, which in turn are implicated for the onset of many diseases. The present data suggest that DG can limit obesity via its dual effects as either an antioxidant and as an anti-inflammatory mediator, confirming that DG can be considered as a promising candidate for future consideration in obesity treatment and regulation of its accompanying metabolic disorders.
Ethical Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the National Hepatology and Tropical Medicine Research Institute Office for IRB, Egypt. The work complied with relevant guidelines for animal handling and welfare (Approval no: 14-2015, Dated: 5 July 2018).
Acknowledgments
The authors greatly thank and acknowledge Taif University, for its support. This research has been supported by Taif University Research Supporting Project number (TURSP-2020/104) Taif University, Taif, Saudi Arabia.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Berenbaum F, Eymard F, Houard X. Osteoarthritis, inflammation and obesity. Curr Opin Rheumatol. 2013;25(1):114–118. doi:10.1097/BOR.0b013e32835a9414
2. Dixon JB. The effect of obesity on health outcomes. Mol Cell Endocrinol. 2010;316(2):104–108. doi:10.1016/j.mce.2009.07.008
3. Popkin BM. Does global obesity represent a global public health challenge? Am J Clin Nutr. 2010;93(2):232–233. doi:10.3945/ajcn.110.008458
4. Fonseca-Alaniz MH, Takada J, Alonso-Vale MI, Lima FB. Adipose tissue as an endocrine organ: from theory to practice. J Pediatr. 2007;83(5 Suppl):S192–203. doi:10.1590/S0021-75572007000700011
5. Fatehi-Hassanabad Z, Chan CB, Furman BL. Reactive oxygen species and endothelial function in diabetes. Eur J Pharmacol. 2010;636(1–3):8–17. doi:10.1016/j.ejphar.2010.03.048
6. Frey RS, Ushio-Fukai M, Malik AB. NADPH oxidase-dependent signaling in endothelial cells: role in physiology and pathophysiology. Antioxid Redox Signal. 2009;11(4):791–810. doi:10.1089/ars.2008.2220
7. Santilli F, Guagnano MT, Vazzana N, La Barba S, Davi G. Oxidative stress drivers and modulators in obesity and cardiovascular disease: from biomarkers to therapeutic approach. Curr Med Chem. 2015;22(5):582–595. doi:10.2174/0929867322666141128163739
8. Furukawa S, Fujita T, Shimabukuro M, et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114(12):1752–1761. doi:10.1172/JCI21625
9. Keaney JF
10. Alsheikh-Ali AA, Kuvin JT, Karas RH. Risk of adverse events with fibrates. Am J Cardiol. 2004;94(7):935–938. doi:10.1016/j.amjcard.2004.06.033
11. Vasudeva N, Yadav N, Sharma SK. Natural products: a safest approach for obesity. Chin J Integr Med. 2012;18(6):473–480. doi:10.1007/s11655-012-1120-0
12. Chen Y, Tang Y-M, Yu S-L, et al. Advances in the pharmacological activities and mechanisms of diosgenin. Chin J Nat Med. 2015;13(8):578–587. doi:10.1016/S1875-5364(15)30053-4
13. Das S, Dey KK, Dey G, et al. Antineoplastic and apoptotic potential of traditional medicines thymoquinone and diosgenin in squamous cell carcinoma. PLoS One. 2012;7(10):e46641. doi:10.1371/journal.pone.0046641
14. Kang TH, Moon E, Hong BN, et al. Diosgenin from Dioscorea nipponica ameliorates diabetic neuropathy by inducing nerve growth factor. Biol Pharm Bull. 2011;34(9):1493–1498. doi:10.1248/bpb.34.1493
15. Trouillas P, Corbière C, Liagre B, Duroux JL, Beneytout JL. Structure-function relationship for saponin effects on cell cycle arrest and apoptosis in the human 1547 osteosarcoma cells: a molecular modelling approach of natural molecules structurally close to diosgenin. Bioorg Med Chem. 2005;13(4):1141–1149. doi:10.1016/j.bmc.2004.11.031
16. Corbiere C, Liagre B, Bianchi A, et al. Different contribution of apoptosis to the antiproliferative effects of diosgenin and other plant steroids, hecogenin and tigogenin, on human 1547 osteosarcoma cells. Int J Oncol. 2003;22(4):899–905.
17. Sato K, Fujita S, Iemitsu M. Acute administration of diosgenin or Dioscorea improves hyperglycemia with increases muscular steroidogenesis in STZ-induced type 1 diabetic rats. J Steroid Biochem Mol Biol. 2014;143:152–159. doi:10.1016/j.jsbmb.2014.02.020
18. Sirotkin AV, Alexa R, Alwasel S, Harrath AH. The phytoestrogen, diosgenin, directly stimulates ovarian cell functions in two farm animal species. Domest Anim Endocrinol. 2019;69:35–41. doi:10.1016/j.domaniend.2019.04.002
19. Khateeb S, Albalawi A, Alkhedaide A. Regulatory effect of diosgenin on lipogenic genes expression in high-fat diet-induced obesity in mice. Saudi J Biol Sci. 2021;28(1):1026–1032. doi:10.1016/j.sjbs.2020.11.045
20. Wang L, Ma T, Zheng Y, Lv S, Li Y, Liu S. Diosgenin inhibits IL-1β-induced expression of inflammatory mediators in human osteoarthritis chondrocytes. Int J Clin Exp Pathol. 2015;8(5):4830–4836.
21. Cai B, Zhang Y, Wang Z, et al. Therapeutic potential of diosgenin and its major derivatives against neurological diseases: recent advances. Oxid Med Cell Longev. 2020;2020:3153082. doi:10.1155/2020/3153082
22. Liu TW, Heden TD, Matthew Morris E, Fritsche KL, Vieira-Potter VJ, Thyfault JP. High-fat diet alters serum fatty acid profiles in obesity prone rats: implications for in vitro studies. Lipids. 2015;50(10):997–1008. doi:10.1007/s11745-015-4061-5
23. Downie T. Histopathology. In: editors, Bancroft JD, Stevens A. Theory and Practice of Histological Techniques. Churchill Livingstone, Edinburgh; Vol. 17, 1990:386. 740 pages, £55.00.
24. Youn JY, Siu KL, Lob HE, Itani H, Harrison DG, Cai H. Role of vascular oxidative stress in obesity and metabolic syndrome. Diabetes. 2014;63(7):2344–2355. doi:10.2337/db13-0719
25. Chen Q, Tang L, Xin G, et al. Oxidative stress mediated by lipid metabolism contributes to high glucose-induced senescence in retinal pigment epithelium. Free Radic Biol Med. 2019;130:48–58. doi:10.1016/j.freeradbiomed.2018.10.419
26. Jesus M, Martins AP, Gallardo E, Silvestre S. Diosgenin: recent highlights on pharmacology and analytical methodology. J Anal Methods Chem. 2016;2016:4156293. doi:10.1155/2016/4156293
27. Manivannan J, Arunagiri P, Sivasubramanian J, Balamurugan E. Diosgenin prevents hepatic oxidative stress, lipid peroxidation and molecular alterations in chronic renal failure rats. Int J Nutr Pharmacol Neurol Dis. 2013;3(3):289–293. doi:10.4103/2231-0738.114870
28. Matsuzawa Y. The metabolic syndrome and adipocytokines. FEBS Lett. 2006;580(12):2917–2921. doi:10.1016/j.febslet.2006.04.028
29. Cao K, Xu J, Zou X, et al. Hydroxytyrosol prevents diet-induced metabolic syndrome and attenuates mitochondrial abnormalities in obese mice. Free Radic Biol Med. 2014;67:396–407. doi:10.1016/j.freeradbiomed.2013.11.029
30. Hua S, Li Y, Su L, Liu X. Diosgenin ameliorates gestational diabetes through inhibition of sterol regulatory element-binding protein-1. Biomed Pharmacother. 2016;84:1460–1465. doi:10.1016/j.biopha.2016.10.049
31. Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature. 2006;440(7086):944–948. doi:10.1038/nature04634
32. Wannamethee SG, Whincup PH, Rumley A, Lowe GD. Inter-relationships of interleukin-6, cardiovascular risk factors and the metabolic syndrome among older men. J Thromb Haemost. 2007;5(8):1637–1643. doi:10.1111/j.1538-7836.2007.02643.x
33. Ellulu MS, Patimah I, Khaza’ai H, Rahmat A, Abed Y. Obesity and inflammation: the linking mechanism and the complications. Arch med sci. 2017;13(4):851–863. doi:10.5114/aoms.2016.58928
34. Rocha VZ, Libby P. Obesity, inflammation, and atherosclerosis. Nat Rev Cardiol. 2009;6(6):399–409. doi:10.1038/nrcardio.2009.55
35. Chen X, Xun K, Chen L, Wang Y. TNF-alpha, a potent lipid metabolism regulator. Cell Biochem Funct. 2009;27(7):407–416. doi:10.1002/cbf.1596
36. Castro AM, Macedo-de la Concha LE, Pantoja-Meléndez CA. Low-grade inflammation and its relation to obesity and chronic degenerative diseases. Rev Medica Del Hosp Gen de Mex. 2017;80(2):101–105. doi:10.1016/j.hgmx.2016.06.011
37. Popko K, Gorska E, Stelmaszczyk-Emmel A, et al. Proinflammatory cytokines Il-6 and TNF-α and the development of inflammation in obese subjects. Eur J Med Res. 2010;15 Suppl 2(Suppl2):120–122. doi:10.1186/2047-783X-15-S2-120
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.