Back to Journals » Journal of Inflammation Research » Volume 15
Dimethyl Fumarate as the Peripheral Blood Inflammatory Mediators Inhibitor in Prevention of Streptozotocin-Induced Neuroinflammation in Aged Rats
Authors Wrona D , Majkutewicz I , Świątek G , Dunacka J , Grembecka B , Glac W
Received 1 October 2021
Accepted for publication 23 November 2021
Published 6 January 2022 Volume 2022:15 Pages 33—52
DOI https://doi.org/10.2147/JIR.S342280
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Danuta Wrona, Irena Majkutewicz, Grzegorz Świątek, Joanna Dunacka, Beata Grembecka, Wojciech Glac
Department of Animal and Human Physiology, Faculty of Biology, University of Gdansk, Gdansk, 80-308, Poland
Correspondence: Danuta Wrona
Department of Animal and Human Physiology, Faculty of Biology, University of Gdansk, 59 Wita Stwosza Str, Gdansk, 80-308, Poland
Tel +4858 523 61 21
Fax +4858 523 61 21
Email [email protected]
Purpose: Intracerebroventricular-(ICV)-streptozotocin-(STZ)-induced neuroinflammation is a model of Alzheimer’s disease (AD) compatible with the inflammation hypothesis of ageing (“inflammaging” state). Previously, we observed age-dependent (young vs aged) dimethyl fumarate (DMF)-induced anti-inflammatory and neuroprotective effects in the brain along with improvement in cognitive functions in rats with the ICV-STZ-induced model of AD. To evaluate whether DMF reduces neuroinflammation based on the peripheral inflammatory response inhibition, we determined peripheral inflammatory mediators in young and aged rats with the ICV-STZ-induced AD pathology following DMF therapy.
Materials and Methods: Young (4-month-old) and aged (22-month-old) rats were fed with 0.4% DMF rat chow for 21 consecutive days after ICV-STZ (3 mg/ventricle) injections. After behavioral testing, blood and spleens were collected to determine the numbers of leukocytes (WBC), lymphocytes and their subpopulations, haematological parameters, the concanavalin (Con)-A-induced production and plasma concentration of interferon (IFN)-γ, interleukin (IL)-6, IL-10 and corticosterone (COR).
Results: Age-dependent anti-inflammatory effect of the DMF treatment in rats with ICV-STZ injections manifested as decreased peripheral WBC and lymphocyte numbers, including TCD3+CD4+CD8−, TCD3+CD4−CD8+, B (CD45RA+) and NK (161a+), in aged rats. Furthermore, DMF lowered the blood and spleen lymphocyte production of pro-inflammatory IFN-γ and IL-6 in young and aged rats, whereas it enhanced the plasma level of anti-inflammatory IL-10 and lymphocyte’s ability to produce it in aged rats only. In parallel to changes in peripheral WBC numbers in the model of AD, DMF decreased the red blood cell number, haemoglobin concentration, haematocrit and mean platelet volume in aged, but not young, rats. In contrast to controls, DMF did not influence the COR response in STZ groups.
Conclusion: Besides preventing neuroinflammation, DMF acts on the pro-/anti-inflammatory balance in the periphery and causes an anti-inflammatory shift in T lymphocytes which could contribute to DMF’s therapeutic effects in the ICV-STZ-induced model of AD, in particular, in aged rats.
Keywords: inflammaging, intracerebroventricular-streptozotocin-induced Alzheimer’s disease model, peripheral immunohaematological parameters, neurotherapeutics, inflammation
Graphical Abstract:
Plain Language Summary
Dimethyl fumarate (DMF) is known as therapeutic agent used in the treatment of multiple sclerosis and psoriasis. It works by blocking of inflammatory response and has anti-oxidative properties. Both inflammatory and oxidative stress responses are increased with ageing and that has been recently called “inflammaging state”. Ageing and chronic inflammation in the brain (neuroinflammation) are the main risk factor for Alzheimer’s disease (AD) and memory impairment. Therefore, we used oral DMF therapy in a model of the AD pathology based on neuroinflammation which we induced by injections of streptozotocin into the brain. We observed that DMF treatment reduced neuroinflammation and alleviated memory impairment, in particular, in aged rats. We hypothesized that those therapeutic effects of DMF could be attributed to the DMF-induced inhibition of neuroinflammation and peripheral inflammatory response. In the present study, we looked at the peripheral mediators of inflammation following the DMF treatment. To evaluate DMF-induced age-dependent effects, we used aged (22-month-old) and young (4-month-old) rats. We found that DMF lowered the numbers of leukocytes , including lymphocytes and T lymphocyte subpopulations, and decreased the production of pro-inflammatory cytokines in young and aged rats with the AD pathology. We noticed that the anti-inflammatory effect of DMF therapy was more effective in aged rather than young animals and it did not depend on the peripheral level of such anti-inflammatory hormone as corticosterone. We suggest that DMF therapy alleviated memory disorders in the AD model by the inhibition of inflammatory mediators in the brain and periphery, more effectively in aged rats.
Introduction
The greatest known risk factor for Alzheimer’s disease (AD) is ageing. It has been proposed that ageing is a chronic and low-grade inflammatory state (“inflammaging” state)1 and a combination of chronic neuroinflammation and ageing - the so-called “neuroinflammaging” state2 - play a major role in the mechanism of neurodegenerative disorders, including AD.3,4 Neuropathological studies have supported early involvement of neuroinflammation in AD by demonstrating the accumulation of activated microglia and inflammatory mediators in the cerebral neocortex at a low Braak stage for AD pathology.5,6 Chronic neuroinflammation driven by cells of the innate immunity7 is mainly due to age-related impairment of anti-inflammatory and anti-oxidant mechanisms that lead to the “inflammaging” state.8–10 It causes subtle clinical symptoms which may persist for years prior to clinical manifestation as AD.11 Neuroinflammation is considered as the driving force of AD pathology12 and starts early in the course of the disease, prior to tau hyperphosphorylation13 and amyloid plaque formation.14–16 Neuroinflammation also is considered the driving force of AD-like cognitive deficits17,18 and reduction of neuronal cell counts.19 According to the inflammation hypothesis of AD16 in pathological aging microglia become hyper-reactive with increased release of pro-inflammatory cytokines and dysfunctional phagocytosis.16 These pro-inflammatory factors released by the pathological activation of microglia are neurotoxic especially if accumulated during chronic neuroinflammatory process.20 Secondary to this neuronal degeneration, Aβ plaques will trigger further release of pro-inflammatory molecules leading to a vicious circle of neurotoxic pro-inflammatory response.16 Recent studies have revealed that the ageing process is associated with the activation of the immunosuppressive network, especially the function of monocytic-derived suppressor cells, which is induced by inflammatory mediators.21 Correspondingly, there are clear age-related changes in peripheral blood haematological parameters, including decreased content of nuclear factor erythroid 2-related factor 2 (NRF2) and anti-oxidant protection in peripheral blood mononuclear cells,10 reduced erythrocyte and platelet counts,22 function, and reactivity. Those changes are driven by changes in haematopoietic tissue, the composition of the blood, and vascular health.23,24 Increased reactivity of platelets, which are an important part of inflammation and immune response, was observed with ageing22 and the activated platelets in AD retain greater amounts of amyloid precursor protein (APP) while showing increased platelet adhesion and thrombus formation.25
It has been actually very well established that molecular changes in AD patients do not occur only in the brain tissue, but also in blood cells and blood vessels.24–26 Lymphocytes are mediators which play a role in AD neuroinflammatory processes and migrate from the blood through the blood-brain barrier (BBB) to the brain. Recent reports have also highlighted the role of peripheral T lymphocytes in the innate immunity of AD neuroinflammatory process,27,28 and changes in the distribution and function of different types of blood lymphocytes from AD patients.29
In both our earlier study and this one, we used the intracerebroventricular (ICV)-streptozotocin (STZ)-induced neuroinflammation suitable for studying the inflammation hypothesis of the AD pathology.12 In our previous study, we found that dimethyl fumarate (DMF), anti-oxidant, immunomodulatory, and anti-inflammatory drug30 used in therapy of multiple sclerosis31,32 and psoriasis,33 had beneficial properties for treatment of rats with the ICV-STZ-induced AD model.34,35 The behavioral results showed that oral DMF therapy prevented ICV-STZ-induced disruption of spatial memory, neurodegeneration in all hippocampal areas, induction of expression of interleukin (IL)-6 and interleukin (IL)-10 in the hippocampus, deficits in cholinergic neurons, and reduced microglia activation in the dentate gyrus. DMF-induced effects were more pronounced in aged vs young rats.35 We suggested that this age-related beneficial effect of DMF treatment after ICV-STZ injections may be the result of reduced central neuroinflammation leading to alleviation of memory impairments. Furthermore, we hypothesized that DMF may target also peripheral blood cell profiles. We therefore analyzed such peripheral inflammatory markers as changes in the peripheral blood leukocyte and lymphocyte numbers, lymphocyte populations and TCD4/TCD8 lymphocyte subsets, blood and spleen pro-/anti-inflammatory cytokine profile, haematological parameters, and anti-inflammatory hormone, corticosterone (COR) in response to DMF therapy of STZ-induced AD pathology. To understand the immune differences between the effects of ageing and AD and age-dependent therapeutic effect of DMF, in this study, we used young (4-month-old) vs aged (22-month-old) rats with the ICV-STZ-induced neuroinflammation compatible with the inflammation hypothesis of AD.
Materials and Methods
Animals
All animal experiments were carried out in accordance with the European Communities Council Directive of 24 November 1986 (86/609/EEC), and under the authority of the Local Ethical Committee for the Care and Use of Laboratory Animals at the Medical University of Gdansk, Poland (No. 6/2014). We used 4-month-old (young group, n = 38) and 22-month-old (aged group, n = 40) Male Wistar rats. Four-month-old rats weighted 330±80 g and 22-month-old rats weighted 600±100 g (Tri-City Central Animal Laboratory, Research and Service Centre of the Medical University of Gdansk, Poland). For the duration of the experiment, rats were housed separately in polycarbonate cages (20 cm width, 37 cm length, 18 cm height) on a 12-hr light/dark cycle (lights on at 06:00), in an air conditioned constant temperature (22 ± 2°C) room, could visually observe other subjects, and were indirectly exposed to other subjects’ cage odors. Water and food were available ad libitum. Animals were allowed to adapt to the laboratory conditions for one week before the beginning of the experimental procedure. Then, rats were subjected to daily handling for about 5 minutes each day for two weeks in order to minimize stress caused by the procedure.
Experimental Procedure
Changing the feed to DMF containing (0.4% by weight) chow or standard fodder was established the first day of the experimental procedure. On the second day, surgical ICV injection of STZ or vehicle was carried out. ICV injections were repeated after 2 days in order to divide the total dose of STZ (3 mg/kg) into two injections thereby minimizing mortality.29,30 Within each age group, young and aged rats were separated into four experimental groups: 1) STZCTR: provision of standard chow from day 0 and STZ ICV injections on days 2 and 4 (young, n = 9; aged n = 10); 2) STZDMF: provision of 0.4% DMF chow from day 0 and STZ ICV injections on days 2 and 4 (young, n = 9; aged, n = 10); 3) VEHCTR: provision of standard chow from day 0 and vehicle ICV injections on days 2 and 4 (n = 10 in both age groups); 4) VEHDMF: provision of 0.4% DMF chow on day 0 and vehicle ICV injections on days 2 and 4 (n = 10 in both age groups).
Oral Dimethyl Fumarate (DMF) Therapy
Rats were fed either standard rat chow or rat chow containing 0.4% dimethyl fumarate (DMF) by weight (prepared by Sniff, Germany) starting two days prior to the first ICV injection through the end of the experiment (three weeks later). Both feeds were in pellet form. Food intake and body weights were measured daily. Dose selection was based on earlier study on rats.34–37 We used 0.4% DMF rat chow because a 0.2–0.5% concentration of DMF in feed is well tolerated and does not cause a substantial loss of body weight in rats (7% after two weeks).36 0.4% of DMF in the diet has a therapeutic value as has been supported by 1) attenuation of the STZ-induced neuroinflammation, spatial memory impairment, hippocampal neurodegeneration, and the depletion of basal forebrain cholinergic cells in Wistar rats,34,35 2) elevation of anti-oxidative enzymes activity in variety organs of Sprague-Dawley rats,36 3) normalization of infiltration of macrophages and lymphocytes in kidneys after nephrotoxin administration in Wistar rats.37 Moreover, no significant difference was observed on locomotor activity in an actophotometer after the 4-week oral DMF treatment with a dose of 15, 30 and 60 mg/kg in Sprague-Dawley rats.38 In line with these findings, we did not observe locomotor sensitization after the 3-week oral DMF treatment (data not shown), as indicated by the distance swum and velocity in the Morris Water Maze without platform (probe test).
Intracerebroventricular (ICV) Injections of Streptozotocin (STZ)
ICV injections of streptozotocin (STZ) or vehicle (VEH: 0.02M citrate buffer pH 4.5) were performed according to our previous studies with some modifications.34,35 Briefly, rats under 2.5% isoflurane (airflow: 0.5 L per min) anesthesia using an isoflurane pump (Bitmos OXY 6000, Bitmos GmbH Düsseldorf, Germany) were prepared for surgery and the head of the animal was fixed using a stereotaxic apparatus (Hugo Sachs Elektronik, Germany). A stainless steel guide cannula (22GA, 9 mm long, Plastic One, USA) was implanted into each lateral ventricle (coordinates: AP:-1.3 mm, L:±2 mm, D:+3.6 mm according to bregma). The cannulae were permanently anchored to three stainless steel skull screws with dental acrylic (Duracryl, spofa Dental a.s., Czech Republic). STZ was administered through the implanted cannulae in a cumulative dose of 3 mg/kg over two injections on days 2 and 4 (2 x 1.5 mg/kg, dissolved in 0.02 M citrate buffer pH 4.5) with separate injections into each lateral ventricle (0.75 mg/kg in 2 µL of vehicle) at a rate of 1 μL/min. The total dose of STZ was divided in order to reduce procedure-associated mortality.17 During the infusion procedure, the animal under 2.5% isoflurane anaesthesia was held gently by hand. The injections were performed using a microinfusion pump (Legato-100 - Series Syringe Pump, KD SCIENTIFIC, Holliston, Massachutetts, USA) and a Hamilton syringe (10 μL) connected via a polyethylene tube to an injection cannula (28GA, 11 mm long, Plastic One, USA) which was placed into a guide cannula, and 2 mm below its tip. In order to avoid a backflow of the solution, the injection cannula was left inside the guide for an additional 60 s. The control groups received vehicle injections at the same volumes and by the same procedure. As the stability of STZ solution is maximum at pH close to 4, we used citrate buffer pH 4.5 as a vehicle. After the surgery, the animals were transferred to a warm room, where they stayed until regaining consciousness. Then, rats were taken to their home cages where they were allowed to rest after surgery. Previous findings39 indicated that locomotor activity in the open field test between ICV-STZ treated (3 mg/kg) and sham operated Lewis rats were similar. Like other authors, we did not observe locomotor sensitization after ICV-STZ injections (data not shown), as indicated by the distance swum and velocity in the Morris Water Maze without platform (probe test).
Test of Spatial Memory in Morris Water Maze (MWM)
Morris Water Maze (MWM) testing began two weeks after DMF or control treatment (12 days after the first STZ or VEH injections). Spatial reference memory was tested first (from day 14 to 17) followed by spatial working memory (from day 18 to 21) according to the procedure that we described previously.34,35 Briefly, MWM training with a visible platform was performed initially. If the rat did not locate the platform within 60 sec, it was gently steered towards the platform by hand. After 5 min, a screening trial with a visible platform was performed in order to exclude rats with motivational or sensory-motor deficits. The spatial memory testing phase occurred over 8 days and was performed in two stages: reference memory testing with platform position remaining constant for all training sessions (3 days, four 120-s trials a day and a probe test without a platform on the 4th day) and working memory testing with the platform position changed every day (4 days, 4 trials a day). Inter-trial interval (ITI) was10 min. Three MWM parameters were measured with the use of a video-tracking digitizing device (EthoVision XT, Noldus, Netherlands): latency to reach the platform (in most trials) or the critical annulus (CA - virtual contour of the removed platform) in the probe test, distance swum (path length), and the percentage of time spent in the critical quadrant (CQ) of the pool (where the platform was located).
Blood and Spleen Collection
One hour after the last session of Morris test, blood samples in a volume of 5 mL were collected by a cardiac puncture under 2.5% isoflurane (airflow: 0.5 L per min) anesthesia using an isoflurane pump (Bitmos OXY 6000, Bitmos GmbH Düsseldorf, Germany). Then, rats were euthanized with Morbital (2 mL/kg) and spleens were harvested from all groups of rats.
Peripheral Blood Haematological Parameters in Young and Aged Rats
Peripheral blood haematological parameters: red blood cells (RBC), platelets (PLT), haemoglobin concentration (HGB), mean haemoglobin concentration in the red blood cell (MCHC), mean mass of the haemoglobin in the red blood cell (MCH), mean corpuscular volume (MCV), hematocrit (HCT), mean platelet volume (MPV), red cell distribution width (RDW) were determined in the heparinized whole blood with the haematology analyzer (Cell- Dyn 3700 Abbott, USA).
Total Blood Leukocyte and Leukocyte Subset Number
The total white blood cell (WBC) number and percentage of leukocyte subsets were determined in the heparinized whole blood according to the procedure that we previously described.40 The total WBC number and percentage of lymphocytes, neutrophils, eosinophils, basophils and monocytes were determined with the hematology analyzer (Cell-Dyn 3700 Abbott, USA). The total number of each leukocyte subset was calculated as the total leukocyte number x percentage of the respective leukocyte subpopulation.
Cytometric Analysis of Lymphocyte Populations and Subpopulations
Peripheral blood mononuclear cells (PBMC) were obtained from heparinized whole blood by the Ficoll 400 (Pharmacia, Uppsala Sweden) and Uropolinum (Polfa, Starogard, Poland) density centrifugation method. The spleen tissue was placed into Hank’s balanced salt solution (HBSS Sigma-Aldrich Manufacturer Life Technologies Paisley, Scotland) containing sodium bicarbonate and HEPES, then dissociated using a blender and passed through nylon mesh to remove large aggregates. After the centrifugation (1113×g, 30 min at 4°C), the isolated cells were collected with a Pasteur pipette, washed with phosphate-buffered saline. PBMC and spleen mononuclear cells (SMC) suspension in RPMI-1640 with a 10% calf bovine serum were seeded at a concentration of 4×106 cells/mL and used in the flow cytometry analysis.
A three-color combination of fluorescent monoclonal antibodies was used in the blood and spleen lymphocyte study to identify T (CD3+), B (CD45RA+), NK (CD161a+) lymphocytes and T lymphocyte subsets of the T helper (CD3+CD4+CD8−) and T cytotoxic (CD3+CD4−CD8+) lymphocyte percentage according to the method that we previously described41–43 with some modifications. Twenty five µL of the whole blood or splenocytes were added to 25 µL of IOTest CD3-FITC/CD45RA-PC7/CD161a-APC or CD3-FITC/CD4-PC7/CD8-APC (Beckman Coulter, USA) according to the manufacturer’s instructions. Erythrocytes were lysed (Versalyse, Beckman Coulter) and then blood and spleen samples were mixed and incubated at room temperature for 20 min in darkness. After incubation, 25 μL of Fixative Solution (Beckman Coulter, USA) and 700 μL of PBS was added to the separate sample. Samples were protected from light and stored at 4°C until flow cytometry had been performed with a Cytomics FC 500 flow cytometer (Beckman Coulter, USA) and MXP Software. The percentages of lymphocyte population and T lymphocyte subsets were assessed during the assay, gaiting on forward and side scatter characteristics (Figure 1). Then, total numbers of the lymphocyte population and their subsets were calculated on respective counts of the total WBC number and a percentage of the T (CD3+), B (CD45RA+), NK (CD161a+), CD3+CD4+CD8− T and CD3+CD4−CD8+T lymphocytes.
Determination of Plasma and Peripheral Blood Mononuclear Cells (PBMC)- or Spleen Mononuclear Cells (SMC)-Derived Production of the Pro-Inflammatory Interferon (IFN)-γ, Interleukin (IL)-6 and Anti-Inflammatory Interleukin (IL)-10
Peripheral blood mononuclear cells (PBMC) and spleen mononuclear cells (SMC) were examined for their ability to produce pro-inflammatory IFN-γ, IL-6 and anti-inflammatory IL-10 in response to concanavalin A (Con-A) stimulation according to the procedure described previously.40 Briefly, PBMC and SMC were seeded at a concentration of 4×106 cells/mL in 24-well Corning tissue culture plates, and then stimulated with a Con-A solution (2.5 μg/mL) or remained non-stimulated (control). The PBMC and SMC were incubated in 37°C. Cell-free supernatants were collected 48 h (for IL-6) and 72 h (for IFN- γ and IL-10) later and stored at −80°C until assayed.
The IFN-γ, IL-6 and IL-10 concentrations in the supernatants and plasma were determined by enzyme-linked immunoassay (ELISA) using a commercially available kit (Rat-IFN-γ and Rat-IL-4 ELISA kits R&D, USA) according to the manufacture’s instructions and our previous study.40 Briefly, 50 µL of standards or samples were dispensed into 96 wells coated with rat IFN-γ, IL-6 and IL-10 antibody, respectively, and incubated for 2 h (IFN-γ) or 1 h (IL-4) at room temperature. After extensive washing, 100 µL of the biotinylated anti-IFN-γ or anti-IL-4 were added to each well, and the plates were incubated for 30 min (IFN-γ) or 1h (IL-4) at room temperature. The wells were again washed 3 times, 100 µL of Streptavidin-HRP was added and incubation was carried out for 30 min. 3.3´,5,5´-tetramethylbenzidine (TMB) (100 μL/well) was used as the chromogen for the colorimetric assay. The reaction was stopped after 10 min by adding a 100 µL/well of stop solution and the absorbance was determined using the DTX 880 Multimode Detector (Beckman Coulter, USA) system set to 450 nm. Cytokine concentrations were calculated based on the standard curve generated by Beckman Coulter´s Biomek software program, based on the absorbance of standard samples. The sensitivity of detection was 2 pg/mL for IFN-γ, 16 pg/mL for IL-6 and 3 pg/mL for IL-10.
Determination of Plasma Corticosterone (COR) Concentration
Concomitantly with the immune parameters, in the same animals, the plasma corticosterone (COR) concentrations were measured. Blood samples were collected by heart puncture under 2.5% isoflurane (airflow: 0.5 L per min) anesthesia using an isoflurane pump (Bitmos OXY 6000, Bitmos GmbH Düsseldorf, Germany) between 09.00 and 10.00 a.m. when COR levels are at their lowest.44 The plasma COR concentration was measured by radioimmunoassay using a commercially available kit (rCorticosterone [125I RIA KIT, isotop Institute of Isotopes Co, LTD, Budapest, Hungary] and Wizard 1470 gamma counter (Pharmacia – LKB, Turku, Finland). The measures were made in a duplicate. The sensitivity of the assay was 0.01 ng/tube.
Data Analysis
The data is presented as mean ± SD. The normality of the distribution of the variables was checked with Kołmogorow-Smirnov test and the homogeneity of the variances with a Levene test. As the outcome of Kołmogorow-Smirnov test indicated that all data was not distributed normally, we used for further statistical evaluation of blood, spleen and plasma parameters, non-parametric tests for further analysis. Data was evaluated using the Kruskal–Wallis one way ANOVA with factors AD-like induction (STZ_VEH), therapy (DMF_standard chow), age (young_aged), and the Mann–Whitney U-test for group comparison. A P value lower than 0.05 was considered statistically significant.
Results
Morris Water Maze (MWM) Test of Spatial Memory in Young and Aged Rats
Results of behavioral testing were similar to those described previously.34,35 Briefly, all rats were able to locate and climb the platform within 60 s during a screening trial using a visible platform. At the acquisition phase of the reference memory test, STZ groups in both young and aged rats showed the highest latency to reach the platform and the longest distance swum. The impairment of reference memory acquisition was greater in aged STZ rats compared to young STZ rats as indicated by greater the latency to reach the platform and distance to reach platform. Latency to reach the platform for aged STZ rats was higher than in both VEHCTR and VEHDMF groups. Aged STZ rats not receiving DMF treatment swam longer distances than aged STZDMF rats and aged VEHCTR rats. STZ rats spent less time in the critical quadrant (CQ) than VEHDMF rats on day 1, and spent less time compared to each other group on day 3. Aged VEHDMF rats were the only group that improved its performance during the test, showing a reduced latency and distance on day 3 compared to day 1. There was also a significant effect of age on the percentage of time spent in the CQ: aged VEHDMF rats spent more time in the CQ than young VEHDMF rats during the whole acquisition phase of the reference memory MWM test.
In the probe test for spatial reference memory, working memory impairment was greater in aged STZ rats compared to young STZ rats: values for latency to reach the platform in trials 2–4, as well as distance swum and the percent of time in the CQ in trial 4, were greater for aged STZ rats. Latency to reach the platform and the distance swum by aged STZ rats were longer compared to aged VEHCTR and VEHDMF groups. Moreover, there were significant differences between STZ and STZDMF groups in aged rats. Aged STZ rats took longer to reach the platform in trials 1–2, and swam a longer distance in trial 2, than aged STZDMF rats. Differences in the percent of time in the CQ were found only in aged rats. Aged STZ rats spent a lower percent of time in the CQ than aged VEHDMF rats in trials 2–4 as well as less percent time than aged VEHCTR animals in trials 3–4. VEHCTR group was the only group that improved its performance in consecutive trials within the young group of rats.
Total Blood Leukocytes, Leukocyte Subset Percentage and Number, Relative Thymus and Spleen Weight in Young and Aged Rats
Total White Blood Cell (WBC) Number
There was a significantly (P < 0.05) higher the total number of white blood cells (WBC) in the STZDMF group in comparison with the VEHDMF and STZCTR young rats (Table 1). In the aged rats (Table 2), the total WBC number within the STZDMF group was significantly lower rather than in the VEHDMF aged rats. The WBC number was significantly (P < 0.001) higher in the VEHDMF and STZCTR in the aged rather than young rats (Table 2).
Percentage and Total Lymphocyte Number
The percentage of blood lymphocytes was significantly (P < 0.05) higher in the VEHDMF rather than VEHCTR young rats (Table 1). In the aged animals, both the percentage and total lymphocyte numbers were significantly (P < 0.05) lower in the STZDMF rather than STZCTR group (Table 2). In comparison with the young animals, numbers of lymphocytes were significantly lower within the VEHDMF (P < 0.001 and P < 0.01 for percentage and total number, respectively), STZDMF (P < 0.001, percentage and total number) and STZCTR (P < 0.05, percentage number) aged rats (Table 2).
Percentage and Total Neutrophil Number
A significantly (P < 0.05) lower the percentage and total number of blood neutrophils in the STZDMF in comparison with the VEHCTR was observed in young rats (Table 1). In the aged rats, the number of blood neutrophils was significantly lower within the STZCTR (P < 0.01 for percentage and total number) and VEHDMF (P < 0.05 for percentage number) in comparison with the VEHCTR group. Moreover, within the STZDMF aged rats a significantly (P <0.05) lower percentage of neutrophils in comparison with the VEHDMF aged animals, was noticed (Table 2). Within all the aged rats, the percentage and total number of neutrophils was significantly (P < 0.001, Table 2) lower in comparison with the respective groups of young animals.
Percentage and Total Eosinophil Number
There were no significant differences in the percentage and total number of eosinophils between all groups of young or aged animals (Tables 1 and 2). In the VEHDMF (P < 0.001) and STZDMF (P < 0.05) aged rats, a significantly higher total number of eosinophils in comparison with the respective groups of young animals, were observed (Table 2).
Percentage and Total Basophil Number
In comparison with the young VEHCTR group, a significantly lower total number (STZCTR, P < 0.01) and percentage (STZDMF, P < 0.05) of basophils, was observed (Table 1). Moreover, in the STZDMF animals, the basophil percentage was significantly lower rather than VEHDMF group in young and aged (P < 0.05) animals and STZCTR group in aged rats (P < 0.01). The percentage and total numbers of eosinophils were significantly higher in all groups of aged rats (Table 2) in comparison with young animals.
Percentage and Total Monocyte Number
There was a significantly lower percentage and total monocyte number in the VEHDMF rather than VEHCTR group (P < 0.05) and in the STZDMF animals in comparison with the STZCTR (P < 0.05) and VEHDMF (P < 0.05) group of the young rats (Table 1). The monocyte percentage was significantly higher in the STZDMF aged animals in comparison with the VEHDMF (P < 0.05) and STZCTR (P < 0.01) group (Table 2). In comparison with young rats, significant differences in the percentage of monocytes were observed between all the aged groups of rats (Table 2), with the higher level of monocytes within the VEHDMF and STZDMF and lower monocyte percentage within the VEHCTR and STZCTR in aged animals.
Cytometric Analysis of Peripheral Blood Lymphocytes and Lymphocyte Subpopulations in Aged Rats
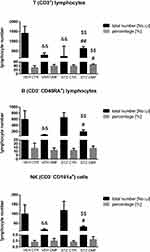
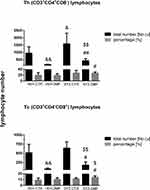
The percentage and total T (CD3+), B (CD45RA+), NK (CD161a+), TCD4+ (CD3+CD4+CD8−) and TCD8+(CD3+CD4−CD8+) lymphocyte numbers in the peripheral blood mononuclear cells (PBMC) in the aged rats are presented in Figures 2 and 3. As shown in Figure 2, in the STZDMF group, a significantly higher the total and percentage of T (CD3+) lymphocytes rather than in the STZCTR (P < 0.01) and VEHDMF (P < 0.05) was noticed. There was a significantly (P < 0.01) lower the total T (CD3+) lymphocyte number in the STZCTR and VEHDMF in comparison with the VEHCTR group. The total number of B (CD45RA+) lymphocytes was lower within the STZDMF animals in comparison with the STZCTR (P < 0.01) and VEHDMF (P < 0.05) groups (Figure 2). The total B (CD45RA+) lymphocyte number was significantly lower within the VEHDMF group rather than VEHCTR animals (P < 0.01). There was a significantly lower the total number of NK (CD161a+) cells (Figure 2) within the STZDMF rather than STZCTR (P < 0.01) and VEHDMF (P < 0.05) groups, whereas the total number of NK (CD161a+) cells was lower (P < 0.01) within the VEHDMF in comparison with the VEHCTR animals. As shown in Figure 3, within the STZDMF group, a significantly higher (P < 0.05) percentage of T CD3+CD4−CD8+ lymphocytes, whereas lower total numbers of T CD3+CD4+CD8− and T CD3+CD4−CD8+ lymphocytes (P < 0.01) in comparison with the STZCTR group were observed. In comparison with the VEHCTR group, the total number of T CD3+CD4+CD8− was significantly higher in the STZCTR animals (P < 0.05), whereas total numbers of T CD3+CD4+CD8− and T CD3+CD4−CD8+ lymphocytes were lower within the VEHDMF group (P < 0.01).
Relative Thymus and Spleen Weight in Young and Aged Rats
There were no significant differences in the relative thymus and spleen weights between all young rats (Table 1). In aged rats (Table 2), a significantly lower relative thymus and spleen weights in the VEHDMF group in comparison with the VEHCTR animals (P < 0.05) and in the STZDMF group in comparison with the VEHDMF rats (P < 0.05) were observed. Moreover, the relative thymus weight in the aged rats was significantly (P < 0.001) lower rather than the respective groups of young animals.
Peripheral Blood Haematological Parameters in Young and Aged Rats
Total Number of Red Blood Cells (RBC) in Young and Aged Rats
There was a significant decrease in the total number of red blood cells (RBC) in the STZDMF group in comparison with the VEHDMF (P < 0.05) and STZCTR (P < 0.01) groups in the aged animals (Table 3). The total RBC number did not differ between all the groups of the young animals or between young and aged rats.
Total Number of Platelet (PLT) in Young and Aged Rats
In the young animals, a significantly (P < 0.05) lower platelet (PLT) total number was observed in the STZDMF group, in comparison with the VEHDMF animals. In comparison with the young rats, the PLT number was significantly higher in the VEHDMF (P < 0.05), STZCTR and STZDMF (P < 0.01) aged rats (Table 3).
Haemoglobin Concentration (HGB) in Young and Aged Rats
There was a significantly (P < 0.05) lower haemoglobin concentration (HGB) in the STZDMF aged rats in comparison with the STZCTR animals and higher HGB level in the aged VEHDMF and STZCTR (P < 0.01) rather than the respective young animals (Table 3).
Hematocrit (HCT) and Mean Platelet Volume (MPV) in Young and Aged Rats
In the aged STZDMF rats, a significantly lower hematocrit (HCT), in comparison with the STZCTR and VEHDMF (P < 0.05) group as well as the mean platelet volume (MPV), in comparison with the STZCTR aged (P < 0.05) and young STZDMF (P < 0.01) animals, were observed (Table 3).
Mean Haemoglobin Concentration in the Red Blood Cell (MCHC), Mean Mass of the Haemoglobin in the Red Blood Cell (MCH), Mean Corpuscular Volume (MCV), and Red Cell Distribution Width (RDW) in Young and Aged Rats
There were no significant differences in the mean haemoglobin concentration in the red blood cell (MCHC), mean mass of the haemoglobin in the red blood cell (MCH), mean corpuscular volume (MCV) and red cell distribution width (RDW) in the young and aged rats (Table 3). In comparison with the young animals, the higher MCHC in the STZDMF (P < 0.05), higher MCH and MCV in the STZCTR (P < 0.05), and higher RDW in the VEHDMF (P < 0.05), STZCTR (P < 0.01) and STZDMF (P < 0.001) aged rats were noticed.
Pro-/Anti-Inflammatory Cytokines
Plasma Interleukin (IL)-6/Interleukin (IL)-10 Concentration in Young and Aged Rats
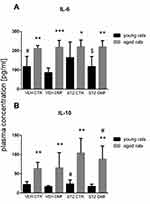
There were significant differences in the plasma IL-6 concentration between young groups of animals with the ICV-STZ-induced model of AD (STZDMF vs STZCTR) and between the control VEHCTR and VEHDMF groups (P < 0.05), with a significantly lower the cytokine level in the animals treated with the DMF (Figure 4A). Moreover, a significantly higher plasma IL-6 concentration within the STZDMF and STZCTR groups rather than control VEHDMF or VEHCTR, respectively, in young rats was observed. There were no significant differences in the plasma IL-6 concentration between all groups of aged animals. However, as compared to the young animals, all groups of the aged rats had a significantly higher level of IL-6 in the plasma (STZCTR, P < 0.05; VEHCTR, P < 0.01; STZDMF, P < 0.01, VEHDMF, P < 0.001).
As shown in Figure 4B, there were no significant differences in the plasma IL-10 concentration between all groups of the young rats. In the aged rats, a significantly higher cytokine levels in the STZCTR group in comparison with the VEHCTR and VEHDMF groups (P < 0.05) and in the STZDMF animals rather than VEHDMF group (P < 0.05) were observed. Moreover, as compared to the young rats, plasma IL-10 concentration was significantly higher (P < 0.01) within all aged groups.
Spleen Mononuclear Cell (SMC) Interferon (IFN)-γ/Interleukin (IL)-10 Production in Young Rats
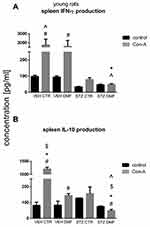
Figure 5A presents production of IFN-γ by SMC stimulated by concanavalin (Con)-A and without Con-A stimulation (control) in young rats. Con-A-induced splenocyte production of IFN-γ was significantly increased (P < 0.05) in the VEHCTR and VEHDMF groups in comparison with the respective control values without mitogenic stimulation. There were no significant differences in the IFN-γ production by Con-A stimulated splenocytes from both STZCTR and STZDMF groups in comparison with the control non-stimulated splenocytes. In comparison with both VEHCTR and VEHDMF animals, a significantly lower (P < 0.05) splenocyte ability to production of IFN-γ following Con-A stimulation in the STZCTR and STZDMF group, with the lowest (P < 0.05) cytokine production in the STZDMF animals, was noticed.
As shown in Figure 5B, production of IL-10 by Con-A stimulated SMC was significantly (P < 0.05) higher in the VEHCTR and VEHDMF groups in comparison with their respective control non-stimulated splenocytes. Moreover, a significantly lower (P < 0.05) IL-10 production after Con-A stimulation in the STZDMF animals, in comparison with splenocytes without Con-A stimulation (controls), was observed. Con-A stimulation of splenocytes induced the highest (P < 0.05) IL-10 production within the VEHCTR group whereas it was the lowest (P < 0.05) IL-10 production within the STZDMF animals. In comparison with the DMF-treated groups, there was a significantly (P < 0.05) higher Con-A-stimulated ability of splenocytes to IL-10 production within the STZCTR and VEHCTR groups.
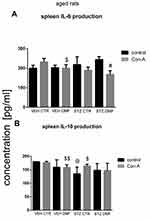
Spleen Mononuclear Cell (SMC) Interleukin (IL)-6/Interleukin (IL)-10 Production in Aged Rats
Figure 6A shows production of IL-6 by SMC stimulated by Con-A and without Con-A stimulation (control) in aged rats. Con-A-induced splenocyte production of IL-6 was significantly (P < 0.05) lower in comparison with the non-stimulated splenocytes in the STZDMF group. There was a significantly lower (P < 0.05) production of IL-6 after Con-A stimulation of splenocytes in the VEHDMF, STZCTR and STZDMF groups rather than VEHCTR animals, with the lowest cytokine production within the STZDMF group.
As shown in Figure 6B, there were no significant differences in the IL-10 production between the Con-A stimulated and non-stimulated SMC within all the investigated groups of aged animals. Furthermore, there were no significant differences in IL-10 production between STZCTR and STZDMF groups.
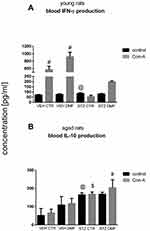
Peripheral Blood Mononuclear Cell (PBMC) Interferon (IFN)-γ/Interleukin (IL)-10 Production in Young and Aged Rats
Figure 7 presents the production of IFN-γ by Con-A stimulated and non-stimulated peripheral blood mononuclear (PBMC) in young rats (Figure 7A) and PBMC-induced production of IL-10 after Con-A stimulation or without Con-A stimulation in aged rats (Figure 7B). As shown in Figure 7A, there was a significantly (P < 0.05) lower IFN-γ production by PBMC after mitogenic stimulation in the STZCTR rather than VEHCTR young rats. Moreover, a significantly (P < 0.05) higher IFN-γ production by Con-A stimulated blood lymphocytes in the control VEHCTR and VEHDMF groups, in comparison with the non-stimulated lymphocytes in young rats were observed. The lowest ability of blood lymphocytes to produce IFN-γ after Con-A stimulation in the STZCTR animals was noticed.
PBMC IL-10 production in control conditions (without Con-A stimulation) in aged rats (Figure 7B) was significantly (P < 0.05) higher in both STZCTR and STZDMF groups in comparison with the VEHCTR animals. In comparison with the control value of IL-10 production by non-stimulated lymphocytes, a significant (P < 0.05) increase in IL-10 production after Con-A stimulation in the STZDMF group was observed. Moreover, the higher ability (P < 0.05) to produce of IL-10 by stimulated PBMC in the STZCTR and STZDMF groups, with the highest level in the STZDMF animals, in comparison with the VEHCTR groups, was noticed.
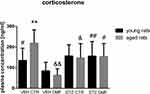
Plasma Corticosterone Concentration in Young and Aged Rats
As shown in Figure 8, there were no significant differences in the plasma COR concentration between STZDMF and STZCTR groups, neither in the young nor in the aged rats. However, as compared with the control VEHDMF animals, a significantly higher COR concentrations in the STZDMF (P < 0.01) and VEHCTR (P < 0.05) groups in the young rats were noticed. In the aged rats, plasma concentrations of COR in the both STZCTR and STZDMF groups were significantly (P < 0.05) higher in comparison with their respective control VEHCTR and VEHDMF groups. Moreover, the significant difference (P < 0.01) in the COR level between both control group of aged rats was noticed, with the lower hormone concentration in the VEHDMF rather than VEHCTR animals. There was a significant difference (P < 0.01) in the plasma COR concentration between young and aged rats within the control VEHCTR groups.
Discussion
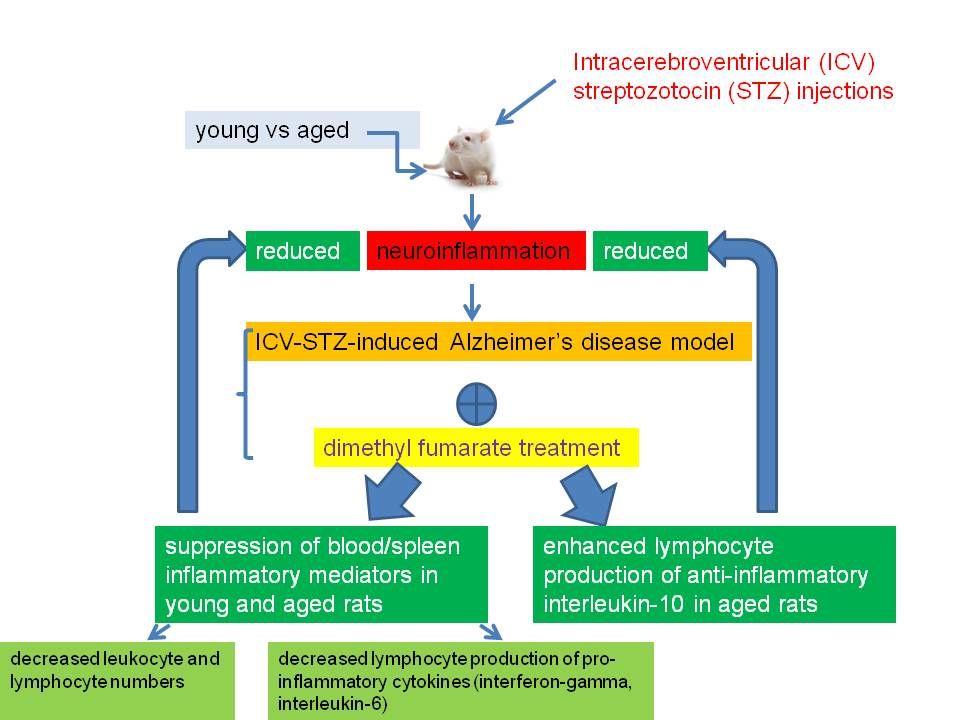
As far as we know, this is the first report on the peripheral DMF effect in rats with the ICV-STZ-induced neuroinflammation which is compatible with the model of AD. The main findings of the present study are that DMF used as a therapeutic factor in rats with the AD model prevented the peripheral pro-inflammatory response and memory impairments in young and aged animals. These effects were more pronounced in aged rather than young rats. Moreover, DMF decreased peripheral blood haematological parameters and led to the blood and spleen anti-inflammatory response in aged but not in young rats. It suggests that beneficial, age-dependent effect of DMF can be mediated by inhibition of the peripheral inflammatory mediators, including changes in the distribution and function of peripheral lymphocytes.
The proposed mechanisms of the DMF-induced reduction of inflammatory mediators in the ICV-STZ model of AD are the following: (A) a change in the peripheral immune cell composition, (B) a change in the peripheral immune cell phenotype, (C) a change in the balance of pro-/anti-inflammatory cytokines or (D) suppression of migration of leukocytes through the blood-brain-barrier (BBB).
The change in blood immune cell composition is one of the most important mechanisms by which DMF skews the immune response towards an anti-inflammatory state in the ICV-STZ model of AD. After 21 days of DMF therapy, the decreased total numbers of B (CD45RA+) lymphocytes, NK (CD161a+) cells, TCD3+CD4+CD8− and T TCD3+CD4− CD8+ lymphocytes were noted which may account for the observed peripheral lymphopenia and leukopenia in aged rats after ICV-STZ injections. Notably, the DMF-induced age-dependent (young vs aged) effect concerned, in particular, lowered numbers of peripheral leukocytes, lymphocytes, B, NK, TCD3+CD4+CD8− and TCD3+CD4−CD8+ lymphocytes and increased total T CD3+ and monocyte numbers in aged as compared to young rats. Our results are consistent with previous reports showing reduced numbers of lymphocytes, including blood T cells, CD8+ T cells, CD4+ T cells, and CD19+ B cells in DMF-treated RRMS patients.45,46 In contrast to these findings, we found an increased total number and percentage of peripheral T (CD3+) lymphocytes in aged rats with the ICV-STZ-induced AD model after DMF treatment. Regarding the increased T CD3+ lymphocyte number, these results were not confirmed when exploring total cell numbers of TCD3+CD4+CD8− and TCD3+CD4−CD8+ subpopulations. It could show that this relative increase may be a consequence of a decrease in other lymphoid populations such as B (CD45RA+) lymphocytes and NK (CD161a+) cells. The decreased NK cell (CD161a+) number observed in the present study is also in contrast to results reported in studies on DMF effects in MS patients.47,48 Concerning the fact that NK cells exhibit regulatory function and use different strategies to limit T cell function49 another explanation of the increased number of TCD3+ lymphocytes could be opposite effect of DMF treatment on T (CD3+) and NK (CD161a+) cell numbers observed in the ICV-STZ-induced pathology.
Interestingly, in response to the DMF treatment, increased peripheral monocyte number in aged but not young rats after STZ injections was found. DMF is suggested to act by activating the nuclear factor (erythroid-derived 2)-like 2 (Nrf2),50 which is essential in redox homeostasis and responses to reactive oxygen species (ROS). However, it has been demonstrated that older animals have decreased nuclear content of Nrf-251 and therefore functionally reduced antioxidant and anti-inflammatory Nrf-2 activity which is likely reflecting the phenomenon of “inflammaging” state.10 Moreover, monocytes are potent producers of ROS primarily via NADPH oxidases32,52 suggesting Nrf-2-independent mechanisms in the anti-inflammatory activity of DMF in aged rats. Other reports demonstrate that the beneficial effect of DMF treatment on macrophages/monocytes was accompanied by the induction of anti-inflammatory M2 monocytes and reduced frequencies of IFN-ɤ and IL-17-producing CD4+ cells, indicating that DMF can promote M2 monocytes in Nrf-2-independent manner. DMF can also affect the phenotype of monocytes/macrophages by affecting the level of microRNA. Recently, it has been shown that DMF can reduce the expression of the pro-inflammatory miR-155 in monocytes from RRMS patients that is known to induce the production of TNF-α and IL-6 in human macrophages. Our results are in line with those studies showing that DMF increased peripheral blood monocyte counts and monocytic ROS generation and that epigenetic methylation changes in monocytes precede those occurring in TCD4+ in RRMS patients starting DMF therapy.32 Thus, our data suggest that this early and beneficial effect of DMF on monocytes could be of importance for subsequent modulation of adaptive immune response in aged rats.
The suggested modes of DMF action on lymphocyte numbers include differential susceptibility of distinct lymphocyte subpopulations to DMF-induced apoptosis which may contribute to efficacy and safety profile of this drug.53,54 Among T cells, DMF treatment selectively depletes highly glycolytic effector T cells while sparing oxidative naïve T cells and Treg cells,55,56 therefore affecting cell-mediated antiviral immunity and CNS immunosurveillance.57 Similarly, in MS, the DMF treatment was associated with a shift in the immunophenotypes of circulating T cells, reducing circulating central and effector memory CD4+ and CD8+ T cells.58 DMF-induced apoptosis is greater for CD8+ T than CD4+ T cells, memory than naïve, and conventional than regulatory T cell subsets.54 Another mode of immunomodulative action of DMF which could evoke these changes in peripheral lymphocyte composition could be differential effects of this drug on lymphocyte proliferation. Stronger anti-proliferative effect on TCD8+ rather than TCD4+ lymphocytes upon DMF stimulation was observed. DMF inhibited proliferation of T cells in vitro by reducing the activation status of antigen presenting cells via nuclear factor kappa-light chain enhancer of activated B cells (NFκB) inactivation. Activation of NFκB is crucial for T cell survival whereas NFκB inactivation in-vivo led to oxidative stress in T cells in multiple sclerosis patients.31 Our findings are consistent with these studies. We observed a decrease in the peripheral number of TCD3+CD4+CD8− and TCD3+CD4−CD8+ lymphocytes in DMF treated rats with the ICV-STZ-induced AD model, which was more pronounced for TCD3+CD4−CD8+ lymphocytes.
DMF-induced peripheral immunomodulatory effects in rats with the ICV-STZ model of AD in the present study might have resulted from a Th1 to Th2 shift and the modulation of TCD3+CD4+CD8− function. Our findings are in accordance with studies that reported an anti-inflammatory shift in T cells after the DMF treatment.59,60 DMF has been shown to inhibit the synthesis of many pro-inflammatory mediators including NOS, TNF-α, IFN-ɤ, IL-1β, IL-6, IL-2, IL-12, and IL-17.61 Apart from a decline in the absolute cell number of Tregs (CD4+), DMF can reduce the capacity of T cells to produce pro-inflammatory cytokines in MS patients.46 Similarly, treatment with DMF significantly decreased IL-17 mRNA levels in PBMCs of psoriasis patients.33 Activation of the cAMP-dependent signaling pathway may contribute to the generation of Th2 lymphocytes, since it has been shown that this pathway provides a strong inhibitory signal for Th1 cytokines, whereas Th2 cytokines are not affected or are upregulated.62 As DMF stimulates cAMP signaling in human PBMC,63 and cAMP regulates T and NK cell functions, we further analyzed ability of TCD3+CD4+ CD8− lymphocytes to produce pro-/anti-inflammatory cytokines. We found that peripheral immunomodulatory effects of DMF not only alter lymphocyte composition, but also have profound age-dependent effects on cytokine production. DMF reduced the number of blood TCD3+CD4+CD8− lymphocytes and simultaneously decreased the production of such pro-inflammatory cytokines as IFN-ɤ or IL-6 in young and aged animals, whereas it enhanced the ability of PBMC and SMC to produce an anti-inflammatory IL-10, as well as it increased plasma IL-10 concentration in the aged, but not young, rats with the STZ-induced AD pathology. These effects are compatible with our previous findings that the DMF treatment after ICV- STZ injections induces a decrease in IL-6 and increase in IL-10 expression in the rat brain.34 Other authors reported that increased levels of cAMP inhibited the expression of pro-inflammatory cytokines such as IL-2, IFN-ɤ and TNF-α, and stimulated the production of anti-inflammatory cytokines such as IL-10.64 Together, these effects indicate that DMF leads to the anti-inflammatory phenotype and favors a shift from Th1 to Th2 response. This shift in TCD3+CD4+CD8− lymphocytes likewise contributes to the DMF therapeutic effects. Furthermore, our results indicate that this age-dependent anti-inflammatory effect does not depend on the differences in the hypothalamic-pituitary-adrenal axis response between young and aged rats measured as plasma concentration of COR. In fact, we observed similar increases in COR concentrations in young and aged rats with STZ injections.
We also found the DMF treatment to be associated with a decrease in several peripheral haematological parameters, including the red blood cell number (RBC), haemoglobin concentration (HGB), haematocrit (HCT) and mean platelet volume (MPV) after ICV-STZ injections, in particular in aged rats. As compared to young animals, all those parameters were significantly lower, except for platelet number (PLT), in aged rats with AD pathology. Simultaneously with the decreased RBC number, DMF-treated aged animals presented a lowering of red cell distribution width (RDW) which could indicate normalization of RBC size distribution. Regarding the influence of age on RBC function, the available data suggest that plasma β-amyloid peptides bind to ageing erythrocytes, implying a pathogenic role of erythrocyte amyloid complex in AD. This complex induces changes in the red blood cell morphology, adhesion to endothelium and influences vessel activity.65 Our findings are in contrast with previous reports that indicated that chronic treatment with DMF has improved haematological parameters by activation of Nrf2 in stem-cell-derived erythroid progenitors in tissue culture and in murine and primate models.66 Moreover, it has been shown that Nrf-2 is also a positive regulator of gamma-globin expression during erythropoiesis and regulates expression of cytoprotective proteins, including heme-oxygenase-1.67 Thus, in the present study, decreased haematological parameters after DMF treatment observed in aged animals can be explained by the lack of such a therapeutic target for DMF as peripheral blood Nrf-2 expression which is suppressed with ageing.51
Furthermore, coagulation system, and platelets in particular, are involved in AD pathology24 and correspondingly abnormal clotting was found in AD patients which was correlated with decline in cognitive ability.25,68 Platelets, which are an important part of the inflammation and immune response, are hyper-activated in AD and these hyper-activated cells more aggressively damage healthy vessels.23 In the present study, PLT number was significantly higher whereas MPV was lower in aged rather than young rats after STZ injections and DMF treatment. Our findings are in line with results demonstrating that platelet count and likely their function are augmented with ageing. Our results also suggest that there is a failure of Nrf-2-activating intervention of DMF, and there are Nrf-2-independent mechanisms in the anti-inflammatory activity of DMF in aged rats with the AD pathology.
Besides the effect on the immune cell composition and phenotypes, DMF may protect the brain by decreasing the expression of adhesion molecules and suppressing the activation state of the immune systems,69 and by inhibiting the brain infiltration of immune cells through their retention within secondary lymphoid tissues or within the peripheral blood compartment itself. Furthermore, DMF may protect the BBB integrity70 that could possibly interrupt the recruitment and migration of immune cells to the site of inflammation.71
Conclusions
In summary, our study showed that the DMF-induced attenuation of the response of inflammatory mediators, including changes in the distribution and function of peripheral lymphocytes, and memory impairment was more pronounced in aged rats with the ICV-STZ injections as compared to younger animals. The decreased number of TCD3+CD4+CD8− lymphocytes in parallel to the decreased production of pro-inflammatory cytokines (IFN-ɤ, IL-6), and concomitantly with unaffected or even enhanced production of anti-inflammatory cytokine (IL-10) in aged rats suggests that DMF acts on pro-/anti-inflammatory balance in the periphery and leads to an anti-inflammatory shift in T lymphocytes. Those effects could contribute to therapeutic effects of DMF in the ICV-STZ-induced model of AD (graphical abstract). The obtained results extend our previous findings on beneficial brain anti-inflammatory effects of DMF along with memory improvement and suggest that DMF could be included in the therapeutic strategy for treating Alzheimer’s disease, in particular, through the modulation of the central and peripheral immune system, favoring the anti-inflammatory response. However, the DMF action may also lead to more prevalent viral infections.
Acknowledgments
We express our appreciation to Ewa Laska, Aleksandra Chomik, Justyna Marchewka, Marcin Wyroślak, Mariola Grałek for their technical help in our study. The authors thank Emilia Leszkowicz for her help with proofreading.
Author Contributions
Danuta Wrona designed the study, carried out the literature review, and drafted the manuscript; Irena Majkutewicz conceived the study and participated in its design and coordination. All the authors a) made substantial contributions to acquisition of data, or analysis and interpretation of data; b) took part in revising it critically for important intellectual content; c) agreed to submit the manuscript to the current journal; d) gave their final approval of the manuscript’s version to be published, and agreed to be accountable for all aspects of their work.
Funding
This project was partially supported by the grant from the Ministry of Science and Higher Education of Poland for young scientists from the University of Gdansk (538-L124-B145-18) and by funding for statutory R&D activities of the Department of Animal and Human Physiology of the University of Gdansk.
Disclosure
The authors report no conflict of interest in this work.
References
1. Franceschi C, Capri M, Monti D, et al. Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev. 2007;28:92–105. doi:10.1016/j.mad.2006.11.016
2. Pizza V, Agresta A, D’Acunto CW, Festa M, Capasso A. Neuroinflamm-aging and neurodegenerative diseases: an overview. CNS Neurol Disord Drug Targets. 2011;10(5):621–634. doi:10.2174/187152711796235014
3. Wyss-Coray T. Inflammation in Alzheimer disease: driving force, bystander or beneficial response? Nat Med. 2006;12:1005–1015. doi:10.1038/nm1484
4. Stefanova NA, Ershov NI, Maksimowa KY, Muraleva NA, Tyumentsev MA, Kolosova NG. The rat prefrontal-cortex transcriptome: effects of aging and sporadic Alzheimer’s disease-like pathology. J Gerontol A Biol Sci Med Sci. 2019;74(1):33–43. doi:10.1093/gerona/gly198
5. Eikelenboom P, van Exel E, Hoozemans JJ, Veerhuis R, Rozemuller AJ, van Gool WA. Neuroinflammation – an early event in both the history and pathogenesis of Alzheimer’s disease. Neurodegener Dis. 2010;7(1–3):38–41. doi:10.1159/000283480
6. Heneka MT, Carson MJ, Khoury EJ, et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015;14:388–405. doi:10.1016/S1474-4422(15)70016-5
7. Lehnardt S, Massilon L, Follett P, et al. Activation of innate immunity in the CNS triggers neurodegeneration through a toll-like receptor 4-dependent pathway. Proc Natl Acad Sci U S A. 2003;100(14):8514–8519. doi:10.1073/pnas.1432609100
8. Walker DG, Dalsing-Hemandez JE, Campbell NA, Lue LF. Decreased expression of CD 200 and CD 200 receptor in Alzheimer’s disease: a potential mechanism leading to chronic inflammation. Exp Neurol. 2009;215(1):5–19. doi:10.1016/j.expneurol.2008.09.003
9. Naj AC, Jun G, Beecham GW, et al. Common variants at MS4A4/MS4A6E, CD2AP, CD33 and EPHA1 are associated with late-onset Alzheimer’s disease. Nat Genet. 2011;43(5):436–441. doi:10.1038/ng.801
10. Neilson LE, Quinn JF, Gray NE. Peripheral blood NRF2 expression as a biomarker in human health and disease. Antioxidants. 2021;10(1):28. doi:10.3390/antiox10010028
11. Johnson VE, Stewart JE, Begbie FD, Trojanowski JQ, Smith DH, Stewart W. Inflammation and white matter degeneration persist for years after a single traumatic brain injury. Brain. 2013;136(1):28–42. doi:10.1093/brain/aws322
12. Nazem A, Sankowski R, Bacher M, Al-Abed Y. Rodent models of neuroinflammation for Alzheimer’s disease. J Neuroinflammation. 2015;17(12):74. doi:10.1186/s12974-015-0291-y
13. Chen Y, Liang Z, Blanchard J, et al. A nontransgenic mouse (icv-STZ mouse) of Alzheimer’s disease: similarities to and differences from the transgenic model (3xTg-AD mouse). Mol Neurobiol. 2013;47:711–725. doi:10.1007/s12035-012-8375-5
14. Ferretti MT, Cuello AC. Does a pro-inflammatory process precede Alzheimer’s disease and mild cognitive impairment? Curr Alzheimer Res. 2011;8(2):164–174. doi:10.2174/156720511795255982
15. Kraska A, Santin MD, Dorieux O, et al. In vivo cross-sectional characterization of cerebral alterations induced by intracerebroventricular administration of streptozotocin. PLoS One. 2012;7(9):e46196. doi:10.1371/journal.pone.0046196
16. Krstic D, Knuesel I. Deciphering the mechanism underlying late-onset Alzheimer disease. Nat Rev Neurol. 2013;9(1):25–34. doi:10.1038/nrneurol.2012.236
17. Salkovic-Petrisic M, Osmanovic-Barilar J, Brückner M, Hoyer S, Arendt T, Riederer P. Cerebral amyloid angiopathy in streptozotocin rat model of sporadic Alzheimer’s disease: a long-term follow up study. J Neural Transm. 2011;118(5):765–772. doi:10.1007/s00702-011-0651-4
18. Mishra SK, Singh S, Shukla S, Shukla R. Intracerebroventricular streptozotocin impairs adult neurogenesis and cognitive functions via regulating neuroinflammation and insulin signaling in adult rats. Neurochem Int. 2018;113:56–68. doi:10.1016/j.neuint.2017.11.012
19. Kamat P, Kalani A, Rai S, et al. Streptozotocin intracerebroventricular induced neurotoxicity and brain insulin resistance therapeutic intervention for treatment of Sporadic Alzheimer’s Disease (sAD)-like pathology. Mol Neurobiol. 2016;53(7):4548–4562. doi:10.1007/s12035-015-9384-y
20. Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity, uncovering the molecular mechanicms. Nat Rev Neurosci. 2007;8(1):57–69. doi:10.1038/nrn2038
21. Salminen A. Activation of immunosuppression network in the aging process. Ageing Res Rev. 2020;57:100998. doi:10.1016/j.arr.2019.100998
22. Jones CI. Platelet function and ageing. Mamm Genome. 2016;27(7–8):358–366. doi:10.1007/s00335-016-9629-8
23. Kniewallner KM, Foidl BM, Humpel C. Platelets isolated from an Alzheimer’s mouse damage healthy cortical vessels and cause inflammation in an organotypic ex vivo brain slice model. Sci Rep. 2018;8(1):15483. doi:10.1038/s41598-018-33768-2
24. Pluta R, Ułamek-Kozioł M, Januszewski S, Czuczwar SJ. Platelets, lymphocytes and erythrocytes from Alzheimer’s disease patients: the quest for blood cell-based biomarkers. Folia Neuropathol. 2018;56(1):14–20. doi:10.5114/fn.2018.74655
25. Canobbio I, Abubaker AA, Viscante C, Torti M, Paula G. Role of amyloid peptides in vascular dysfunction and platelet dysregulation in Alzheimer’s disease. Front Cell Neurosci. 2015;9:65. doi:10.3389/fncel.2015.00065
26. Stevenson A, Lopez D, Khoo P, Kalaria RN, Mukaetova-Ladinska EB. Exploring erythrocytes as blood biomarkers for Alzheimer’s disease. J Alzheimers Dis. 2017;60:845–857. doi:10.3233/JAD-170363
27. Gonzalez H, Pacheco R. T-cell-mediated regulation of neuroinflammation involved in neurodegenerative diseases. J Neuroinflammation. 2014;11(1):201. doi:10.1186/s12974-014-0201-8
28. Mietelska-Porowska A, Wojda U. T lymphocytes and inflammatory mediators in the interplay between brain and blood in Alzheimer’s disease: potential pools of new biomarkers. J Immunol Res. 2017;2017:4626540. doi:10.1155/2017/4626540
29. Richartz-Salzburger E, Batra A, Stransky E, et al. Altered lymphocyte distribution in Alzheimer’s disease. J Psychiatr Res. 2007;41(1–2):174–178. doi:10.1016/j.jpsychires.2006.01.010
30. Saidu NEB, Kavian N, Leroy K, Jakob C, Nicco C, Batteux F. Dimethyl fumarate, a two-edged drug: current status and future directions. Med Res Rev. 2019;39(5):1923–1952. doi:10.1002/med.21567
31. Diebold M, Sievers C, Bantug G, et al. T Dimethyl fumarate influences innate and adaptive immunity in multiple sclerosis. J Autoimmun. 2018;86:39–50. doi:10.1016/j.jaut.2017.09.009
32. Carlström KE, Ewing E, Granquist M, et al. Therapeutic efficacy of dimethyl fumarate in relapsing-remitting multiple sclerosis associates with ROS pathway in monocytes. Nature Commun. 2019;10:3081. doi:10.1038/s41467-019-11139-3
33. Tahvili S, Zandich B, Amirghofran Z. The effect of dimethyl fumarate on gene expression and the level of cytokines related to different T helper cell subsets in peripheral blood mononuclear cells of patients with psoriasis. Int J Dermatol. 2015;54(7):e254–e260. doi:10.1111/ijd.12834
34. Majkutewicz I, Kurowska E, Podlacha M, et al. Dimethyl fumarate attenuates intracerebroventricular streptozotocin-induced spatial memory impairment and hippocampal neurodegeneration in rats. Behav Brain Res. 2016;308:24–37. doi:10.1016/j.bbr.2016.04.012
35. Majkutewicz I, Kurowska E, Podlacha M, et al. Age-dependent effects of dimethyl fumarate on cognitive and neuropathological features in the streptozotocin-induced rat model of Alzheimer’s disease. Brain Res. 2018;1686:19–33. doi:10.1016/j.brainres.2018.02.016
36. Spencer SR, Wilczak CA, Talalay P. Induction of glutathione transferases and NAD(P)H: quinonereductase by fumaric acid derivatives in rodent cells and tissues. Cancer Res. 1990;50(24):7871–7875.
37. Sasaki A, Koike N, Murakami T, Suzuki K. Dimethyl fumarate ameliorates cisplatin-induced renal tubulointerstitial lesions. J Toxicol Pathol. 2019;39:79–89. doi:10.1293/tox.2018-0049
38. Dhaliwal N, Dhaliwal J, Singh A, Chopra K. Dimethyl fumarate attenuates 2-VO-induced vascular dementia via activating the Nrf-2 signaling pathway in rats. Inflammopharmacology. 2021;29:537–547. doi:10.1007/s10787-020-00785-5
39. Bloch K, Gil-Ad I, Vanichkin A, et al. Intracerebroventricular streptozotocin induces obesity and dementia in Luis rats. J Alzheimers Dis. 2017;60:121–136. doi:10.3233/JAD-161289
40. Wrona D, Listowska M, Kubera M, et al. Effects of chronic desipramine pretreatment on open field-induced suppression of blood natural killer cell activity and cytokine response depend on the rat’s behavioral characteristics. J Neuroimmunol. 2014;268:13–24. doi:10.1016/j.jneuroim.2013.10.001
41. Listowska M, Glac W, Grembecka B, Grzybowska M, Wrona D. Change in blood CD4+T and CD8+T lymphocytes in stressed rats pretreated chronically with desipramine are more pronounced after chronic open field stress challenge. J Neuroimmunol. 2015;282:54–62. doi:10.1016/j.jneuroim.2015.02.015
42. Glac W, Dunacka J, Grembecka B, Światek G, Majkutewicz I, Wrona D. Prolonged peripheral immunosuppressive responses as consequence of random amphetamine treatment, amphetamine withdrawal and subsequent amphetamine challenges in rats. J Neuroimmune Pharmacol. 2021. doi:10.1007/s11481-021-09988-1
43. Grembecka B, Glac W, Listowska M, et al. Subthalamic deep brain stimulation affects plasma corticosterone concentration and peripheral immunity changes in rat model of Parkinson’s disease. J Neuroimmune Pharmacol. 2021;16(2):454–469. doi:10.1007/s11481-020-09934-7
44. Ulrich-LAI YM, Herman JP. Neural regulation of endocrine and autonomic stress response. Nat Rev Neurosci. 2009;10:397–409. doi:10.1038/nrn2647
45. Montes D, Fraussen J, Wijmeersch B, Hupperts R, Somers V. Dimethyl fumarate induces a persistent change in the composition of the innate and adaptive immune system in multiple sclerosis patients. Sci Rep. 2018;8:8194. doi:10.1038/s41598-018-26519-w
46. Longbrake EE, Cantoni C, Chahin S, Cignarella F, Cross AH, Piccio L. Dimethyl fumarate induces changes in B- and T-lymphocyte function independent of the effects on absolute lymphocyte count. Mult Scler. 2018;24(6):728–738. doi:10.1177/1352458517707069
47. Vego H, Sand KL, Hoglund RA, et al. Monomethyl fumarate augments NK cell lysis of tumor cells through degranulation and the upregulation of NKp46 and CD107a. Cell Mol Immunol. 2016;13(1):57–64. doi:10.1038/cmi.2014.114
48. Smith MD, Calabresi P, Bhargava P. Dimethyl fumarate treatment alters NK cell function in multiple sclerosis. Eur J Immunol. 2018;48(2):380–383. doi:10.1002/eji.201747277
49. Crome SQ, Lang PA, Lang KS, Ohashi PS. Natural killer cells regulate diverse T cell responses. Trends Immunol. 2013;34(7):342–349. doi:10.1016/j.it.2013.03.002
50. Linker RA, Lee DH, Ryan S, et al. Fumaric acid esters exert neuroprotective effects in neuroinflammation via activation of the Nrf2 antioxidant pathway. Brain. 2011;134(3):678–692. doi:10.1093/brain/awq386
51. Safdar A, deBeer J, Tarnopolsky MA. Dysfunctional Nrf-2-Keap1 redox signaling in skeletal muscle of the sedentary old. Free Radic Biol Med. 2010;49:1487–1493. doi:10.1016/j.freeradbiomed.2010.08.010
52. Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases, physiology and pathology. Physiol Rev. 2007;87:245–313. doi:10.1152/physrev.00044.2005
53. Ghadiri M, Rezk A, Li R, et al. Dimethyl fumarate-induced lymphopenia in MS due to differential T-cell subset apoptosis. Neurol Neuroimmunol Neuroinflamm. 2017;4:e340. doi:10.1212/NXI.0000000000000340
54. Sainz de la Maza S, Medina S, Villarrubia N, et al. Factors associated with dimethyl fumarate-induced lymphopenia. J Neurol Sci. 2019;398(4):4–8. doi:10.1016/j.jns.2019.01.007
55. Spencer CM, Crabtree-Hartman EC, Lehman-Horn K, Cree BA, Zamvil SS. Reduction of CD8+ T lymphocyte in multiple sclerosis patients treated with dimethyl fumarate. Neurol Neuroimmunol Neuroinflamm. 2015;2(3):e76. doi:10.1212/NXI.0000000000000076
56. Berkovich R, Weiner IP. Effects of dimethyl fumarate (Teefidera) on lymphocyte subsets. Mult Scler Relat Disord. 2015;4(4):339–341. doi:10.1016/j.msard.2015.06.002
57. Chaves C, Ganguly R, Ceresia C, Camac A. Lymphocyte subtypes in relapsing-remitting multiple sclerosis patients treated with dimethyl fumarate. Mult Scler J Exp Transl Clin. 2017;3(2):2055217317702933. doi:10.1177/2055217317702933
58. Longbrake EE, Ramsbottom MJ, Cantoni C, Ghezzi L, Cross AH, Piccio L. Dimethyl fumarate selectively reduces memory T cells in multiple sclerosis patients. Mult Scler. 2016;22(8):1061–1070. doi:10.1177/1352458515608961
59. Medina S, Villarrubia N, Sainz de la Maza S, et al. Optimal response to dimethyl fumarate associates in MS with a shift from an inflammatory to a tolerogenic blood cell profile. Mult Scler. 2018;24(10):1317–1327. doi:10.1177/1352458517717088
60. Najjar E, Staun-Ram E, Volkowich A, Miller A. Dimethyl fumarate promotes B cell-mediated anti-inflammatory cytokine profile in B and T cells, and inhibits immune cell migration in patients with MS. J Neuroimmunol. 2020;343:577230. doi:10.1016/j.jneuroim.2020.577230
61. Albrecht P, Bouchachia I, Goebels N, et al. Effects of dimethyl fumarate on neuroprotection and immunomodulation. J Neuroinflammation. 2012;9(1):163. doi:10.1186/1742-2094-9-163
62. Borger P, Postma DS, Vellenga E, Kaufman HF. Regulation of asthma-related T-cell cytokines by the cyclic AMP-dependent signaling pathway. Clin Exp Allergy. 2000;30:920–926. doi:10.1046/j.1365-2222.2000.00794.x
63. Fiedler SE, Kerns AR, Tsang C, Tsang V, Bourdette D, Salinthone S. Dimethyl fumarate activates the prostaglandin EP2 receptor and stimulates cAMP signaling in human peripheral blood mononuclear cells. Biochem Biophys Res Commun. 2016;475:19–24. doi:10.1016/j.bbrc.2016.05.021
64. Zidek Z. Adenosine-cyclic AMP pathway and cytokine expression. Eur Cytokine Netw. 1999;10:319–328.
65. Nakagawa K, Kiko T, Kuriwada S, Miyazawa T, Kimura F, Miyazawa T. Amyloid β induces adhesion of erythrocytes to endothelial cells and affects endothelial viability and functionality. Biosci Biotechnol Biochem. 2011;75(10):2030–2033. doi:10.1271/bbb.110318
66. Krishnamoorthy S, Pace B, Gupta D, et al. Dimethyl fumarate increases fetal hemoglobin, provides heme detoxification, and corrects anemia in sickle cell disease. JCI Insight. 2017;2(20):e96409. doi:10.1172/jci.insight.96409
67. Macari ER, Lowrey CH. Induction of human fetal hemoglobin via NRF-2 antioxidant response signaling pathway. Blood. 2011;117(22):5987–5997. doi:10.1182/blood-2010-10-314096
68. Suidan GL, Singh PK, Patel-Hett S, et al. Abnormal clotting of the intrinsic/contact pathway in Alzheimer disease patients is related to cognitive ability. Blood Adv. 2018;2(9):954–963. doi:10.1182/bloodadvances.2018017798
69. Yadav SK, Soin D, Ito K, Dhib-Jalbut S. Insight into the mechanism of action of dimethyl fumarate in multiple sclerosis. J Mol Med. 2019;97(4):463–472. doi:10.1007/s00109-019-01761-5
70. Kunze R, Urrutia A, Hoffmann A, et al. Dimethyl fumarate attenuates cerebral edema formation by protecting the blood-brain barrier integrity. Exp Neurol. 2015;266:99–111. doi:10.1016/j.expneurol.2015.02.022
71. Lim JL, van der Pol SM, Di Dio F, et al. Protective effects of monomethyl fumarate at the inflamed blood-brain barrier. Microvasc Res. 2016;105:61–69. doi:10.1016/j.mvr.2015.12.003
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.