Back to Journals » Clinical Ophthalmology » Volume 14
Difluprednate 0.05% versus Prednisolone Acetate Post-Phacoemulsification for Inflammation and Pain: An Efficacy and Safety Clinical Trial
Authors Palacio-Pastrana C , Chávez-Mondragón E , Soto-Gómez A , Suárez-Velasco R, Montes-Salcedo M, Fernández de Ortega L, Nasser-Nasser L , Baiza-Durán L, Olvera-Montaño O , Muñoz-Villegas P
Received 20 March 2020
Accepted for publication 20 May 2020
Published 12 June 2020 Volume 2020:14 Pages 1581—1589
DOI https://doi.org/10.2147/OPTH.S254705
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Claudia Palacio-Pastrana,1 Eduardo Chávez-Mondragón,2 Abraham Soto-Gómez,3 Rubén Suárez-Velasco,4 Miguel Montes-Salcedo,5 Lourdes Fernández de Ortega,6 Linda Nasser-Nasser,7 Leopoldo Baiza-Durán,8 Oscar Olvera-Montaño,8 Patricia Muñoz-Villegas8
1Fundación Hospital Nuestra Señora de la Luz, IAP, CDMX, Mexico; 2Fundación de Asistencia Privada Conde de Valenciana, IAP, CDMX, Mexico; 3Catarata y Glaucoma de Occidente SA de CV, Guadalajara, Jalisco, Mexico; 4Private Office, Guadalajara, Jalisco, Mexico; 5Aris Vision Institute, Guadalajara, Jalisco, Mexico; 6Asociación Para Evitar la Ceguera en México, IAP, CDMX, Mexico; 7Visión Cirugía Ambulatoria, Monterrey, Nuevo Leon, Mexico; 8Medical Affairs, Laboratorios Sophia, SA de CV, Zapopan, Jalisco, Mexico
Correspondence: Patricia Muñoz-Villegas Tel +52 3001 4200 ext 1018
Email [email protected]
Background: The purpose of this study was to compare the efficacy and safety of difluprednate 0.05% (PRO-145) versus prednisolone acetate 1% (Prednefrin® SF), for management of postoperative inflammation and pain, after cataract surgery.
Methods: This was a Phase III, multicenter, prospective, double-blind, clinical trial. Intent-to-treat population included 178 post-phacoemulsification patients that were assigned to receive either PRO-145, or prednisolone. One day after unilateral eye surgery, patients instilled a drop 4 times a day for 14 days (then tapering the dose downward for 14 days). The primary efficacy endpoints were anterior chamber (AC) cell grade and flare. Other parameters measured included: retinal central thickness (measured via OCT), conjunctival hyperemia, edema, pain and photophobia. Tolerability and safety were assessed through burning, itching, foreign body sensation, visual acuity (VA), intraocular pressure (IOP) and incidence of adverse events (AE).
Results: A total of 171 subjects were randomized (1:1) and completed the study. Compared to day 1, there was a significant improvement in the AC cell count and flare in both groups by the final visit (80.2% vs 88.4%, p=1.000). Conjunctival hyperemia improved in a similar fashion (81.2% vs 79%, p=0.234) in both PRO-145 and prednisolone groups, without differences between them. This was also observed for edema (82.4% vs 82.5%, p=0.246), pain (15.3% vs 7%, p=0.497) and photophobia (16.4% vs 15.1%, p=0.246), respectively. There was no significant difference between treatments for any tolerability parameter studied. Finally, at the 4-week postoperative visit, there were no significant differences between treatments for VA, IOP and AE results (p-values; 0.095, 0.053 and 0.099, respectively).
Conclusion: The results of this study suggest that PRO-145 is as effective and safe as prednisolone acetate in treating postoperative inflammation and pain in patients undergoing phacoemulsification. The study was registered at ClinicalTrials.gov as NCT03693989.
Keywords: difluprednate, dose-reduction, cataract surgery, ophthalmic corticosteroids
Introduction
Cataract surgery is one of the most common ophthalmologic surgical procedures. In Latin America, this procedure’s rate has increased by 70% since 2005.1 Ocular inflammation is common after ocular surgery, and can lead to complications such as pain, discomfort, corneal edema, and elevation in intraocular pressure (IOP), among others.2–5 Topical corticosteroids are routinely used as postoperative ocular anti-inflammatory drugs.6,7 Several studies have demonstrated that topical steroids are safe and effective in reducing ocular inflammation when administered during surgery and throughout the immediate 4 weeks following cataract extraction.8,9 Difluprednate ophthalmic emulsion is a topical ocular corticosteroid that has been approved by the US Food and Drug Administration (FDA) since 2008 as treatment for the inflammation and pain associated with ocular surgery.4,7,9–11 However, in Mexico and Latin America, it is not currently commercially available. Difluprednate is an ophthalmic steroid with high glucocorticoid receptor-binding affinity and high tissue penetration, therefore it is considered more potent than other topically applied steroids.12–14 Ocular inflammation is assessable through clinical signs such as anterior chamber (AC) cellularity and flare, corneal edema, elevated IOP, and cystoid macular edema (CME). Symptoms such as ocular pain, photophobia, tearing, blurry vision, and itching may also be associated with ocular inflammation.11,13,15 Steroids have been proven to cause IOP elevation; however, it has been reported that difluprednate administered 2 times daily for 30 days, resulted in no significant differences in the mean or IOP elevation percentages compared to prednisolone acetate after cataract surgery. These results can be attributed to a low-frequency dosing during a short period of steroid use.4 The present study used prednisolone acetate 1% as the reference article because it is considered as the gold standard treatment for post-operative ocular inflammation.6 A homogeneous population of patients suffering cataract, but no other ocular comorbidities, was included in this study.
The current study was designed to evaluate the efficacy and safety of difluprednate 0.05% (PRO-145) ophthalmic emulsion as treatment for postoperative inflammation and pain following phacoemulsification surgery.
Methods
Study Design
This was a phase III, parallel, prospective, double-blind, multicenter clinical trial. The study was registered in ClinicalTrials.gov as NCT03693989. It was conducted in seven centers in Mexico. An ethics committee in each center reviewed and approved the study’s protocol and the informed consent form (see Ethics approval section). This study was conducted in compliance with the Declaration of Helsinki and in accordance with Good Clinical Practices Standards. All patients that participated provided written and signed informed consent. Patients were recruited between October 2018 and August 2019 (FPFV: 10/04/18 and LPLV:08/07/19). The patients were enrolled during the post-operative visit on day 1.
Participants
Inclusion criteria included patients, men and women (aged ≥18 years), who underwent unilateral phacoemulsification (LOCS III cataract classification; NO ≥ 2 and NC ≥ 2). Exclusion criteria included patients with history of diabetes, hypertension, IOP ≥ 24 mmHg, glaucoma or patients with active or chronic systemic diseases and/or under pharmacological treatment including immunomodulators, corticoids, topical ocular drops and systemic medication that may have affected the study’s outcomes, patients with ocular surface alterations such as pterygium and pinguecula, any type of corneal ulcers, history of uveitis, eye infectious diseases, any illness that could interfere with study parameters, history of penetrating keratoplasty, macular diseases, simultaneous bilateral cataract surgery, subjects with a single functional eye, individuals with any known hypersensitivity to drugs used in this study and patients either pregnant, breastfeeding or with high childbearing potential and no birth control method.
Treatment and Evaluations
One hundred and seventy-eight patients were randomized in a 1:1 ratio to receive either difluprednate emulsion 0.05% (PRO-145, Laboratorios Sophia, SA de CV, Zapopan, Jalisco, Mexico; n=88) or prednisolone acetate 1% (Prednefrin® SF, Allergan, Inc., Irvine, CA, USA; n=90) through computer software randomization numbers (SAS Institute, Inc, Cary, NC, USA). Patients instilled a drop of the study drug topically in the inferior conjunctival sac QID for 14 days after surgery, followed by tapering for 14 days, this regimen depended on the investigator’s assessment of the subject’s individual response to treatment. All the researchers and other sponsoring team members were blind to treatment assignment throughout the study. Follow-up visits took place on days 1, 7, 14 and 29 ± 3 after surgery (randomization). A safety call was carried out 2 weeks after the final visit (43 ± 3 days). The study drug was discontinued if either the principal investigator or the patient judged that it was not in the latter’s best interest to continue, or if a female patient got pregnant.
Study Endpoints
Efficacy Assessments
The primary efficacy endpoint was the anterior chamber (AC) cell grade and flare compared to day 1, evaluated by biomicroscopy. Using a light beam of 0.2 x 0.2 mm directed obliquely to the AC with a forward inclination of the light source (slit lamp tower) the degree of flare and cellularity were determined according to the standardization of the uveitis nomenclature, see Table 1.16 Other parameters measured included retinal central thickness measured via optical coherence tomography (OCT) performed using a Zeiss OCT Stratus scanner (Carl Zeiss Meditec, Inc., Dublin, CA, USA), a Zeiss OCT Cirrus scanner (Carl Zeiss Meditec, Inc., Dublin, CA, USA) or a Heidelberg OCT Spectralis scanner (Heidelberg Engineering, Inc., Franklin, MA, USA). Changes in conjunctival hyperemia and corneal edema were assayed with the Efron scale. Inflammatory symptoms assessed were pain and photophobia. Tolerability assessments: Patients were questioned about their satisfaction with their treatment. The tolerability was measured through the ocular symptomatology post-installation determined by burning, itching and foreign body sensation.
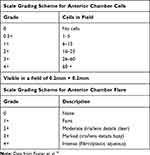 |
Table 1 Standardized Grading Scales for Uveitis |
Safety Assessments
Safety was evaluated through visual acuity (VA), intraocular pressure (IOP), and the incidence of adverse events (AE). IOP was measured using a calibrated Goldmann applanation tonometer. Visual acuity was determined with a Snellen chart and expressed in LogMAR values.
Statistical Analysis
Statistical analysis was carried out using SPSS 19.0 software for Windows (SPSS Inc., Chicago, IL, USA). Efficacy evaluations were performed only in per-protocol population (PP), established as a randomized patient with no major deviation from the protocol after conducting a bivariate analysis. All the patients who were enrolled in the study were included in the tolerability and safety analyses (intent-to treat population, ITT). Sample size calculation was performed to test the increase in the number and percentage of patients with AC cell grade of 0 (i.e., no cells) at the end of 14-day treatment period, with a complete clearing of AC cells count of 78.9% in the difluprednate group versus 77.5% in the prednisolone group.9,17 A sample size of 148 cases (eyes) was determined, with an alpha of 0.05 and 80% power. Therefore, 89 eyes were considered per group, allowing as much as 20% of excluded cases in the event of major protocol deviations. The Kolmogorov–Smirnov and Shapiro–Wilks tests were performed to evaluate normality of the continuous data (details not shown). The continuous variables were analyzed using the Student’s t-test. The ordinal variables were analyzed using 2 x 2 contingency tables and the differences were calculated with Pearson Chi-square test or Fisher’s exact test. All statistical analyses performed in this study were 2 sided (p≤0.05).
Results
Characteristics of the participants
A total of 178 patients who underwent unilateral phacoemulsification (≤24 hrs after surgery) were enrolled. Out of this group, 6 discontinued their participation because of the presentation of either adverse events (myocardial infarction and urinary tract infection, 2/6), or protocol deviations (4/6). Another patient was excluded from the efficacy assessment due to poor adherence, determined as <80% of indicated treatment (prednisolone group). Therefore, 85 (85/88, 96.6%) patients in group PRO-145 (difluprednate 0.05%), and 86 (86/90, 95.5%) in group prednisolone (Prednefrin® SF) completed the entire protocol without deviations up to day 29 ± 3, see Figure 1.
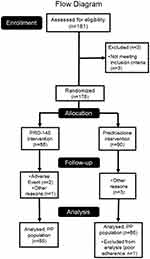 |
Figure 1 Current flow diagram of patients enrolled in the study. |
There were no demographic or clinically relevant differences at day 1 between treatment groups. Mean age ± standard deviation (SD) was 65.3 ± 12.0 years (range 18–89); 54.4% of patients were female. Clinical signs and symptoms were similar between groups, see Table 2.
 |
Table 2 Initial Characteristics of Each Group (n=171 Completed Patients) |
Drug administration was discontinued after day 14 for 69 patients while 102 followed a dose-reduction regime (PP population), with the following administration scheme: 2 times daily for one week (8.8%), 2 times daily for the first week and once daily for the second week (49%), once daily for two weeks (15.7%), and other regimes (26.5%). Remarkably, there were no significant differences between treatment groups (X2(3) =1.663, p=0.645), and the regime selection was dependent on the investigator’s criteria.
Efficacy
Anterior Chamber Cell Count and Flare
In the PP population (n=171), the percentage of patients with grade 0 (no cells) at day 1 (primary efficacy endpoint) was similar between PRO-145 and prednisolone groups (3.5% vs 2.3%, X2(1)=0.218, p=0.682). By the final visit, there was a significant improvement in AC cell grade in both groups, compared to day 1 (p=0.0001). The percentage of patients with AC cell grade of 0 (i.e., no cells), increased for the PRO-145 group by D7 (41.2% [n=35] vs 3.5% [n=3], p=0.0001), D14 (77.6% [n=66] vs 3.5%, p=0.0001) and by the end of 29-day protocol period (88.2% [n=69] vs 3.5%, p=0.0001). Meanwhile, for the prednisolone group this increase was also observed by D7 (27.9% [n=24] vs 2.3%, p=0.0001), D14 (80.2% [n=69] vs 2.3%, p=0.0001) and D29 (88.4% [n=76] vs 2.3%, p=0.0001). No significant differences were observed between groups at any time point (p-values; 0.078, 0.711 and 1.000 at D7, D14 and D29, respectively).
Similar findings were observed in the analysis of AC flare; the percentage of patients with a grade 0 (none) was statistically similar between PRO-145 (44.7%, n=38) vs prednisolone (41.9%, n=36), at day 1 (X2(1)=0.141, p=0.759). The treatment groups had a similar increase in the percentage of patients with flare grade 0 by D7 (96.5% [n=82] vs 90.7% [n=78], X2(1)=2.367, p=0.211), D14 (98.8% [n=84] vs 100% [n=86], X2(1)=1.018, p=0.497) and final visit (98.8% [n=84] vs 100% [n=86], X2(1)=1.018, p=0.497), see Table 3.
 |
Table 3 Assessment of Inflammation on Day 29 (PP Population) |
Retinal Central Thickness
At day 29, there was a slight increase on retinal thickness (by OCT) compared to day 1 in both PRO-145 and prednisolone groups (t(1)=4.329, p=0.144). At the final visit, the retinal thickness had increased in 149 patients (87.1%, 149/171) in both groups, 134 patients had an increase between 1–25 µm (66 on PRO-145 and 68 on prednisolone group), 10 patients had an increase between 26–50 µm (5 on each group), and 5 patients had an increase between 51–70 µm (1 on PRO-145 and 4 on prednisolone). The mean ± SD was 253.59 ± 26.97 µm for PRO-145 vs 261.33 ± 29.42 µm for the prednisolone group, at the final visit (t(169)=−1.792, p=0.075). The increase at the final visit for PRO-145 was 10.25 ± 11.96 µm vs 13.15 ± 14.58 µm in the prednisolone group; however, these findings were not statistically different in the analysis between groups (t(169)=−1.423, p=0.156).
Conjunctival Hyperemia and Corneal Edema
After 1 week of treatment, compared to day 1, there was a significant reduction in the conjunctival hyperemia (Efron scale) in both groups (p=0.0001); 40% for the PRO-145 group and 30.2% for the prednisolone group. By day 14, the reduction on hyperemia’s prevalence was 58.8% and 57%, and by day 29, it was 81.2% versus 75.6% for both groups, respectively. There were no significant differences between treatments in the incidence of conjunctival hyperemia at each visit (p-values; 0.804, 0.168, 0.744 and 0.234, respectively).
Similar improvement was observed in corneal edema, after 7 days of treatment compared to day 1, PRO-145 group had a reduction of 50.6% vs 54.6% in the prednisolone group (p=0.0001 vs D1); on day 14 the reduction was 73% for PRO-145 and 70.9% in the prednisolone group; and on day 29 it was 84.2% for the PRO-145 group vs 82.5% in the prednisolone group. At each visit, there were no differences between PRO-145 and prednisolone groups (p-values; 0.536, 1.000, 0.352 and 0.246, respectively), see Table 3.
Pain and Photophobia
All groups showed a significant reduction of ocular symptomatology throughout the study. At day 1 after cataract surgery, 16.5% in difluprednate group and 7% in prednisolone reported pain (X2(1)=3.731, p=0.060). As shown in Table 3, the decline in the presence of ocular pain was 15.3% for the PRO-145 group vs 7% in prednisolone. An 18.8% in the PRO-145 group vs 15.1% in the prednisolone group presented photophobia at day 1 (X2(1)=0.417, p=0.547). However, by day 29, PRO-145 and prednisolone both presented a similar decrease of photophobia, 16.4% vs 15.1%, respectively. By the final visit, 29 days after surgery, the ocular symptomatology showed significant improvement in both groups (p=0.0001). No significant differences were observed between groups (p-values; 0.497 and 0.246, respectively).
Tolerability
Discomfort
Burning, itching and FBS, were considered the parameters of tolerability. Both study drugs were well tolerated. For the ITT population at day 29, the decline in burning sensation reported was 15.4% for the PRO-145 vs 7.7% for the prednisolone group (p=0.346). Findings were similar for the analysis of itching and FBS between treatments. More than 58% of patients in each treatment group reported no discomfort after drop instillation.
Safety
Visual Acuity (LogMAR)
Data on safety were analyzed for the to-be-treated population (ITT). After the intervention time, the VA increased from day 1 (0.622 ± 0.3 vs 0.609 ± 0.2) to final visit (0.917 ± 0.1 vs 0.875 ± 0.2) in PRO-145 and prednisolone groups, respectively, p=0.0001. There were no significant differences between treatments at any visit (p-values; 0.510, 0.178, 0.122 and 0.095, D1, 7, 14 and 29, respectively).
Intraocular Pressure (IOP)
At day 7, one patient showed an IOP >30 mmHg, and at day 14, another patient showed an IOP of 25 mmHg (both reported as an AE); both patients showed normal IOP by day 29 and belonged to the PRO-145 group. For the PRO-145 group, the initial IOP was 14.9 ± 3.6 mmHg, decreasing to 13.9 ± 3.0 mmHg after 29 days of treatment, a reduction of 1.04 mmHg. Meanwhile, for the patients in the prednisolone group, the initial IOP was 15.1 ± 3.1 mmHg, decreasing to 13.0 ± 2.4 mmHg, 2.11 mmHg lower than that of visit 1. No differences were observed between groups at any visit (p-values; 0.152, 0.071, and 0.053, respectively), see Figure 2.
 |
Figure 2 Change in intraocular pressure (mmHg) on ITT population. Mean ± SD following PRO-145 (black circle) and prednisolone (black square) treatment after phacoemulsification, p>0.05. |
Adverse Events (AE)
Two patients discontinued their participation because of AE, but they were non-related to their treatment (PRO-145 group). Both study drugs were safe, and only 17 AE were related to PRO-145 and prednisolone. A total of 182 AE were reported by 56.2% (100/178) of the patients randomized during the protocol. There were no significant differences between treatments in the incidence of AE (X2(1)=2.824, p=0.099). A total of 93 AE were reported for PRO-145 (89 ocular-EA and 4 non-ocular), and 89 for prednisolone (79 ocular-EA and 10 non-ocular); there were no significant differences in the ocular or non-ocular AE between treatments (X2(1)=3.080, p=0.098). AE were classified as mild (90.7%), moderate (8.8%) or severe (0.5%). Only one serious AE occurred during the study; however, it was not related to the products evaluated (PRO-145, myocardial infarction). On day 29, one patient presented diabetic macular edema, and another patient CME (Irvine-Gass syndrome), both AE were recognized as non-serious (prednisolone group). The AE are shown in Table 4.
 |
Table 4 Treatment-Related Adverse Events |
Discussion
Phacoemulsification as a cataract surgery technique was proposed over 40 years ago, and advances in surgical approaches and equipment have increased its safety and efficiency.13,18,19 However, post-operative ocular inflammation remains as a cause of visual impairment and pain. The degree of postoperative inflammation following cataract surgery is related to several surgery-dependent factors such as technique used, intraocular lens type, and to patient-dependent factors such as degree of iris pigmentation, and history of inflammatory disease, among others.5,11 Difluprednate is a prednisolone-derived molecule used for treating postoperative ocular inflammation and pain.4,7,9,10,13 Advantages of this formulation include dose uniformity and better bioavailability.14,20 Difluprednate was formulated as a stable oil-in-water emulsion to bring optimum dosage consistency.7 The pharmacokinetics of a single ocular instillation of generic 0.05% difluprednate emulsion (PRO-145) have been evaluated in a preclinical study in New Zealand white rabbits. PRO-145 penetrates different ocular tissues (conjunctiva, cornea and aqueous humor) and has a similar pharmacokinetic profile compared to currently commercially available difluprednate (Durezol®, Alcon Laboratories, Inc., Fort Worth, TX, USA).14 It is distributed to the anterior and posterior segments via both trans-corneal and non-corneal absorption routes.14,21 Even though results in animal models cannot be extrapolated directly to administration in humans, the results of this study were consistent with other similar studies.21
On the other hand, during 2014, the rate of cataract surgery in Mexico was estimated at 1530 surgeries per million inhabitants.22 Therefore, controlled research in Mexican population after ocular surgery to determine the security and efficacy of PRO-145 was needed. The current study included a homogeneous population of patients affected by cataract but with no other ocular comorbidities, since the cataract surgery rate has increased by almost a 70% since 2005 in Latin America,1,22 where difluprednate emulsion is not commercially available. Even though previous clinical studies examined difluprednate versus placebo, prednisolone acetate, loteprednol or in pediatric population,9,11,13,20,23 prednisolone acetate 1% was used in this study since it is considered standard choice of the treatment of inflammation following ocular surgery.6,13,23 In the current study, we found that the anti-inflammatory effects of PRO-145 and prednisolone in eyes subjected to phacoemulsification were statistically equivalent. Both treatment groups showed similar resolution of AC cell grade, as well AC flare. Also, there was a slight retinal central thickness increase in both PRO-145 and prednisolone groups as measured by OCT on day 29, but this finding was not considered statistically or clinically significant. The increase was higher for the prednisolone group at the final visit (13.15 µm vs 10.25 µm, p=0.156). In addition, two patients in the prednisolone group developed CME. There were no significant differences between PRO-145 and prednisolone for the reduction of hyperemia and corneal edema. By day 29, this reduction in presence of hyperemia was 81.2% for PRO-145 versus 75.6% in the prednisolone group. These results were in concordance with previous studies of difluprednate administered after phacoemulsification.6,11,13,20 Throughout their postoperative course, patients in both treatments also showed a statistically similar resolution of pain/discomfort and photophobia.
For this study, the dosage for both treatments was through a fixed schedule for the first 14 days after surgery; this was followed by a 14-day tapering period on 60% of patients in both treatments. This might be an important variable to try to control. However, the difference in dosage between the most common tapering drug administration regimes was not statistically different between difluprednate and prednisolone. A potential concern with a high-dose of potent topical steroids is the possibility of IOP elevation.6,11 Elevation of IOP in the early postoperative period is a common result of cataract removal surgery, which could be aggravated by steroid-induced hypertension.4,7,11 IOP was not significantly elevated from day 1 during treatment with neither PRO-145 or prednisolone acetate, but was higher at day 7 and 14 for two PRO-145 patients (39 and 25 mmHg); however, by the final visit both patients’ IOP had normalized. Hence, we are in agreement with previous studies, regarding suggested additional vigilance when using corticosteroids in treating patients with ocular hypertension risk factor. On day 29 after surgery, the differences between both drugs were less than 1 mmHg (13.9 ± 3.0 mmHg vs 13.0 ± 2.4 mmHg). This is consistent with the results produced by Donnenfeld et al,11 who evaluated the efficacy of difluprednate emulsion 0.05% in a pulse-dosed versus prednisolone acetate in cataract surgery. The investigators concluded that a high-dose pulsed-therapy regimen reduced inflammation without any unexpected IOP elevation.
When comparing the efficacy and safety of difluprednate and prednisolone acetate, differences in drug delivery formulations and regimens should be taken into consideration. In our study, both study drugs were safe, effective and well tolerated, in accordance with other similar studies.9,10 Regardless of literature reports of AE due to the use of steroids, the presence of AE in both groups showed no statistically significant differences. PRO-145 and prednisolone acetate are equally safe. In our study, PRO-145 was found to have a similar safety profile to prednisolone in all follow-up visits. These findings are consistent with those reported by a study of 438 patients with AC cell grade of 2 or higher, which showed that difluprednate emulsion 0.05% given 2 or 4 times a day reduced postoperative ocular inflammation and pain safely and effectively, compared to placebo.7,20
Meanwhile, diverse steroids have been introduced over the last years, difluprednate is considered more potent that prednisolone acetate, and it has enjoyed a status as the “go-to” steroid for many inflammatory conditions, such as post-operative ocular inflammation after cataract surgery.7
One limitation of this study is that the Standardization of Uveitis Nomenclature Working Group measured AC flare using a grading scale based on visibility of the iris details using slit-lamp examination.16,23 These grading systems might be inappropriate to evaluate clinical conditions where the amount of postoperative inflammation is minimal.19 Further studies should investigate the effectiveness of difluprednate emulsion using other techniques such as laser flare photometry. In addition, patients who have excessive postoperative inflammation or patients who might not be able to adhere to QID dosing postoperatively for a long period of time might benefit further from difluprednate use.6
In Mexico and Latin America, generic difluprednate emulsion 0.05% (PRO-145) is a welcome addition to the available topical steroid options to manage inflammatory response after cataract surgery. PRO-145 is as effective and safe as prednisolone acetate 1% (Prednefrin® SF) for postoperative inflammation and pain in patients undergoing phacoemulsification.
Abbreviations
AC, anterior chamber; AE, adverse events; IOP, intraocular pressure; OCT, optical coherence tomography; VA, visual acuity.
Ethics Approval
The study was approved by Comité de Ética en Investigación Centro Hospitalario Vicor, SA de CV, CHG Hospitales; Comité de Investigación Instituto Jalisciense de Investigación Clínica SA de CV; Comité de Ética en Investigación Médica Sur, SAB de CV; Comité de Ética en Investigación Asociación para Evitar la Ceguera en México, IAP and Comité de Ética en Investigación Fundación de Asistencia Privada Conde de Valenciana, IAP.
Acknowledgments
This study was sponsored by Laboratorios Sophia, SA de CV (Zapopan, Jalisco, México). The funder provided support in the form of salaries for authors (LBD, OOM and PMV), but did not have any additional role in the data collection. The authors thank Alejandra Sánchez Rios, MD for the medical writing support. The data that supports the findings of this study are openly available in Open Science Framework (https://osf.io) as DOI 10.17605/OSF.IO/ZA5TM.
Author Contributions
All authors made substantial contributions to conception and design, acquisition data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
LBD, OOM, and PMV are employee of Laboratorios Sophia, SA de CV. The authors report no other conflicts of interest in this work.
References
1. Batlle JF, Lansingh VC, Reskinoff S, et al. The cataract simulation in Latin America: barriers to cataract surgery. Am J Ophthalmol. 2014;158(2):242–250.e1. doi:10.1016/j.ajo.2014.04.019
2. Chaudhary N, Arora I, Gupta DC, Gupta CP. Comparison of efficacy and safety of dexamethasone 0.1% and difluprednate 0.05% in the management of ocular inflammation after phacoemulsification. J Evol Med Dent Sci. 2015;4(74):12899–12903. doi:10.14260/jemds/2015/1860
3. Apple DJ, Solomon KD, Kostick AM, et al. Posterior capsule opacification. Surv Ophthalmol. 1992;37(2):73–116. doi:10.1016/0039-6257(92)90073-3
4. Tijunelis MA, Person E, Shahzad IM, et al. Comparison of prednisolone acetate 1.0% and difluprednate ophthalmic emulsion 0.05% after cataract surgery: incidence of postoperative steroid-induced ocular hypertension. J Cataract Refract Surg. 2017;43(2):223–227. doi:10.1016/j.jcrs.2017.02.002
5. El-Harazi SM, Feldman RM. Control of intra-ocular inflammation associated with cataract surgery. Curr Opin Ophthalmol. 2001;12(1):4–8. doi:10.1097/00055735-200102000-00002
6. Garg P, Tuteja N, Qayum S. To study the efficacy of difluprednate ophthalmic emulsion and prednisolone acetate ophthalmic suspension on post-operative inflammation in cataract surgery. J Clin Diagn Res. 2016;10(12):NC05–NC08. doi:10.7860/JCDR/2016/21690.9035
7. Donnenfeld ED. Difluprednate for the prevention of ocular inflammation postsurgery: an update. Clin Ophthalmol. 2011;5:811–816. doi:10.2147/OPTH.S6541
8. Rajpal RK, Fong R, Comstock TL. Loteprednol etabonate ophthalmic gel 0.05% following cataract surgery: integrated analysis of two clinical studies. Adv Ther. 2013;30(10):907–923. doi:10.1007/s12325-013-0059-7
9. Wilson ME, O’Halloran H, Lambert SR, et al. Difluprednate versus prednisolone acetate for inflammation following cataract surgery in pediatric patients: a randomized safety and efficacy study. Eye (Lond). 2016;30(9):1187–1194. doi:10.1038/eye.2016.132
10. Demco TA, Sutton H, Demco CJ, Raj PS. Topical diclofenac sodium compared with prednisolone acetate after phacoemulsification-lens implant surgery. Eur J Ophthalmol. 1997;7(3):236–240. doi:10.1177/112067219700700306
11. Donnenfeld ED, Holland EJ, Perry HD, et al. A multicenter randomized controlled fellow eye trial of pulse-dose difluprednate 0.05% versus prednisolone acetate 1% in cataract surgery. Am J Ophthalmol. 2011;152(4):609–617.e1. doi:10.1016/j.ajo.2011.03.018
12. Kakimoto H, Takamura Y, Inatani M, et al. Effect of 0.05% difluprednate ophthalmic emulsion on proinflammatory cytokine levels after retinal laser photocoagulation in rabbits. J Ocul Pharmacol Ther. 2018;34(5):410–415. doi:10.1089/jop.2017.0109
13. Abessi B, Brooksby L, Schultze RL. Comparison of efficacy of difluprednate 0.05% and loteprednol gel 0.5% after cataract surgery. Eye Contact Lens. 2018;44(2):S37–S42. doi:10.1097/ICL.0000000000000407
14. Mercado-Sesma A, Contreras-Rubio A, Bonilla-García J, et al. Bioavailability of generic 0.05% difluprednate emulsion in the aqueous humor, cornea, and conjunctiva of New Zealand rabbits after a single dose compared with commercial difluprednate. J Ophthalmic Inflamm Infect. 2017;7(1):10. doi:10.1186/s12348-017-0127-2
15. Lyseng-Williamson KA. Loteprednol etabonate ophthalmic gel 0.5%: a review of its use in post-operative inflammation and pain following ocular surgery. Drugs. 2013;73(9):948–958. doi:10.1007/s40265-013-0073-8
16. Foster CS, Vitale AT, editors. Diagnosis and Treatment of Uveitis. Philadelphia: WB Saunders Company; 2002.
17. Chow S-C, Shao J, Wang H. Sample Size Calculations in Clinical Research (2nd Ed). Boca Raton, FL: Chapman and Hall/CRC; 2008.
18. Linebarger EJ, Hardten DR, Shah G, Lindstrom RL. Phacoemulsification and modern cataract surgery. Surv Ophthalmol. 1999;44(2):123–147. doi:10.1016/S0039-6257(99)00085-5
19. Coassin M, De Maria M, Fontana L, et al. Anterior chamber inflammation after cataract surgery: a randomized clinical trial comparing bromfenac 0.09% to dexamethasone 0.1. Adv Ther. 2019;36(10):2712–2722. doi:10.1007/s12325-019-01076-4
20. Korenfeld MS, Silverstein SM. Difluprednate ophthalmic emulsion 0.05% for postoperative inflammation and pain. J Cataract Refract Surg. 2009;35(1):26–34. doi:10.1016/j.jcrs.2008.09.024
21. Novack GD, Robin AL. Ocular pharmacology. J Clin Pharmacol. 2016;56(5):517–527. doi:10.1002/jcph.634
22. Gomez Bastar PA, Lansingh VC, López Star EM, et al. Cataract remains the primary cause of blindness in emerging economies, including Mexico. Rev Mex Oftalmol. 2014;88(4):208–209.
23. kaur S, Sukhija J. Difluprednate versus prednisolone acetate for inflammation following cataract surgery in pediatric patients: a randomized safety and efficacy study. Eye (Lond). 2017;31(3):506. doi:10.1038/eye.2016.243
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
