Back to Journals » Infection and Drug Resistance » Volume 12
Diffuse multibacillary leprosy patient with Lucio’s phenomenon and positive anticardiolipin antibody misdiagnosed as lupus erythematosus panniculitis in the People’s Republic of China
Authors Gao W , Chen Z, Jiang H, Shi Y , Zhang W, Wang H
Received 21 January 2019
Accepted for publication 21 February 2019
Published 2 April 2019 Volume 2019:12 Pages 741—744
DOI https://doi.org/10.2147/IDR.S202386
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sahil Khanna
Wei Gao, Zhiming Chen, Haiqin Jiang, Ying Shi, Wenyue Zhang, Hongsheng Wang
Institute of Dermatology, Chinese Academy of Medical Sciences and Peking Union Medical College, Jiangsu Key Laboratory of Molecular Biology for Skin Diseases and STIs, Nanjing 210042, People’s Republic of China
Abstract: Lucio’s phenomenon (LP) is a special reactional state associated with diffuse multibacillary leprosy; both exhibit a limitative global distribution mainly in Mexico and Central America. We report a case of a 28-year-old female leprosy patient in the People’s Republic of China, together with LP and positive anticardiolipin antibody, characterized by vascular thrombosis and invasion of blood vessel walls by leprosy bacilli, causing extensive skin ulcers and followed by a large number of atrophic scars.
Keywords: Leprosy, Lucio’s phenomenon, anticardiolipin antibody
Introduction
Leprosy is a chronic granulomatous disease caused by Mycobacterium leprae that can affect the skin and peripheral nerves, and whose histopathological manifestations depend on the immunological status of the patient at the time.1 Leprosy patients with misdiagnosis and delayed treatment may suffer from peripheral nerve injury and systematic disability. According to the WHO official records, 211,973 new cases were reported globally in the year 2015, and China contributed 678 (0.32%) new cases since the disease is still a public health problem in the southwest of China.2 Lucio’s phenomenon (LP) was first described in 1852 by Lucio and Alvarado, as a diffuse form of leprosmatous leprosy, common in Mexico (23%) and Central America, but quite rare in the rest of the world.3,4 Leprosy reactions may be separated clinically and histopathologically into different types: reverse reaction (typeⅠ), erythema nodosum leprosum (typeⅡ), and LP.5 As a type Ⅲ hypersensitivity, LP can be characterized by the existence of immune complex, necrotizing vasculitis on medium-sized vessels, and invasion of Mycobacterium leprea resulting in endothelial cell proliferation, vascular wall thickening, vascular obstruction and thrombosis. We report a case of a 28-year-old female diffuse multibacillary leprosy patient with LPand positive anticardiolipin antibody in the People's Republic of China.
Case presentation
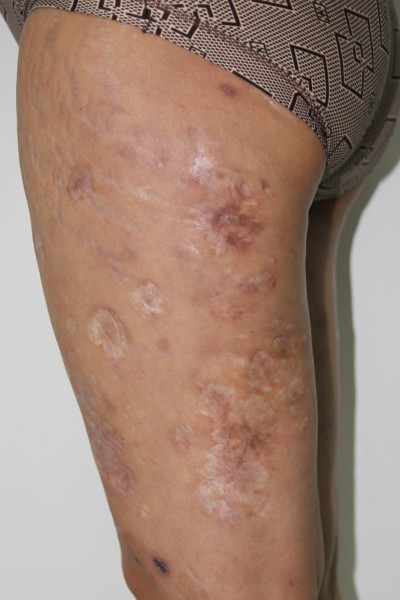
A 28-year-old female worker from the south center of China, who presented with nodules nearly all over the body for 1 year. At first, one dark-red nodule appeared on the left shoulder, gradually developed into painful ulceration and weeping, followed by more erythema, nodules, and erosions, along with recurrent fevers (the maximum temperature up to 41°C), and arthralgia at the same time. Sequentially, large amount of atrophic scars of old lesions were present, predominant on the face, lower back and extremities. At that time, the bone marrow showed normal, and laboratory tests displayed the presence of anticardiolipin (ACA) antibodies (40 RU/mL; normal <12 RU/mL) and slight increasing of erythrocyte sedimentation (ESR) and C-reaction protein (CRP), while, other laboratory results were within the normal range. The patient had been misdiagnosed as nodular panniculitis or lupus erythematosus panniculitis (LEP) in many hospitals previously and was treated with prednisone (the exact dosage is unknown), hydroxychloroquine irregularly, during the period, as nodules, ulceration and necrosis faded away, leaving atrophic scars, yet lesions relapsed frequently.
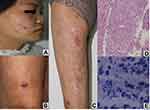
On physical examination, the patient presented dark-red soybean- to peanut-sized macula, nodules over the face and lower extremities, together with large areas of atrophic scars. she had alopecia of the lateral eyebrows, no disability degree (Figure 1A–C). Neurological examination revealed that she had mild hypoaesthesia on the extensor side of lower legs, ankles and lateral plantar area, with no tangibly thickened peripheral nerves.
Laboratory tests showed negative results for serological assays, such as HIV, syphilis and hepatitis B and C. Slit-skin smear examination of the ears and elbows revealed a bacteriological index of 4+ (indicating at least 10 bacilli per field). Serological tests of specific antibodies, including NOD-BSA and LID-1 (Infectious Disease Research Institute, Seattle, Washington, USA) by ELISA was strongly positive. And skin biopsy of one nodule on the right arm was taken and sent for histopathological examination and polymerase chain reaction (PCR). Therefore, a direct sequencing protocol targeting 16S rRNA genes of mycobacterium was applied in the tissue specimen. Sequence analysis of 16S rRNA genes indicated that 100% homology with the Mycobacterium leprae strain MRHRU-235-G chromosome. Gene sequences were analyzed using BLAST V2.0 software available at
Pathological examination on the right arm showed epidermal and dermal ulcer, necrosis, vascular wall thickening, with foam cells, vascular obstruction and thrombosis. The acid-fast staining revealed numerous uniformly stained acid fast bacilli (AFB) (Figure 1D–E).
Subsequently, combined with clinical manifestation and cutaneous biopsy and pathology manifestations, we made a diagnosis of diffuse multibacillary leprosy patient with LP. The patient first denied a history of leprosy exposure, but later remembered that during her childhood, her parents had leprosy.
With negative detections of HLA-B*1301 and negative mutations in Mycobacterium leprae drug resistance-related genes folP1, gyrA, rpoB, a proper therapy was started according to the guidelines of WHO. A MDT (multibacillary) regimen, with prednisolone at doses of 1 mg/kg per day as described in the literature for the Lucio’s phenomenon, gave partial resolution of the skin lesions. The dosage of prednisolone was gradually reduced over the next 6 months while MDT was continued until smears were negative.
Discussion
There are various clinical manifestations of leprosy, according to different types of immunological status and amount of bacillus indexes, the most well-known classified ways considered as two polar forms, including two terminal types: tuberculoid and lepromatous, among which there are three intermediate forms.6 There are three types of reactions that affect 30–50% of patients with leprosy: type Ⅰ reaction; type Ⅱ and the third or LP that was initially described in 1852 by Rafael Lucio, in collaboration with Alvarado. The latter is relatively rare and sometimes life-threatening, and there is only one case about lepromatous leprosy with LP reported in China published this year.7
The clinical manifestations of lepromatous leprosy include macular, popular, nodular, or even diffuse. The diffuse lesions of LP have a preference for the extremities, can include nodules, and heal with atrophic star-like scars.8 LP, a reaction with acute onset, is thought to happen in the lepromatous stage of the illness, and may present as a new feature in already well-established, or even untreated leprosy patients. Initially, as a new leprosy patient, without correct recognition of her expressions, severe systemic symptoms, fever and arthralgia, along with a large area of cutaneous necrosis, the leprosy disease contact history was ignored, causing a long time of misdiagnosis, even though she showed positive autoantibody, she had been given the diagnosis of LEP, which also involves the subcutaneous fat.9 Necrotic lesions mainly affect extremities, while face and trunk involvement is rarely found,6 but the lesions of our patient spread over her face and trunk as dark red macules and nodules together with specific stellate atrophic scars.
Histologically, LP has two types of patterns, one involving leukocytoclastic vasculitis as the underlying pathologic change, another referring to endothelial cell proliferation, thrombosis, a mild mononuclear cell infiltrate and ischemic necrosis. The former is believed to be caused by the deposition of immune complex caused by Mycobacterium leprae, while, in the latter pattern, vascular damage is thought to be due to the direct invasion and destruction of Mycobacterium leprae.10 It has been reported that cryoglobulin can be detected by ELISA of the leprosy patient with LP, and IgG, C3 and immune complex deposition in the dermal vascular wall, implying that LP may be an immune response mediated by the immune complex.11 However, others believe that endothelial cell injury appears to be the main event in the pathogenesis of diffuse leprosy of LP, Mycobacterium leprae entering the endothelial cell, and damaging the blood vessels directly.12 In our patient, necrosis of epidermis and dermis, marked neutrophil infiltration, thickening of vascular walls, with local foam cells, swelling of endothelial cells, and intraluminal thrombus may result in positive anticardiolipin of our patient.
It is known that leprosy can produce IgM anticardiolipin antibodies, resulting in thrombotic changes by a co-factor β2 glycosylphosphatidylinositol (GPI)-dependent mechanism.13 Recently, a study including 30 lepromatous cases showed the presence of antiphospholipid antibodies, may be useful for the early diagnosis of lepromatous cases.14 Our patient also presented positive autoantibody, which maybe related to the increased humoral immunity response. Both molecular imitation and activation of polyclonal B-cell can contribute to autoantibody production in leprosy patients.15
Nowadays, the diagnosis methods of leprosy, besides slit-skin smear test and pathological examination, molecular ways, involving PCRs technique, and leprosy specific antibody monitoring, not only help diagnosis, but also the evaluation of therapeutic effects.16 Early diagnosis and expeditious treatment is beneficial as it can improve the outcome of the disease.17 During her history, our patient accepted systemic corticosteroids, for the diagnosis of LEP, which also helped to control the condition by coincidence, but without MDT regimen, nerve impairment still progressed.
Finally, it is not easy to diagnose LP in the early stage, in low-endemic areas, because of the strong destruction of the epidermis and subcutaneous layers, including peripheral nerves, vascular walls, and other appendages, sometimes with severe systemic symptoms, so we need to keep a wary eye on the disease.
Acknowledgment
This study was supported by grants from the CAMS Innovation Fund for Medical Sciences (2016-I2M-1-005 and 2017-I2M-B&R-14).
Disclosure
The authors report no conflicts of interest in this work. The patient gave written informed consent and institutional approval is not required to publish the case details.
References
1. Walker LS, Lockwood D. Leprosy. Clin Dermatol. 2007;25:165–172. doi:10.1016/j.clindermatol.2006.05.012
2. Le W, Haiqin J, Danfeng H, et al. Monitoring and detection of leprosy patients in Southwest China: a retrospective study, 2010–2014. Sci Rep. 2018;8(1):11407. doi:10.1038/s41598-018-29753-4
3. Latapi F, Chevez Zamoro A. The ‘spotted’ leprosy of Lucio. An introduction to its clinical and histological study. Int J Leprosy. 1948;16:421.
4. Sehgal VN, Srivastava G, Sharma VK. Contemplative immune mechanism of Lucio phenomenon and its global status. J Dermatol. 1987;14:580–585.
5. Cuevas J, Rodríguez-Peralto JL, Carrillo R, Contreras F. Erythema nodosum leprosum: reactional leprosy. Semin Cutan Med Surg. 2007;26:126–130. doi:10.1016/j.sder.2007.02.010
6. Rocha RH, Emerich PS, Diniz LM, et al. Lucio’s phenomenon: exuberant case report and review of Brazilian cases. An Bras Dermatol. 2016;91(5 suppl 1):60–63. doi:10.1590/abd1806-4841.20164370
7. Wang YF, Wang P, Huang H. Lepromatous leprosy with Lucio’s phenomenon. Australas J Dermatol. 2018;59(1):62–63. doi:10.1111/ajd.12639
8. Curi PF, Villaroel JS, Migliore N, et al. Lucio’s phenomenon: report of five cases. Clin Rheumatol. 2016;35(5):1397–1401. doi:10.1007/s10067-014-2683-2
9. Massone C, Kodama K, Salmhofer W, et al. Lupus erythematosus panniculitis (lupus profundus): clinical, histopathological, and molecular analysis of nine cases. J Cutan Pathol. 2005;32(6):396–404. doi:10.1111/j.0303-6987.2005.00351.x
10. Azulay-Abulafia L, Pereira Spinelli L, Hardmann D, et al. Lucio phenomenon. Vasculitis or occlusive vasculopathy? Hautarzt. 2006;57(12):1101–1105. doi:10.1007/s00105-005-1086-3
11. Drosos AA, Brennan PJ, Elisaf MS, Stefanou SG, Papadimitriou CS, Moutsopoulos HM. Specific antigen and antibody to Mycobacterium leprae in the cryoprecipitate of a patient with Lucio phenomenon. Rheumatol Int. 1986;6(2):93–94.
12. Vargas-Ocampo F. Diffuse leprosy of Lucio and Latapí: a histologic study. Lepr Rev. 2007;78(3):248–260.
13. Ordi J, Selva A, Monegal F, Porcel JM, Martinez-Costa X, Vilardell M. Anticardiolipin antibodies and dependence of a serum cofactor. A mechanism of thrombosis. J Rheumatol. 1993;20:1321–1324.
14. Baeza I, Wong-Baeza C, Valerdi E, et al. Lepromatous leprosy patients produce antibodies that recognise non-bilayer lipid arrangements containing mycolic acids. Mem Inst Oswaldo Cruz. 2012;107:95–103.
15. Singh I, Yadav AR, Mohanty KK, et al. Molecular mimicry between HSP 65 of Mycobacterium leprae and cytokeratin 10 of the host keratin; role in pathogenesis of leprosy. Cell Immunol. 2012;278:63–75. doi:10.1016/j.cellimm.2012.06.011
16. Vera-Cabrera L, Escalante-Fuentes WG, Gomez-Flores M, et al. Case of diffuse lepromatous leprosy associated with “Mycobacterium lepromatosis”. J Clin Microbiol. 2011;49:4366–4368. doi:10.1128/JCM.05634-11
17. Choon SE, Tey KE. Lucio’s phenomenon: a report of three cases seen in Johor, Malaysia. Int J Dermatol. 2009;48(9):984–988. doi:10.1111/j.1365-4632.2009.04078.x
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.