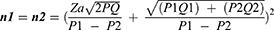Back to Journals » Vascular Health and Risk Management » Volume 18
Difference in GFAP Levels in POCD and Non-POCD Patients After on Pump CABG
Authors Nurcahyo WI, Hadisaputro S , Muttaqin Z, Boom CE, Manapa CH, Pramadika T, Tugasworo D
Received 18 August 2022
Accepted for publication 16 November 2022
Published 30 December 2022 Volume 2022:18 Pages 915—925
DOI https://doi.org/10.2147/VHRM.S386791
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Harry Struijker-Boudier
Widya Istanto Nurcahyo,1 Suharyo Hadisaputro,2 Zainal Muttaqin,3 Cindy Elfira Boom,4 Chandra Hermawan Manapa,1 Taufan Pramadika,1 Dodik Tugasworo5
1Anaesthesiology Department and Intensive Therapy, Faculty of Medicine, Diponegoro University, Semarang, Central Java, Indonesia; 2Postgraduate Faculty of Medicine, Diponegoro University, Semarang, Central Java, Indonesia; 3Neurosurgery Department, Faculty of Medicine, Diponegoro University/Kariadi General Hospital, Semarang, Central Java, Indonesia; 4Anaesthesiology Department and Intensive Therapy, National Cardiovascular Center, Harapan Kita Hospital, Jakarta, Indonesia; 5Neurology Department, Faculty of Medicine, Diponegoro University/Kariadi General Hospital, Semarang, Central Java, Indonesia
Correspondence: Widya Istanto Nurcahyo, Anaesthesiology Department and Intensive Therapy, Faculty of Medicine, Diponegoro University, Semarang, Central Java, 50725, Indonesia, Fax +62 2476928010, Email [email protected]; [email protected]
Introduction: On-pump, coronary artery bypass grafting (CABG) is the most common cause of postoperative cognitive dysfunction (POCD) after cardiac surgery. Previous studies showed that the incidence of POCD after cardiac surgery was 60%, higher than non-cardiac surgery with 11.7%. Glial fibrillary acid protein (GFAP) is one of the sensitive biomarkers of brain damage. Previous studies have found that elevated GFAP serum is associated with cognitive impairment. This study aims to measure the difference in GFAP levels in POCD and non-POCD patients after CABG on-pump surgery.
Methods: This study is a retrospective cohort design study. The data were obtained from 56 subjects undergoing elective CABG on the pump surgery enrolled into two groups consisting of 28 POCD as a case group and 28 non-POCD as a control group. In this study, the ELISA method measured the levels of GFAP biomarkers within 24 hours after surgery. After 72 hours, the patient received a MoCA-INA examination to determine cognitive impairment. Data analysis was carried out by SPSS 23.00 software.
Results: The mean age of patients in both groups was 60 years and was dominated by males (> 85%). POCD patients were found to have a significantly longer duration of cardiopulmonary bypass (CPB) and cross-clamp surgery than non-POCD patients (p = 0.002 and p = 0.004). Postoperative GFAP levels in POCD patients were significantly higher than in non-POCD patients (12.95 ± 7.47 vs 3.80 ± 2.77, p < 0.001). There was a significant increase in GFAP levels compared with non-POCD (8.28 ± 7.24 vs − 1.5 ± 3.03, p < 0.001). The area under the curve (AUC) value of GFAP against POCD was 0.887, cut-off GFAP 4.750 with a sensitivity of 92.9% and a specificity of 71.4%.
Conclusion: POCD patients had higher GFAP levels than non-POCD patients. There are differences in GFAP levels in patients with POCD and non-POCD post-CABG surgery.
Keywords: GFAP, POCD, CPB, MoCa-INA
Introduction
Postoperative Cognitive Dysfunction (POCD) is an impairment or decline in cognitive function measured objectively postoperative compared to preoperative cognitive function.1 POCD is a transient condition, this condition occurs for a few weeks to several months after surgery.2,3 POCD is characterized by symptoms of dementia such as memory impairment, loss of concentration, inability to plan, and difficulty switching between tasks.2,3
Symptoms experienced by POCD patients have a significant clinical and social impact, which is associated with an increase in length of hospitalization, risk of death, inability to work, and social dependence that occurs in all age groups, especially in the elderly.4 The incidence of POCD after cardiac surgery is higher than in non-cardiac surgery. Previous studies showed that the incidence of POCD after non-cardiac surgery was 11.7%, whereas the incidence of POCD after cardiac surgery was 60%.5 Coronary Artery Bypass Grafting (CABG) on the pump is a surgical procedure that aims to improve the quality of life and prognosis of patients suffering from ischemic heart disease.1 CABG is the most frequent operation in cardiac surgery cases, with 62 per 100,000 population in Western European countries. The prevalence of CABG is quite high in Indonesia. In 2014, Dr. Kariadi General Hospital reported on 28 CABG operations. Meanwhile, the National Cardiovascular Center of Harapan Kita Hospital reported 748 CABG patients from 1510 cases of Coronary Heart Disease in 2016.6–8
CABG on the pump has more severe neurological deficit complications compared to CABG off-pump. An earlier study from Dominici et al stated that CABG off-pump in patients with a history of TIA or stroke was associated with a reduced risk of stroke, delirium, and postoperative neurological side effects compared to CABG on the pump.9 CABG on the pump surgery is associated with an increased risk of POCD.10
The occurrence of POCD following cardiac surgery is most frequently caused by CABG on the pump, with prevalence rates of 37.6% at 7 days and 20.8% at 3 months postoperatively.11 The microembolization of atheroma plaque, air, and clot from the aorta or cardiopulmonary bypass (CPB), brain hypoperfusion during cardiopulmonary bypass (CPB), and brain hypoperfusion due to systemic inflammation are the mechanisms causing the neurologic impairment of POCD after cardiac surgery.12 Previous studies at Dr. Cipto Mangunkusumo General Hospital Jakarta on cardiopulmonary bypass (CPB) patients showed that the prevalence of POCD on the last day of hospitalization is 40.7%. Other previous studies also showed the same results with 39.8% and 44.5% of COPD prevalence.13
Guidelines are not generally known in the diagnosis and management of POCD. Detection of POCD requires a sensitive neuropsychological bedside test. A reliable neuropsychological bedside test has not yet been found. Examination of biomarkers has the potential to understand the pathophysiology, diagnose, and determine the prognosis of POCD. Current diagnostic criteria for POCD are undefined.14
Glial fibrillary acidic protein (GFAP) is one of the sensitive biomarkers of brain damage.12 In a previous study, GFAP was elevated in rats with cerebral ischemia and neurotoxic damage. Elevated GFAP indicates the presence of astrogliosis.15 Regarding specificity, GFAP is an intermediate filament derived from astrocytes.12 Previous studies have also found that an elevated GFAP serum is associated with cognitive impairment in patients with Alzheimer’s disease.16 Moreover, GFAP is also used in patients who recovered from sepsis as an early marker for cognitive impairment.17
In this study, the authors will examine the difference in the elevated GFAP levels within 24 hours post-CABG surgery in patients diagnosed with POCD and non-POCD after CABG on the pump surgery. This study aims to prove that GFAP can be used as an early marker in patients with the potential to develop POCD after CABG surgery or not start prophylactic therapy.
Materials and Methods
Patient
This study is a retrospective cohort study conducted at the National Cardiovascular Center Harapan Kita Hospital, Indonesia, from 10 December 2020 to 1 September 2021. Patients undergoing CABG were observed 72 hours longitudinally to observe the incidence of POCD.
The selection of research subjects was carried out using a nonrandom consecutive sampling method. A total of 56 patients who met the research criteria enrolled into 2 groups consisting of 28 POCD patients as a case group and 28 non-POCD patients as a control group (Figure 1).
The inclusion criteria used in this study were male and female patients >18 years old undergoing elective CABG on the pump surgery who were approved to participate. Exclusion criteria in this study were patients with previous cerebrovascular accident (CVA) based on history and medical records, history of previous cardiothoracic surgery, history of preoperative cognitive impairment assessed by Montreal Cognitive Assessment Indonesia Version (MoCA-INA) <26, patients requiring preoperative transfusion, patients with renal impairment (serum creatinine level >2 mg/dl), and patients on prolonged ventilator (duration of mechanical ventilation ≥3 days).
Sample Size
Note
a.Zα: The value in formula Z for the magnitude of the error type 1 (α) = 0.05, which is 1.96.
b.Zβ: The value in formula Z for the magnitude of type 2 error (β) = 0.2, which is 0.842.
Research power = 1 - = 1–0.2 = 0.8 (research power = 80%).
c.P1= the proportion of POCD on the third day after CABG in the group with risk factors.
d.P2= the proportion of POCD on the third day in the group without risk factors
Based on the results of the calculation of the sample size for categorical and continuous scale variables as above, the largest number of samples is 32 subjects for each group. If there is a possibility of a drop-out of 10% (do = 0.1) then the sample size with drop-out correction is:
Based on the calculation of the sample size with drop-out correction, the sample size is 36 people with POCD and 36 without POCD. The total sample is 72 people.
Variable
GFAP serum levels within 24 hours postoperative were measured using the ELISA Kit Elab Science Cat. No: E-EL-h6093 made in the United States, America, where the GFAP serum levels were taken preoperatively. After 72 hours postoperatively, the patient received a MoCA-INA examination to determine cognitive impairment.29 Assessment of cognitive function by trained personnel with MoCA-INA preoperative and postoperative.
Measurement Results
- POCD is negative if the MoCA-INA value is 26 to 30.
- POCD is positive if the value of MoCA-INA <26 patients with MoCA-INA scores >24 was categorized into POCD.
Diagnosis Procedure of POCD Using MoCA-INA
- Provide explanations to research participants who will participate in this study. The explanation given includes the background and purpose of conducting the research as well as asking for approval after giving informed consent or providing a previous explanation.
b. The first data collection using anamnesis and medical records to obtain data on preoperative risk factors (elderly age, hypertension, DM, and atherosclerosis). Preoperative cognitive function was assessed with MoCA-INA as baseline, and patients with MoCA-INA score <26 were excluded from the study. After the MoCA-INA examination, ± 3 mL of venous blood was taken to measure the levels of preoperative brain damage serum biomarkers (GFAP). The second data is by looking at intraoperative medical records and perfusion to obtain data on intraoperative risk factors (length of CPB, length of cross clamps, Hb levels, and MAP). The third data is by looking at postoperative medical records in the ICU to see postoperative risk factors (duration of ventilators and use of sedation) and laboratory tests for measuring levels of brain damage serum biomarkers (GFAP) at 24 hours postoperative CABG on pump.
c. Immediately after the patient finished the CABG on pump surgery procedure, the patient was admitted to the ICU then on every postoperative day in the ICU, HCU or ordinary ward, the patient was reassessed for cognitive function using MoCA-INA to compare with preoperative values.
GFAP Examination Procedure
The steps for the GFAP examination are as follows:
- Prepare all reagents, samples and standard solutions.
- Put 50 L of standard or sample solution into the well.
- Add 50 L of antibody cocktail solution to all wells.
- Incubate at room temperature for 1 hour.
- Aspirate and wash each well three times with 350 L 1X PT wash buffer solution.
- Add 100 L of TMB Development Solution to each and incubate for 10 minutes.
- Add 100 L of Stop Solution.
- Perform reading on the ELISA Reader with a wavelength of 450nm.
Anesthesia Protocol
The standard anesthetic protocol used was following our institutional guidelines. Premedication with 0.07 mg/kg of midazolam was used. Anesthesia was induced by inhaled sevoflurane with a minimal alveolar concentration (MAC) of 1.0, followed by an intravenous injection of fentanyl 4 mcg/kg and rocuronium 1.2 mg/kg. Maintenance was performed with 1.0 MAC of inhaled sevoflurane, intravenous administration of 1 mcg/kg fentanyl per 30 minutes, and rocuronium 0.1 mg/kg per 45 minutes. The mean arterial pressure is set to 55–70 mm Hg during cardiopulmonary bypass (CPB). Heparin (300 IU/kg) was given to maintain an activated clotting time >400 seconds. All patients received 0.02mg/kg infusion of morphine for 48 hours postoperatively.
Data Analysis
Data analysis was carried out by the Statistical Package for the Social Sciences 23.0 software (SPSS Inc.) – IBM SPSS Statistics. Descriptive statistics were expressed in mean and standard deviation. Mann–Whitney test was used to assess the difference in the increase of GFAP levels between two groups with POCD and non-POCD after CABG surgery. Chi-square and Fischer exact tests were carried out to explain the characteristics of the study sample on nominal data. A P-value of <0.005 was considered statistically significant. Wilcoxon and Spearman correlation tests were used to analyze pre and postoperative GFAP levels. Receiving analysis characteristic (ROC) curves were used to determine the 24 hours postoperative GFAP cut-off in POCD patients.
Research Protocol
Ethical Approval
All patients signed informed consent before surgery. The procedures related to this study were reviewed and approved by the Health Research Ethics Commission of the National Cardiovascular Center of Harapan Kita Hospital, Indonesia, with the Number: LB.02.01/VII/484/KEP079/2020 on December 8, 2020. We confirm that our study complies with the Declaration of Helsinki.
Results
Patient Characteristics and Preoperative Data
In this study, the characteristics of both POCD and non-POCD patients tended to be identical, as shown in Table 1. The mean age of patients in both groups (POCD and non-POCD) was 60 years. POCD and non-POCD patients were dominated by males, with 85.7% of POCD and 92.9% of non-POCD. A significant difference was found in the mean of BMI, with a higher BMI found in POCD than non-POCD patients (p = 0.041). After analyzing the data, it was found that the frequency of obesity in POCD patients was higher, but no significant difference was found (p = 0.146).
 |
Table 1 Characteristics of Subjects and Preoperative Sample |
No significant difference was found in smoking, alcohol consumption, and pneumonia history. In preoperative laboratory analysis, non-POCD potassium levels were higher, and non-POCD sodium levels were lower (p=0.022 and p=0.004, respectively). POCD and non-POCD patients had identical characteristics on preoperative MoCA-INA observations, and no significant difference was found (p=0.767).
Intraoperative Data
In Table 2, POCD patients were found to have a significantly longer duration of CPB surgery than non-POCD patients (p=0.002). A significant difference was also found (p=0.004) in the cross-clamp duration with a longer cross-clamp duration observed in POCD patients.
 |
Table 2 Intraoperative Data |
Postoperative Data
Table 3 shows postoperative laboratory analysis, and white blood cell numbers in POCD patients were higher than in non-POCD patients (p=0.004). In addition, a significant difference was found in the MoCA-INA variable (p=0.001), with a lower MoCA-INA score observed in POCD patients. There was a significant difference in postoperative GFAP levels in the POCD and without POCD groups (p = 0.001) (Figure 2).
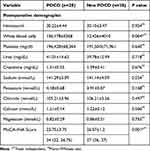 |
Table 3 Postoperative Data |
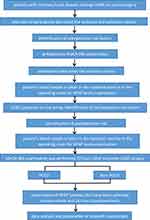 |
Figure 1 Research Protocol. |
Bivariate Analysis
In preoperative GFAP observation, as shown in Table 4, non-POCD patients had higher GFAP scores than POCD patients, but no significant difference was found (p=0.583). Then, in postoperative conditions, POCD patients experienced an increase in GFAP while non-POCD patients experienced a decrease in GFAP levels (Table 4). Postoperative GFAP levels in POCD patients were higher and had larger deltas than in non-POCD patients, and a significant difference was found (p<0.001). The results of the paired T-test analysis showed that the POCD group had a significant increase in GFAP levels (p<0.001), but this increase had a weak positive correlation that was not significant. On the other hand, GFAP levels in the non-POCD group showed a significant decrease (p=0.012), but this decrease had a moderately significant positive correlation.
 |
Table 4 Bivariate Analysis Results |
Receiver Operating Characteristic (ROC) Curves Analysis
The area under the curve (AUC) value is 0.887 (Figure 2). This indicates that GFAP is considered a good biomarker in establishing the diagnosis of POCD. In the value analysis, the cut-off GFAP 4.750 had a sensitivity of 92.9% and a specificity of 71.4%. With this cut-off, the Positive Predictive Value in this study was 26 of 28 POCD patients (92.85%), and 2 POCD patients had a GFAP level of 4.5. Meanwhile, the Negative Predictive Value was 19 patients out of 28 non-POCD patients (67.9%).
 |
Figure 2 Box plot diagram of delta GFAP levels in CABG on-pump patients with and without POCD. |
Discussion
Patients with POCD tend to be older than non-POCD patients. In this study, the patient’s age characteristics of both groups were similar, with an average age of 60 years old. However, previous studies have shown age as a consistent risk factor for the incidence of POCD. An earlier study conducted by Laalou et al showed that the incidence of POCD was related to the patient’s age and time of observation, with an incidence of 23–29% in patients aged 60–69 years and >70 years in the first week and 14% in those aged >70 years in the third month postoperative.18 Baktiar et al reported that 31 patients who underwent open-heart surgery had 31 patients with POCD and 24 non-POCD. It has been proved that in POCD patients aged 55.4 ± 11.7 and non-POCD patients 50.9 ± 13.6, neuronal damage in elderly patients tends to cause neurological manifestations in the form of cognitive impairment.19 However, this study could not prove that age is a risk factor for POCD because both groups had the same mean.
POCD and non-POCD patients were dominated by male patients, namely 85.7% and 92.9%, respectively. A previous study by He et al stated that patients who underwent single valve replacement surgery were found to have POCD, and non-POCD patients were dominated by male patients, which 64.5% and 66.7%, respectively.20 Previous studies found no significant difference between males and females in POCD. However, men with the Apolipoprotein E (APOE4) allele may be more susceptible to developing POCD than the female with the Apolipoprotein E (APOE4).21
The mean BMI of POCD patients was higher than that of non-POCD even though the obese BMI category dominated both groups. However, a study conducted by Baktiar et al found 31 patients with POCD and 24 non-POCD patients who underwent open-heart surgery. No significant difference was found between POCD and non-POCD patients, although POCD patients had a lower mean BMI (24.2 ± 4.0 and 25.4 ± 4.3, respectively).19 In a previous meta-analysis, the obesity category did not find a significant increase in the risk of increasing POCD.22
POCD patients had a longer duration of CPB and cross-clamp surgery. However, a previous study reported that CPB surgery and cross-clamp duration were not risk factors for POCD.23 In a study conducted by He et al, amongst patients undergoing single valve replacement surgery, the duration of CPB was monitored in POCD and non-POCD patients: in patients with POCD, it was found that the duration of CPB was longer, with a mean of 127 minutes (60–400 minutes) compared to non-POCD patients with a mean of 116 minutes (74–186 minutes). However, no significant difference was found (p= 0.36).20 In patients with POCD, the duration of the cross-clamp was found to be faster with a mean of 90 minutes (40–308 minutes) than non-POCD patients with a mean of 93 minutes (41–131 minutes), with no significant difference found (p-value = 0.73).20
The time difference between POCD and non-POCD patients in the previous study was short. However, our study showed that a 1.5–2-fold increase in longer duration was found in POCD patients. This condition may be associated with prolonged inflammatory conditions and ischemia, stimulating significant neuronal damage.24
Patients with POCD had a lower postoperative MoCA-INA score compared to non-POCD patients. In a study conducted by Aykut et al, the effects of pulsatile and non-pulsatile flow on cognitive decline in patients undergoing CABG were compared. Cognitive function was assessed by the Montreal Cognitive Assessment Indonesia Version (MoCA-INA) at 1 day preoperative and 1 month postoperative. The incidence of POCD was 17.3% in pulsatile flow and 35.6% in non-pulsatile flow. Decreased cognitive function was more common in the non-pulsatile group than in the pulsatile group.25,26 In a study by He et al, mental status assessment with MoCA-INA was performed amongst patients undergoing single valve replacement surgery. This assessment was carried out 1 day before surgery and 2 days after surgery, and 9 days after surgery found, 32 patients with POCD and 146 non-POCD patients. On 1 day before surgery, it was found that the MoCA-INA test results were higher in POCD patients (29.6 ± 0.4) than in non-POCD patients (28.7 ± 1.5). After undergoing surgery, 2 days postoperatively, it was found that the results of the MoCA-INA test were significantly lower in POCD patients (22.1 ± 1.8) than in non-POCD patients (27.3 ± 2.6), namely p < 0.05 there was a statistically significant difference.20 In our study, the postoperative MoCA-INA score decreased compared to preoperative condition. However, in non-POCD patients, only a mild decrease in the MoCA-INA score was found; still, >26 was within the normal range.
POCD patients have an increased postoperative GFAP. Meanwhile, in non-POCD patients, GFAP levels were decreased. A study by Wiberg et al on biomarkers of cerebral injury to predict POCD in patients undergoing cardiac surgery observed that postoperative serum GFAP concentrations were significantly increased in patients undergoing cardiac surgery with POCD upon discharge from the hospital. This study included 168 patient subjects who underwent cardiac surgery. A total of 48 subjects (28%) met the POCD criteria. It was found that patients with POCD experienced a significant increase in serum GFAP levels from baseline (p = 0.01). It means that there is a relationship between GFAP levels and the incidence of POCD. This study aimed to determine the power of the biomarkers of neuron-specific enolase (NSE), tau, neurofilament light chain (NFL), and GFAP to predict POCD in patients undergoing cardiac surgery, where only tau serum and GFAP levels were significantly elevated after cardiac surgery. GFAP may be considered as a biomarker for the presence of POCD in patients after cardiac surgery, but more study is still needed to evaluate the GFAP levels as a biomarker.27
Other studies also support the statement that there is a significant increase in serum GFAP levels at the end of surgery in patients who show POCD after surgery. This prospective observational study involves 82 trauma patients undergoing surgery under general anesthesia. Subjects underwent perioperative cognitive assessment and serum GFAP biomarker measurements. It was found that patients who had criteria for the diagnosis of POCD had significantly elevated serum GFAP levels at the end of surgery. This study provides additional evidence that trauma surgery is associated with high postoperative serum GFAP levels, which may indicate significant nerve damage.28
The results of the ROC analysis in this study indicate that GFAP can be used as a good predictor of POCD. Evidence from a previous study showed a similar result, namely the AUC on ROC of 0.64, indicating that GFAP is a fairly good biomarker predictor of POCD.27 The use of GFAP biomarkers as a diagnosis of POCD must continue to be developed together with other risk factors to be used as a diagnostic guide for patients’ potential for postoperative POCD.
The limitation of this study was that the sampling time was not carried out exactly 24 hours after surgery in some of the study samples, and this time difference was generally caused because the patient was undergoing a diagnostic process or other therapeutic management. Blood sampling paths for research subjects were carried out through different veins.
Conclusion
A 24-hour postoperative GFAP can be a good predictor of a POCD biomarker in pump CABG surgery. POCD patients had higher GFAP levels than non-POCD patients. Further research is needed to observe GFAP as an accurate predictor of POCD in terms of timing, method, and patient risk factors.
Abbreviations
CABG, coronary artery bypass grafting; POCD, postoperative cognitive dysfunction; GFAP, glial fibrillary acid protein; MoCA-INA, Montreal cognitive assessment Indonesia version; CPB, cardiopulmonary bypass; AUC, area under the curve; ROC, receiving analysis characteristic; NSE, neuron-specific enolase; NFL, neurofilament light chain.
Acknowledgments
This research was supported by the Anesthesia and Intensive Care Subspecialty Program, Universitas Diponegoro, Semarang, Indonesia. We also thank Dr. Kariadi General Hospital and Harapan Kita Hospital and all parties who have contributed to this research.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The authors received no financial support for this article’s research, authorship, and publication.
Disclosure
The authors declare that they have no conflicts of interest in relation to this work.
References
1. Evered LA, Silbert BS. Postoperative cognitive dysfunction and non-cardiac surgery. Anesth Analg. 2018;127(2):496–505. doi:10.1213/ANE.0000000000003514
2. Oldham MA, Vachon J, Yuh D, Lee HB. Cognitive outcomes after heart valve surgery: a systematic review and meta-analysis. J Am Geriatr Soc. 2018;66(12):2327–2334. doi:10.1111/jgs.15601
3. Pappa M, Theodosiadis N, Tsounis A, Sarafis P. Pathogenesis and treatment of postoperative cognitive dysfunction. Electron Physician. 2017;9(2):3768–3775. doi:10.19082/3768
4. Rundshagen I. Postoperative cognitive dysfunction. Dtsch Arztebl Int. 2014;111(8):119–125. doi:10.3238/arztebl.2014.0119
5. Needham MJ, Webb CE, Bryden DC Postoperative cognitive dysfunction and dementia: what we need to know and do. Br J Anaesth. 119; 2017:i115–i125. 10.1093/bja/aex354
6. OECD, Health at a Glance 2009. Paris; 2010. doi:10.1787/9789264105133-ko
7. Head SJ, Milojevic M, Taggart DP, Puskas JD. Current practice of state-of-the-art surgical coronary revascularization. Circulation. 2017;136(14):1331–1345. doi:10.1161/CIRCULATIONAHA.116.022572
8. Harahap G, Nurcahyo W, Ismail A. Mortalitas operasi jantung coronary artery bypass graft Di Rsup Dr Kariadi Semarang Periode Januari 2014 - Desember 2014. J Kedokt Diponegoro. 2016;5(2):160–166. doi:10.14710/dmj.v5i2.11822
9. Dominici C, Salsano A, Nenna A, et al. Neurological outcomes after on-pump vs off-pump CABG in patients with cerebrovascular disease. J Card Surg. 2019;34(10):941–947. doi:10.1111/jocs.14158
10. Umholtz M, Nader ND. Postoperative delirium and postoperative cognitive dysfunction. Neuromethods. 2020;150:239–253. doi:10.1007/978-1-4939-9891-3_15
11. Van Harten AE, Scheeren TWL, Absalom AR. A review of postoperative cognitive dysfunction and neuroinflammation associated with cardiac surgery and anaesthesia. Anaesthesia. 2012;67(3):280–293. doi:10.1111/j.1365-2044.2011.07008.x
12. Seco M, Edelman JJB, Wilson MK, Bannon PG, Vallely MP. Serum biomarkers of neurologic injury in cardiac operations. Ann Thorac Surg. 2012;94(3):1026–1033. doi:10.1016/j.athoracsur.2012.04.142
13. Aprianti M. Effect of age, education level, diabetes, CPB and cross clamp time on POCD after open heart surgery in RSCM; 2017.
14. Valentin LSS, Pietrobon R, Aguiar Junior W de, et al. Definition and application of neuropsychological test battery to evaluate postoperative cognitive dysfunction. Einstein. 2015;13(1):20–26. doi:10.1590/S1679-45082015AO3152
15. Cechetti F, Pagnussat AS, Worm PV, et al. Chronic brain hypoperfusion causes early glial activation and neuronal death, and subsequent long-term memory impairment. Brain Res Bull. 2012;87:109–116. doi:10.1016/j.brainresbull.2011.10.006
16. Oeckl P, Halbgebauer S, Anderl-Straub S, et al. Glial fibrillary acidic protein in serum is increased in Alzheimer’s disease and correlates with cognitive impairment. J Alzheimers Dis. 2019;67(2):481–488. doi:10.3233/JAD-180325
17. Anderson BJ, Reilly JP, Ittner C, et al. Glial Fibrillary Acidic Protein (GFAP) is an early marker of cognitive impairment in sepsis survivors. B22 Crit Care. 2018;2018:2780–2788.
18. Laalou FZ, Jochum D, Pain L. Dysfonctions cognitives postopératoires: stratégie de prévention, de dépistage et de prise en charge. Ann Fr Anesth Reanim. 2011;30(10):e49–e53. doi:10.1016/j.annfar.2011.08.009
19. Baktiar Y, Soenarto RF, Alatas A, Auerkari AN. S100B as a serologic marker for cognitive dysfunction following open-heart surgery. Int J Appl Pharm. 2020;12(Special Issue 3):50–53. doi:10.22159/ijap.2020.v12s3.39473
20. He X, Wen LJ, Cui C, Li DR, Teng JF. The significance of S100β protein on postoperative cognitive dysfunction in patients who underwent single valve replacement surgery under general anesthesia. Eur Rev Med Pharmacol Sci. 2017;21(9):2192–2198.
21. Schenning KJ, Murchison CF, Mattek NC, Kaye JA, Quinn JF. Sex and genetic differences in postoperative cognitive dysfunction: a longitudinal cohort analysis. Biol Sex Differ. 2019;10(1). doi:10.1186/s13293-019-0228-8
22. Feinkohl I, Winterer G, Pischon T. Obesity and postoperative cognitive dysfunction: a systematic review and meta-analysis. Diabetes Metab Res Rev. 2016;32(6):643–651. doi:10.1002/dmrr.2786
23. Soenarto RF, Mansjoer A, Amir N, Aprianti M, Perdana A. Cardiopulmonary bypass alone does not cause postoperative cognitive dysfunction following open heart surgery. Anesthesiol Pain Med. 2018;8(6). doi:10.5812/aapm.83610
24. Alam A, Hana Z, Jin Z, Suen KC, Ma D. Surgery, neuroinflammation and cognitive impairment. EBioMedicine. 2018;37:547–556. doi:10.1016/j.ebiom.2018.10.021
25. Özhan A, Baştopcu M, Karakaya C, et al. The relationship between aortic calcification on chest radiograph and neurocognitive impairment after coronary artery bypass grafting. Turkish J Thorac Cardiovasc Surg. 2021;29(2):166–173. doi:10.5606/tgkdc.dergisi.2021.21285
26. Aykut K, Albayrak G, Guzeloglu M, Hazan E, Tufekci M, Erdoǧan I. Pulsatile versus non-pulsatile flow to reduce cognitive decline after coronary artery bypass surgery: a randomized prospective clinical trial. J Cardiovasc Dis Res. 2013;4(2):127–129. doi:10.1016/j.jcdr.2013.05.005
27. Wiberg S, Holmgaard F, Zetterberg H, et al. Biomarkers of cerebral injury for prediction of postoperative cognitive dysfunction in patients undergoing cardiac surgery. J Cardiothorac Vasc Anesth. 2021;36(1):125–132. doi:10.1053/j.jvca.2021.05.016
28. Hamzayevna SV, Hasanovich AA, Alihanovich VA. Glial fibrillary acidic protein protein and neuron-specific enolase as predictors of cognitive dysfunction after trauma surgery. Bulletin of Emergency Medicine. 2019;12(4):17–21.
29. Julayanont P, Phillips N, Chertkow H, Nasreddine ZS. The Montreal Cognitive Assessment (MoCA): concept and Clinical review. In: Larner AJ, editor. Cognitive Screening Instruments: A Practical Approach. Springer-Verlag; 2013:111–152.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.