Back to Journals » OncoTargets and Therapy » Volume 7
Dichloroacetate modulates cytokines toward T helper 1 function via induction of the interleukin-12–interferon-γ pathway
Authors Badr M, Qinna N , Qadan F, Matalka K
Received 28 October 2013
Accepted for publication 16 December 2013
Published 7 February 2014 Volume 2014:7 Pages 193—201
DOI https://doi.org/10.2147/OTT.S56688
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 6
Mujtaba M Badr,1,2 Nidal A Qinna,1,2 Fadi Qadan,2 Khalid Z Matalka1,2
1Department of Pharmacology and Biomedical Sciences, Faculty of Pharmacy and Medical Sciences, 2Petra University Pharmaceutical Center, University of Petra, Amman, Jordan
Background: Dichloroacetate (DCA) is one of the new, promising anticancer drugs. DCA restores normal mitochondrial function and enables cancer cells to undergo apoptosis. In addition, DCA was found to modulate certain signaling pathways involving some transcription factors. The latter encouraged us to study DCA immunomodulatory activity on cytokines and their association with increasing DCA cancer cell cytotoxicity.
Methods and results: Cell viability assay was used to determine the effect of different concentrations of DCA on the survival of 3-methylcholanthrene (MCA) fibrosarcoma cell line. DCA decreased the percent survival of MCA fibrosarcoma in a dose-dependent manner (P<0.01). Furthermore, this percent survival was further reduced when MCA fibrosarcoma cells were cocultured with mouse splenocytes. The latter was observed at 10 mM DCA (P<0.01), and the inhibitory concentration at 50% dropped from 23 mM to 15.6 mM DCA (P<0.05). In addition, DCA significantly enhanced interferon (IFN)-γ but not interleukin (IL)-17 production levels in unstimulated and stimulated mouse spleen cells. To investigate the mechanism of DCA on IFN-γ production, DCA cytokine modulatory effect was tested on unstimulated macrophages, T-cells, and natural killer cells. DCA significantly increased IL-12 production from macrophages but did not modulate the production of IFN-γ from either T-cells or natural killer cells. Moreover, the DCA-enhancing effect on IFN-γ production was reversed by anti-IL-12 antibody. Also, the DCA cytokine modulatory effect was tested in vivo after inducing mouse skin inflammation using phorbol 12-myristate 13-acetate (PMA). DCA restored PMA-lowered IFN-γ and IL-12 levels and normalized PMA-increased transforming growth factor-ß level, but it inhibited IL-10 levels even further (P<0.05).
Conclusion: DCA has immunomodulatory activity, mainly via activation of the IL-12–IFN-γ pathway and is able to modulate cytokines toward T helper 1 lymphocyte function. These DCA immunomodulatory effects are promising and further investigations are required to develop protocols for its use in cancer treatment.
Keywords: dichloroacetate, fibrosarcoma, cytokines, IL-12, IFN-γ, inflammation
Introduction
Cancer cells generally rely on anaerobic respiration for energy production,1 which leads to lactate formation, thus making the tumor microenvironment more acidic. Such acidity increases cancer cell chemoresistance, suppresses apoptosis, and facilitates mobility and metastasis.1,2 Although reoxygenation via neovascularization mechanisms of solid tumors occur, glycolysis and its byproducts persists.1,2
Dichloroacetate (DCA) is a simple chemical compound that has been used for years to treat lactic acidosis and other mitochondrial disorder.2–4 It was found to inhibit pyruvate dehydrogenase kinase (PDK), which thereby enables pyruvate dehydrogenase to facilitate pyruvate entering the mitochondria for oxidative phosphorylation. In other words, DCA enables the cell to go through metabolic respiration rather than lactate formation.3–8 Thus, it was thought that DCA could reduce glycolysis-related effects and act as an anticancer drug.
In 2007, Bonnet et al released the first scientific evidence identifying DCA as a potential anticancer compound.2 In comparison to normal cell lines, Bonnet et al showed that DCA lowers apoptosis resistance in several human cancer cell lines (A549, MCF7, and M059K) through lowering mitochondrial membrane potential. It also increases mitochondria-derived hydrogen peroxide, increases potassium (K+) channel Kv1.5 efflux of K+, and lowers cytoplasmic calcium ions. In addition, they showed that oral DCA administration reduces tumor proliferation in athymic nude rats. Similarly, others have shown that DCA induces apoptosis of prostate cancer cell lines and also modulates antiapoptotic genes: BCl-2,9 increases caspases 3 and 9 expression,10 and increases p53 (antisuppressor gene) in endometrial cancer cell lines.11 However, it has been found that DCA concentration-induced cytotoxic effect varies between cancer cell lines. Some cell lines were resistant to DCA,11 and others need concentrations above 25 mM to induce a significant effect.12 Moreover, other studies showed that DCA reduced apoptosis under hypoxic conditions in some colorectal cancer cell lines,13 increased tumor volume in colorectal cancer xenograft mice,13 and at 25 mg/kg/day caused peripheral neuropathy in a clinical trial.14 However, the latter adverse event was not observed in patients who had received DCA at 12.5 mg/kg every 12 hours for 9–16 years.15
Cytokine modulation is a key factor in the progression or suppression of cancer within its microenvironment.16,17 Generally, activation of macrophages and/or dendritic cells results in the local production of cytokines, such as interleukin (IL)-12, which in turn amplify the innate immune response and stimulate T-cell function. Production of IL-12 is a major inducer of T helper 1(Th1) and natural killer (NK) lymphocytes and their cytokines. These produced cytokines polarize macrophages, enhance dendritic cell maturation, and lower regulatory T-cell function by inhibiting regulatory cytokines such as transforming growth factor (TGF)-β and IL-10.16–19 Since it has been shown that DCA inhibits PDK and facilitates signaling pathways that lead to the inhibition of transcription factor nuclear factor of activated T-cells,2 we were encouraged to study DCA immunomodulatory activity. The present study was first based on whether DCA antiproliferative activity and cytotoxicity on 3-methylcholanthrene (MCA) fibrosarcoma could be enhanced in the presence of immune cells, and then determining if this phenomenon is related to DCA’s ability to modulate cytokines in vitro and in vivo. The long-term goal, however, is to understand DCA mechanisms in cancer therapy to develop protocols for its use in cancer treatment.
Materials and methods
Materials
Rosewell Park Memorial Institute (RPMI)-1640, fetal bovine serum, penicillin/streptomycin, amphotericin B (supplemented (s)-RPMI) and trypsin were obtained from (Merck Millipore, Billerica, MA, USA). DCA, MCA 98%, Igepal CA-630, phorbol 12-myristate 13-acetate (PMA), and phytohemagglutinin (PHA) were purchased from Sigma-Aldrich (St Louis, MO, USA). Endotoxin-free Dulbecco’s phosphate buffered saline (PBS) without calcium and magnesium was purchased from EuroClone SpA (Milan, Italy). Tissue culture flasks (25 cm2 and 75 cm2) were obtained from Guangzhou Jet Bio-Filtration Products (Guangzhou, People’s Republic of China), and tissue culture and Maxisorb 96-well flat plates were products of Nalge Nunc International (Rochester, NY, USA). The kit for mouse erythrocytes lysis and the MagCellect mouse NK Cell and cluster of differentiation (CD)3+ T-cell isolation kits were products of R&D Systems (Minneapolis, MN, USA). The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reagent and sodium dodecyl sulfate were purchased from Trevigen (Gaithersburg, MD, USA). A rat monoclonal anti-mouse IL-12 p70 (immunoglobulin G1) was acquired from R&D Systems.
Animals
BALB/c male and female mice were utilized throughout the experiments as indicated. Mice were purchased from Yarmouk University (Irbid, Jordan) and were housed at the University of Petra animal facility in Amman. All mice were housed in a pathogen-free environment at 22°C with 12 hours light/dark cycle and with food and tap water ad libitum. All animal experiments were performed in compliance with the guidelines set by the Federation of European Laboratory Animal Science Association, and the study protocol was approved by the Deanship of Scientific Research at the University of Petra.
MCA-induced tumors and cell culture
MCA fibrosarcoma was induced in mice as described elsewhere.18,19 When the tumor size reached 1–2 cm in diameter, it was removed aseptically, cut in small pieces, minced, cultured in a T75 flask with FBS, and incubated at 37°C in a humidified chamber with 5% carbon dioxide (CO2). Culture medium was changed every 2 days, and cells were split when they reached confluency.
Spleen, macrophage, T-cell, and NK cell isolation
Mice were sacrificed by cervical dislocation. Spleen tissues were collected, squeezed between two slides to generate a single cell suspension, and red blood cells were lysed using M-lyse buffer (R&D Systems). The cells were washed with washing buffer, centrifuged for 10 minutes, and the supernatant was discarded. Cells were then counted and added to a 96-well plate at a density of 105 cells/100 μL/well and incubated at 37ºC with 5% CO2 in a humidified chamber.
The isolation of macrophages was performed according to the protocol described by Wahl and Smith.20 A volume of 15 mL from the above prepared spleen cell suspension was added to a 75 cm2 tissue culture flask and incubated for 1 hour at 37°C with 5% CO2 in a humidified chamber. The supernatant was then decanted, and the flask was washed two times with 10 mL FBS medium to remove nonadherent cells. The adherent macrophage cells were collected by scrapping, and they were resuspended in s-RPMI and counted for further applications. The NK cell and CD3+ T-cell isolation procedures were performed according to the manufacturer’s instructions. Isolated NK cells and CD3+ T-cells were counted, diluted to the proper concentration, and added to the 96-well culture plate.
Cell viability and proliferation assay
Cell viability and proliferation was assessed by MTT assay. Two hours or 24 hours after culturing spleen or MCA fibrosarcoma, respectively, 100 μL of DCA was added to each well and incubated for 48 hours. Following this incubation period, 20 μL MTT reagent was added and formed a purple precipitate. Four hours later 50 μL sodium dodecyl sulfate was added. When spleen was cocultured with the adherent MCA fibrosarcoma, and before adding the MTT reagent, the supernatant content of each well was carefully removed, leaving the adherent MCA-fibrosarcoma in the wells.19 Absorbance was read at 570 nm by GloMax®-Multi Detection System (Promega, Madison, WI, USA).
Cell culture for cytokine analysis
Spleen cells, macrophages, NK cells, or T-cells from healthy mice were treated as above and incubated with DCA. In the case of MCA fibrosarcoma stimulation, MCA fibrosarcoma cells were seeded into 96-well plates at a density of 105 cells/well for 24 hours, followed by the addition of spleen cells at a similar density. DCA was then added, and cells were incubated for an additional 48 hours. While in PMA-stimulation, spleen cells were seeded into 96-well plates at a density of 105 cells/well. After 2 hours, 50 ng/mL PMA was added, followed by the addition of 100 μL DCA for another 48 hours. Following the second incubation, the content of the wells was harvested and added to individual tubes containing 50 μL of 0.1% Igepal nonionic detergent diluted in endotoxin-free PBS for 10 minutes at 4°C.21 The tubes were stored at −30°C for cytokine analysis.
Cytokine analysis
Interferon (IFN)-γ, IL-17, IL-12, TGF-β, or IL-10 levels were assayed using enzyme-linked immunosorbent assay according to the manufacturer’s instructions (mouse Duoset® cytokines; R&D Systems).
Inflammation and histology assessment
Mice weighing 25–30 g were shaved in the dorsal area and left for 3 days, followed by a topical treatment with PMA (2.5 μg) in 200 μL acetone.22 DCA (20 mg/kg) was given orally to one of the PMA-treated groups on the day of application, and another two doses were given after 24 and 48 hours. Mice were sacrificed 48 hours after PMA application, and the skin from shaved areas was removed from each mouse. One skin sample was placed in 10% formalin for further histology examination, and the skin tissues from the other mice were weighed and transferred to 2 mL cold, sterile PBS containing 0.1% Igepal and incubated for 10 minutes. The skin was homogenized, centrifuged, and the supernatant was collected and stored at −25°C for cytokine analysis. Histopathology was performed by a certified histopathologist at Sultan Medical Lab, Amman, Jordan. Tissue sections were 7–9 μm thick and stained using hematoxylin and eosin stain.
Data analysis
Data in the figures are presented as mean ± standard error of the mean and assessed by using one way ANOVA analysis followed by a Tukey’s test (95% confidence) for multiple comparisons (SPSS version 17; IBM Corporation, Armonk, NY, USA). The 50% inhibitory concentration (IC50%) was calculated from each independent experiment, and the average ± standard error is presented. P<0.05 was considered statistically significant.
Results
High concentrations of DCA reduced the viability and proliferation of splenocytes
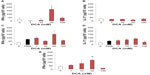
Using cell viability and proliferation assay, DCA reduced the viability and proliferation of unstimulated and PHA-stimulated splenocytes, respectively, in a dose-dependent manner. This reduction was observed at high DCA concentrations (50 mM and 100 mM; P<0.01) (Figure 1). However, the IC50% of DCA was 89±10 mM and 48±5 mM on unstimulated and PHA-stimulated splenocytes, respectively, indicating higher effect of DCA on splenocyte proliferation.
DCA decreased MCA fibrosarcoma survival, and this activity was enhanced by splenocytes
The first question was whether DCA can reduce MCA fibrosarcoma proliferation and viability and secondly, if such activity can be enhanced by splenocytes. Increasing concentrations of DCA significantly decreased the percent survival of MCA fibrosarcoma cells. The percent survival reached 13% and 16% at 50 mM and 100 mM DCA, respectively (P<0.001) (Figure 2). The IC50% of DCA on MCA fibrosarcoma was 23.0±4.0 mM. However, when splenocytes were cocultured with MCA fibrosarcoma cells, the percent survival of MCA fibrosarcoma cells was reduced by 13% and shifted the DCA-induced survival curve to the left. After normalizing the effect of splenocytes, DCA at 10 mM significantly reduced the percent survival in comparison to the cultures without splenocytes (P<0.001) (Figure 2). In addition, the IC50% of DCA dropped to 15.7±2.1 mM in comparison to 23.0±4.0 mM (P<0.05) when MCA fibrosarcoma cells were cultured without splenocytes.
DCA increased IFN-γ but not IL-17 production from stimulated and unstimulated splenocytes
The following sets of experiments were performed to assess the association of DCA in cytokine modulation. First, we evaluated IFN-γ and IL-17 production using MCA-stimulated splenocytes to resemble the behavior of immune cells in vivo when they are in close proximity with cancer cells. In addition, we used a protein kinase C activator, PMA, as another stimulatory model. In PMA and MCA fibrosarcoma-stimulated splenocytes, DCA at 50 mM increased IFN-γ but not IL-17 levels in comparison to stimulated control cells (P<0.01) (Figure 3A–D). Second, we examined whether DCA could induce such cytokines without exogenous stimuli. Herein, DCA was found to induce IFN-γ from unstimulated spleen cells in a concentration-dependent manner (Figure 3E), and the IFN-γ levels were significantly higher at 10 mM and 50 mM of DCA (P<0.05 and P<0.01, respectively).
DCA increased IL-12 production from unstimulated macrophages but did not modulate IFN-γ production from T-cells or NK cells
To evaluate the cytokine modulatory effect of DCA on unstimulated purified subsets of immune cells, T-cells, NK cells, and macrophages were isolated from mouse spleen. DCA significantly increased the production of IL-12 from macrophages, and at a DCA concentration range of 10–100 mM, IL-12 production was significantly increased (P<0.05) (Figure 4A). On the other hand, DCA did not modulate IFN-γ production from NK cell or T-cell cultures (Figure 4B and C).
Anti-IL-12 antibody reversed DCA effect on IFN-γ production from splenocytes
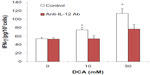
To investigate if the DCA enhancing effect on IFN-γ production was due to induction of IL-12, anti-IL-12 antibody (160 ng/mL) was added to the cultures at the same time as the addition of DCA. The addition of anti-IL-12 antibody reversed the DCA enhancing effect on IFN-γ production. This reversal effect was complete at 10 mM DCA and up to 65% at DCA 50 mM (P<0.01) (Figure 5).
DCA restored IL-12, IFN-γ, and TGF-β levels and reduced IL-10 in PMA- induced skin inflammation in mice
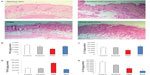
We generated PMA-induced skin inflammation in vivo to assess cytokine modulation following three oral administrations of DCA at 20 mg/kg. Applying PMA once topically induced severe skin inflammation in the deep dermis, reaching fatty tissue. The majority of cells in the affected area consists of neutrophils and macrophages (Figure 6A). The epidermis also showed pustules with areas of hyperkeratosis. In DCA-PMA treated mice, inflammation was still present in the dermis, and the epidermis showed large bullae filled with pustules consisting of neutrophils and macrophages. Such a histopathological pattern was investigated further by determining the type of cytokines produced and whether they reflected the infiltrating immune cells types. PMA lowered the levels of IL-12, IFN-γ, and IL-10 and increased TGF-β in the skin of mice 48 hours after PMA application (P<0.05). However, oral administration of 20 mg DCA/kg reversed the effect of PMA on IL-12, IFN-γ, and TGF-β levels and decreased further IL-10 levels (P<0.05) (Figure 6B–E).
Discussion
All previous studies confirmed that DCA can target cancer cells mainly via inhibiting PDK, followed by increasing cancer cell apoptosis.23 Similarly, the current study showed that DCA reduced the survival of MCA fibrosarcoma cells in a dose-dependent manner. However, the current study also showed that this survival reduction was enhanced when DCA is incubated with spleen cells. The latter suggests that DCA enhances killing of cancer cells through activating immune cells. In addition, although previous published work showed that DCA has no apparent toxicity by measuring hemoglobin, serum transaminases, and creatinine levels following 12-week oral administration of 50–100 mg/kg DCA,2 our data showed that high DCA concentrations (50–100 mM) reduced splenocytes viability and their proliferative ability. However, these latter concentrations are highly unlikely to be reached in vivo.
Recently, Ohashi et al have demonstrated that DCA promotes immunomodulatory activity.24 They have shown that DCA increases the number of IFN-γ producing CD8+ T and NK cells but did not significantly modulate IFN-γ levels from simulated splenocytes.24 However, looking carefully at their data, DCA exhibited a trend toward increasing IFN-γ production from nonlactic acid-treated stimulated splenocytes at the concentrations used (2–10 mM). In the present study, DCA increased the production of IFN-γ but not IL-17 from PMA and MCA fibrosarcoma-stimulated splenocytes. In addition, DCA increased the production of IFN-γ from unstimulated spleen cells. This increase, however, was not observed at 100 mM of DCA, which could be explained by the reduction of splenocytes survival at such concentration.
To look further into DCA immunomodulatory activity, DCA was tested on isolated immune cell subsets: macrophages, NK cells, and T-cells. DCA induced IL-12 production from unstimulated macrophages but not IFN-γ production from NK or T-cells. Moreover, when this IL-12 production is blocked, DCA enhancing IFN-γ production from splenocytes is reversed, indicating that DCA induces IFN-γ via an IL-12-mediated pathway. Previous studies have shown that DCA inhibits downregulation of K+ channels (Kv1.5), and blocking these channels reduces the production of IL-12 from dendritic cells.2,25 Thus, we hypothesize that the DCA effect of inducing the production of IL-12 from macrophages is through the upregulation of Kv1.5 channels.2,25 IL-12 then binds to IL-12 receptors on Th1 cells and activates transcription factor belongs to signal transducer activator transcription gene 4 that ends up in upregulating IFN-γ gene expression.17,26,27 More studies, however, addressing the role of DCA on the IL-12–IFN-γ pathway induction are warranted.
To investigate if DCA can modulate cytokines in vivo, a skin inflammation model using PMA was used.22 DCA was given orally at a concentration based on the currently available preclinical and human DCA studies.28,29 DCA did not modulate the severity of PMA-induced inflammatory pattern as shown by the histopathology study; however, it did restore PMA-lowering IFN-γ and IL-12 levels and PMA-increasing TGF-β levels in mouse skin. In addition, DCA inhibited IL-10 production. These results suggest that DCA polarizes macrophages into M1 type. Such polarization may remodel tumor associated macrophages, which in turn activates IFN-γ production from Th1 and NK cells, leading to enhancing the antitumor activity of NK cells, dendritic cells, and cytotoxic T lymphocytes and suppresses T regulatory and myeloid suppressor cells.17,25–30 Further research in the above areas are warranted to validate such a hypothesis.
In conclusion, our study showed that DCA modulates cytokines in favor of Th1 function, and this activation is associated with enhanced DCA cytotoxicity against MCA fibrosarcoma. Further studies are necessary to explain the action of DCA on macrophages and their polarization in the cancer microenvironment. Finally, DCA immunomodulatory effects are promising and merit further investigation to improve its use as an anticancer drug.
Acknowledgments
The authors gratefully acknowledge the financial support from the Scientific Research Fund (Grant number MPH-2-01-2011), Amman, Jordan and the Deanship of Research and Graduate Studies at the University of Petra (Grants number 1-4-2012 and 1-4-2013), Amman, Jordan.
Disclosure
The authors report no conflicts of interest in this work.
References
Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer. 2004;4(11):891–899. | |
Bonnet S, Archer SL, Allalunis-Turner J, et al. A mitochondria-K+ channel axis is suppressed in cancer and its normalization promotes apoptosis and inhibits cancer growth. Cancer Cell. 2007;11(1):37–51. | |
Michelakis ED, Webster L, Mackey JR. Dichloroacetate (DCA) as a potential metabolic-targeting therapy for cancer. Br J Cancer. 2008;99(7):989–994. | |
Heshe D, Hoogestraat S, Brauckmann C, Karst U, Boos J, Lanvers-Kaminsky C. Dichloroacetate metabolically targeted therapy defeats cytotoxicity of standard anticancer drugs. Cancer Chemother Pharmacol. 2011;67(3):647–655. | |
Madhok BM, Yeluri S, Perry SL, Hughes TA, Jayne DG. Dichloroacetate induces apoptosis and cell-cycle arrest in colorectal cancer cells. Br J Cancer. 2010;102(12):1746–1752. | |
Tong J, Xie G, He J, Li J, Pan F, Liang H. Synergistic antitumor effect of dichloroacetate in combination with 5-fluorouracil in colorectal cancer. J Biomed Biotechnol. 2011;2011:740564. | |
Papandreou I, Goliasova T, Denko NC. Anticancer drugs that target metabolism: Is dichloroacetate the new paradigm? Int J Cancer. 2011;128(5):1001–1008. | |
Robey IF, Martin NK. Bicarbonate and dichloroacetate: evaluating pH altering therapies in a mouse model for metastatic breast cancer. BMC Cancer. 2011;11:235. | |
Cao W, Yacoub S, Shiverick KT, et al. Dichloroacetate (DCA) sensitizes both wild-type and over expressing Bcl-2 prostate cancer cells in vitro to radiation. Prostate. 2008;68(11):1223–1231. | |
Xie J, Wang BS, Yu DH, et al. Dichloroacetate shifts the metabolism from glycolysis to glucose oxidation and exhibits synergistic growth inhibition with cisplatin in HeLa cells. Int J Oncol. 2011;38(2):409–417. | |
Wong JY, Huggins GS, Debidda M, Munshi NC, De Vivo I. Dichloroacetate induces apoptosis in endometrial cancer cells. Gynecol Oncol. 2008;109(3):394–402. | |
Stockwin LH, Yu SX, Borgel S, et al. Sodium dichloroacetate selectively targets cells with defects in the mitochondrial ETC. Int J Cancer. 2010;127(11):2510–2519. | |
Shahrzad S, Lacombe K, Adamcic U, Minhas K, Coomber BL. Sodium dichloroacetate (DCA) reduces apoptosis in colorectal tumor hypoxia. Cancer Lett. 2010;297(1):75–83. | |
Kaufmann P, Engelstad K, Wei Y, et al. Dichloroacetate causes toxic neuropathy in MELAS: a randomized, controlled clinical trial. Neurology. 2006;66(3):324–330. | |
Abdelmalak M, Lew A, Ramezani R, et al. Long-term safety of dichloroacetate in congenital lactic acidosis. Mol Genet Metab. 2013;109(2):139–143. | |
Balwit JM, Hwu P, Urba WJ, Marincola FM. The iSBTc/SITC primer on tumor immunology and biological therapy of cancer: a summary of the 2010 program. J Transl Med. 2011;9:18. | |
Alshaker HA, Matalka KZ. IFN-γ, IL-17 and TGF-β involvement in shaping the tumor microenvironment: The significance of modulating such cytokines in treating malignant solid tumors. Cancer Cell Int. 2011;11:33. | |
Alshaker HA, Qinna NA, Qadan F, Bustami M, Matalka KZ. Eriobotrya japonica hydrophilic extract modulates cytokines in normal tissues, in the tumor of Meth-A-fibrosarcoma bearing mice, and enhances their survival time. BMC Complement Altern Med. 2011;11:9. | |
Matalka KZ, Alsaadi MT, Qinna N, et al. Enhancing doxorubicin-induced MCA-fibrosarcoma cytotoxicity by an Eriobotrya japonica hydrophilic butanol-treated extract through natural killer cells. J Cancer Sci Ther. 2012;S18:003. | |
Wahl LM, Smith PD. Current Protocols in Immunology. Coligan JE, Bierer BE, Margulies DH, Shevach EM, Strober W, editors. Hoboken, NJ, USA: John Wiley & Sons, Inc. 2011. | |
Matalka KZ, Tutunji MF, Abu-Baker M, Abu Baker Y. Measurement of protein cytokines in tissue extracts by enzyme-linked immunosorbent assays: application to lipopolysaccharide-induced differential milieu of cytokines. Neuro Endocrinol Lett. 2005;26(3):231–236. | |
Sung YM, He G, Fischer SM. Lack of expression of the EP2 but not EP3 receptor for prostaglandin E2 results in suppression of skin tumor development. Cancer Res. 2005;65(20):9304–9311. | |
Sutendra G, Michelakis ED. Pyruvate dehydrogenase kinase as a novel therapeutic target in oncology. Front Oncol. 2013;3:38. | |
Ohashi T, Akazawa T, Aoki M, et al. Dichloroacetate improves immune dysfunction caused by tumor-secreted lactic acid and increases antitumor immunoreactivity. Int J Cancer. 2013;133(5):1107–1118. | |
Mullen KM, Rozycka M, Rus H, et al. Potassium channels Kv1.3 and Kv1.5 are expressed on blood-derived dendritic cells in the central nervous system. Ann Neurol. 2006;60(1):118–127. | |
Yang J, Murphy TL, Ouyang W, Murphy KM. Induction of interferon-gamma production in Th1 CD4+ T-cells: evidence for two distinct pathways for promoter activation. Eur J Immunol. 1999;29(2):548–555. | |
Martín-Fontecha A, Thomsen LL, Brett S, et al. Induced recruitment of NK cells to lymph nodes provides IFN-gamma for T(H)1 priming. Nat Immunol. 2004;5(12):1260–1265. | |
Michelakis ED, Sutendra G, Dromparis P, et al. Metabolic modulation of glioblastoma with dichloroacetate. Sci Transl Med. 2010;2(31):31ra34. | |
Strum SB, Adalsteinsson O, Black RR, Segal D, Peress NL, Waldenfels J. Case report: Sodium dichloroacetate (DCA) inhibition of the “Warburg Effect” in a human cancer patient: complete response in non-Hodgkin’s lymphoma after disease progression with rituximab-CHOP. J Bioenerg Biomembr. 2013;45(3):307–315. | |
Martín-Fontecha A, Thomsen LL, Brett S, et al. Induced recruitment of NK cells to lymph nodes provides IFN-gamma for T(H)1 priming. Nat Immunol. 2004;5(12):1260–1265. | |
Komita H, Homma S, Saotome H, Zeniya M, Ohno T, Toda G. Interferon-gamma produced by interleukin-12-activated tumor infiltrating CD8+T-cells directly induces apoptosis of mouse hepatocellular carcinoma. J Hepatol. 2006;45(5):662–672. | |
Martini M, Testi MG, Pasetto M, et al. IFN-gamma-mediated upmodulation of MHC class I expression activates tumor-specific immune response in a mouse model of prostate cancer. Vaccine. 2010;28(20):3548–3557. | |
Yang L, Pang Y, Moses HL. TGF-beta and immune cells: an important regulatory axis in the tumor microenvironment and progression. Trends Immunol. 2010;31(6):220–227. | |
Gorska AE, Jensen RA, Shyr Y, Aakre ME, Bhowmick NA, Moses HL. Transgenic mice expressing a dominant-negative mutant type II transforming growth factor-beta receptor exhibit impaired mammary development and enhanced mammary tumor formation. Am J Pathol. 2003;163(4):1539–1549. | |
Yang L, Huang J, Ren X, et al. Abrogation of TGF beta signaling in mammary carcinomas recruits Gr-1+CD11b+ myeloid cells that promote metastasis. Cancer Cell. 2008;13(1):23–35. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.