Back to Archived Journals » Reports in Medical Imaging » Volume 14
Diagnostic Value of Perfusion-Weighted Magnetic Resonance Imaging as an Adjunct to Routine Magnetic Resonance Protocols for Adults Presenting with Acute Ischemic Stroke
Authors Jaafari O, Gallagher H, Alshehri M, Hakami K, AlShammari M
Received 7 August 2021
Accepted for publication 28 October 2021
Published 16 November 2021 Volume 2021:14 Pages 79—89
DOI https://doi.org/10.2147/RMI.S331876
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Tarik Massoud
Osama Jaafari,1 Helen Gallagher,2 Muhammed Alshehri,1 Khalid Hakami,1 Majedh AlShammari1
1Royal Commission Medical Centre, King Fahd, Al-Nakheel, Yanbu, Saudi Arabia; 2School of Health and Life Sciences, Glasgow Caledonian University, Glasgow, Scotland
Correspondence: Osama Jaafari
Royal Commission Medical Centre, King Fahd, Al-Nakheel, Yanbu, 46451, Saudi Arabia
Tel +966-14-393-7700
Fax +966143922483
Email [email protected]
Background: Stroke is one of the most burdensome neurological conditions affecting young and older adult populations worldwide, and notably it has remained one of the leading causes of disability and premature mortality within its disease category. Timely and accurate diagnosis and characterization of stroke with cross-sectional imaging impacts significantly on treatment planning and patient outcomes, particularly with the advent of early reperfusion therapies. Recently, there has been growing recognition that perfusion-weighted MRI (PW-MRI) may offer a significant advantage in diagnosing and characterizing the ischemia induced by stroke, as it can detect diffusion–perfusion deficits that may influence treatment decisions to prevent infarction. This structured literature review sought to explore the diagnostic utility and accuracy of PW-MRI compared to other MRI protocols.
Methods: A literature search was performed using Medline, Embase, and the Cochrane Library, with articles limited to the English language and publication in the last 15 years. Eligible articles were appraised using the QUADAS-2 framework, and given marked interstudy heterogeneity, the diagnostic outcomes were analyzed using a narrative approach.
Results: A total of ten studies were reviewed — retrospective and one prospective — and found to observe a moderate–high risk of bias. The studies revealed that PW-MRI offers beneficial diagnostic capabilities when compared to other MRI sequences and ability to detect significant diffusion–perfusion mismatches. However, PW-MRI was found to overestimate the degree of oligemia and ischemia in brain tissue. As ischemia does not always progress to infarction, thrombolytic treatment may be unnecessary in some cases and expose patients to adverse effects.
Conclusion: These findings indicate that PW-MRI should be considered for routine imaging as an adjunct to other MRI protocols and an alternative to CT thereby reducing ionizing radiation exposure.
Keywords: perfusion–diffusion deficits, cross-sectional diagnostic imaging
Introduction
Stroke is a highly prevalent and disabling neurological condition that predominantly affects older persons and is one of the leading causes of permanent morbidity and mortality, with life expectancy averaging toward the eighth decade of life.1 On a global level, stroke is reported to affect >13 million people annually. The lifetime risk of stroke is 25%,2 and it is the second-leading cause of premature mortality behind ischemic heart disease.3
Efforts to reduce the burden of stroke have focused upon early reperfusion therapies that rely on timely and accurate diagnosis, ascertained using cross-sectional imaging. The diagnosis of acute ischemic stroke (AIS) is contingent on patient history, clinical examination findings, and radiological evidence of cerebral vascular obstruction. Diagnosis in the first few hours of symptom onset has been informed by CT, given its ready availability and high accuracy in detecting ischemic and hemorrhagic change.4 The CT brain scan is one of the most commonly requested initial investigations and is the first-line investigation for suspected acute stroke (both hemorrhagic and ischemic), because it provides a rapid radiological overview of the brain and its surrounding structures. Indeed, the evidence demonstrates unequivocally that a CT brain scan can confirm or exclude intracranial hemorrhage and stroke, and it can be performed in seconds.5 However, CT is associated with ionizing radiation, which has known stochastic and deterministic effects upon long-term health., It also has poor sensitivity in detecting early loss of gray–white matter differentiation, which can lead to false-negative diagnoses. Unlike MRI, CT is unable to provide information about the functional status of ischemic brain tissue. For these reasons, MRI has been generally thought to be superior to CT for the diagnosis of acute stroke. This is because changes in acute ischemic injury can be detected earlier with MRI than CT, especially when diffusion-weighted imaging is utilised.6 One 2007 study sought to compare CT and MRI for the emergency diagnosis of acute stroke: 365 patients (217 of whom were subsequently diagnosed with acute stroke) were assessed by researchers. MRI detected both AIE and acute hemorrhagic stroke more frequently than CT (p<0.0001). In patients who were subjected to radiological imaging within 3 hours of symptom onset, MRI detected AIE in 46% of patients, while CT detected AIE in only 7% of patients. Based on these data, researchers determined that MRI had a sensitivity of 83% compared to just 26% for CT.6 There are several reasons for the superiority of MRI to CT in terms of detecting acute stroke. MRI can provide detailed images of cerebrovascular perfusion, which is invaluable for understanding AIS pathophysiology and thus — through more informed treatment — improved patient outcomes.7 However, the overall accuracy of MRI can vary based on the different sequences utilized. This can influence decisions on prescribing brain-tissue and lifesaving reperfusion therapy.8 Another benefit of MRI over CT is that there is an absence of radiation and relatively safe contrast that is administered in lower doses. One MRI technique, known as arterial spin labeling, enables imaging of the brain without administering contrast agents. This could be a significant advantage for patients with renal impairment or who have allergies to contrast agents.9 However, it bears mention that MRI is more complex, time-consuming and expensive than CT. One of the most promising MRI sequences has been perfusion-weighted MRI (PW-MRI).2 This modality can evaluate the hemodynamic status of brain tissue and detect variances in perfusion that can help identify, characterize, and discriminate between the ischemic core and the penumbra.10
The use of PW-MRI has recently increased in response to advances in reperfusion therapy, and in particular the approval of thrombolytic agents, which have been among the only treatments to improve AIS outcomes.11 PW-MRI allows the visualization blood flow and thus perfusion of brain tissue at the microvascular level. It is ideal for ascertaining the presence and extent of oligemic, ischemic, and infarcted brain tissue. In DSM PW-MRI, the contrast administered leads to alterations in the magnetic field over time with the dephasing of spins, resulting in signal loss during perfusion. By applying a multiphase acquisition, the perfusion of the contrast agent can be visualized over a temporal-capture phase of 45–90 seconds and viewed as perfusion maps.7 Notably, these maps also permit the quantification of perfusion parameters, such as relative mean transit time and relative cerebral blood flow and volume. Numeric measures of perfusion may facilitate the characterization of ischemic change that can better inform salvageability and treatment in the future.12
However, the short temporal assessment using PW-MRI does not provide a reliable measure and predictor of the final ischemic and infarctive effects, as perfusion during AIS shows marked dynamicity.7 Therefore, using PW-MRI alone to inform diagnosis and treatment may compromise outcomes, as changes in perfusion over time may contraindicate or demand thrombolytic therapy at differing periods. Evidence has shown that even minimal delays between CT and PW-MRI can demonstrate substantial variance in the extent of ischemia and brain perfusion, which can cause clinical uncertainty.13
The use of PW-MRI as complementary imaging to conventional MRI protocols has important clinical benefits. For the purpose of this discussion, conventional MRI protocols comprise T1-weighted, T2-weighted, fluid-attenuated inversion recovery (FLAIR), diffusion-weighted MRI (DW-MRI), and susceptibility-weighted MRI. Parsons et al14 showed that information on diffusion–perfusion mismatch attained using PW-MRI resulted in greater instigation and success of tPA therapy than a historical control cohort who received DW-MRI. Treatment with tPA resulted in considerable sparing of infarcted brain tissue, with a higher proportion of persons failing to progress to infarction. Furthermore, treatment resulted in considerably less expansion of infarct size, which would have accounted for significant variance in patient outcomes. Ryu et al8 conducted a meta-analysis of 13 trials exploring the utility of PW-MRI for stroke patients, and found that it was more effective at informing patient suitability for reperfusion treatment than other imaging. Patients receiving treatment following PW-MRI were twice as likely to achieve a desirable neurological outcome at 3 months.
Clinical guidelines recommend that nonenhanced CT be used to diagnose AIS should patients be suitable for thrombolysis. The acquisition time for CT is significantly faster than MRI and thus more applicable to the acute environment. However, perfusion imaging using CT or an MRI equivalent is essential for those who may be candidates for thrombectomy and presenting beyond 6 hours of symptom onset.15,16 Presently, MRI is reserved as a second-line imaging modality to CT for the diagnosis of AIS, although MRI offers the benefit of avoiding ionizing radiation exposure and specific protocols, and it may provide superior imaging quality to permit accurate diagnosis.4 This systematic literature review employed a central research question derived using the recommended PICO framework.17,18 It aimed to critically evaluate the diagnostic accuracy, sensitivity, specificity, and predictive values of PW-MRI and other MRI protocols for the diagnosis of AIS in adults, determine the implications for clinical practice and guidelines, and identify areas for future research.
Methods
A search query was developed and used in databases (Medline, the Cochrane Library, Embase, PubMed, and Scopus), and journal websites. Search terms were derived from the review question and the PICO elements: population — adults with AIS, intervention — PW-MRI, comparator — other MRI protocols, and outcomes — diagnostic accuracy. To ensure the application of all appropriate terms, a scoping review helped identify additional terms that would improve searchability.19,20 The final terms were combined in a string using Boolean operators with “OR” and “AND.” The search strategy (Table 1) precluded the comparator component, as its inclusion may have led to excessive search precision and a high risk of missing pertinent studies.21 Studies with a relevant comparator of congruence with the review question were identified during study filtering and selection.
 |
Table 1 Search summary |
Inclusion and Exclusion Criteria
Inclusion criteria were based on the PICO elements and are outlined in Table 2. The indices of diagnostic accuracy formerly noted were selected as the primary outcomes, given that these are widely used to define the accuracy of diagnostic tests in the literature, although with a limited number of relevant studies, diagnostic utility also had to be considered.22 In addition, the reports of image interpreters concerning the utility and accuracy of MRI protocols were considered, as this can provide a realistic and pragmatic perspective of radiological tests.23 Notably, the comparator component of diagnostic accuracy reviews involving imaging tests would usually seek to compare accuracy with the gold-reference standard, which is normally histological examination.24 However, histology was not apposite. Therefore, the comparator was deemed to be non–PW-MRI protocols. The results of study search and selection are described and presented using the standard PRISMA28 format in Figure 1.
 |
Table 2 Inclusion and exclusion criteria |
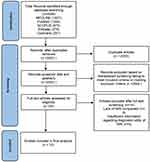 |
Figure 1 PRISMA flowchart. Notes: PRISMA figure adapted from Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. Creative Commons.28 |
Quality Assessment
Quality assessment using the QUADAS-2 tool was undertaken to judge the internal and external validity and generalizability of the diagnostic accuracy data reported. This is a validated and widely used approach to scrutinizing research on diagnostic accuracy design.25 QUADAS-2 details a series of questions designed to elicit bias and other methodological issues across several key domains.26 In studies where one or more quality questions in each domain were rated as yes, no, or unclear, the risk of bias was deemed low, high, or unclear respectively.26
Data Extraction and Analysis
Study data were extracted using a rigorous and reliable process15 into predefined templates, and these were transcribed into physical and electronic databases to permit interstudy comparisons. Diagnostic accuracy data from each study were described briefly using a tabular method, and the collective diagnostic accuracy of PW-MRI was calculated by formulating the mean sensitivity, specificity, negative predictive value (NPV), and positive predictive value (PPV), among other indices. Sensitivity and specificity were calculated as a mean of those reported or were calculated from the raw data of informing studies using the formule provided in Table 3. Similarly, mean NPV and PPV were calculated: NPV = true negatives/(false negatives + true negatives); PPV = true positives/(true positives + false positives).27
 |
Table 3 Calculation of sensitivity and specificity26 |
Results
Following search and selection, ten studies met the criteria. The search results can be seen in Figure 1. The collective sample size was 1,184 subjects; however, there was wide variance across the included studies. A summary of study characteristics can be seen in Table 4. A majority of these studies29,30,32–36 did not define the power attained for the sample sizes analyzed, and thus those with small samples could have been at risk of type II error,39 although the lack of reporting makes this inference unclear. In contrast, three studies had sample sizes >100 subjects, and thus a low risk of type II error was inferred.31,36,37
 |
Table 4 Summary of study characteristics |
All the studies explored the diagnostic utility and/or accuracy of PW-MRI, although there was interstudy variance in terms of the specific technical protocols used to acquire images and comparator imaging.29–38 All studies compared PW-MRI against the appropriate comparator of DW-MRI, and three also compared the protocol with additional MR sequences, including MR angiography31,32 and FLAIR. Three studies failed to define the specific technical parameters used to acquire PW-MRI and DW-MRI, and thus it was not possible to compare the diagnostic utility/accuracy of these protocols with other studies in this review.33,35,36, All studies utilized contrast-enhanced MRI with a magnetic field strength of 1.5 T, although one study included a mix of MR scans taken at 1.5 and 3.0 T, which may have implications for the diagnostic utility.36 There was varied reporting of the diagnostic utility and accuracy of PW-MRI and other MR protocols. The specific outcomes reported included sensitivity, specificity and/or interobserver variability,30,33,36,37, degree of perfusion–diffusion mismatching,31–35,38, lesion size or extent,29,35 and treatment suitability and clinical outcomes.31,32,34–36
QUADAS-2 Quality Assessment
Outcomes from the quality assessment are detailed in Table 5. Overall, the risk of bias was deemed high, and concern over applicability was also high. This is not unexpected, given that all studies employed retrospective methodologies. A major contributor to these findings was the use of DW-MRI as a reference test. No histological gold standard is readily available, and thus the risk of reference-misclassification bias40 was deemed high, given that DW-MRI is not completely sensitive and specific for IE.
 |
Table 5 Summary of risk of bias using QUADAS-2 |
Diagnostic Utility and Accuracy of PW-MRI
Reporting of the diagnostic utility and accuracy of PW-MRI varied across studies, due to the various measures that were employed to ascertain the diagnosis of IE and/or characterize the extent of ischemia. Four studies reported on the usual accepted measures of diagnostic accuracy, which include sensitivity and specificity.30,33,36,37 Butcher et al30 found PW-MRI sensitivity of 78% and specificity of 88% for eliciting perfusion–diffusion mismatches by volume, as well as high interrater reliability, with #x1D705; scores >0.94 for experienced radiologists. However, agreement was much lower among inexperienced radiologists (#x1D705;=0.28–0.49). Despite this, PW-MRI was significantly better at detecting perfusion–diffusion mismatching than DW-MRI (p<0.001). Simonsen et al36 found that the sensitivity and specificity of DW-MRI for diagnostic IE were 92% and 75%, respectively; however, complementing the protocol with PW-MRI increased sensitivity further to 97.5%. They also explored the effect of combined MRI scanning upon clinical outcomes, albeit indirectly, where one patient had a negative DW-MRI scan but a positive PW-MRI scan, and this resulted in a large infarction and a poor neurological outcome. Notably, the poor specificity of DW-MRI was largely observed for persons with posterior circulation strokes, which was significantly likely to be the location of ischemia when imaging was negative (p=0.0019). Straka et al33 also explored the utility of combined diffusion–perfusion MRI using an automated RAPID protocol to calculate diffusion–perfusion mismatching. DW-MRI showed a slightly greater correlation than PW-MRI for acute stroke (0.99 vs 0.96), although the difference was not significant. Overall, the sensitivity and specificity of combined imaging was 100% and 91%, respectively, which is similar to accuracy reported previously. Wolman et al37 even showed that DW-MRI and PW-MRI provided comparable sensitivity (95.9%) and specificity (98.4%) for the accurate triage of patients with IE to endovascular therapy, and notably this imaging approach was associated with successful recanalization of the obstructed cerebral vasculature in 96% of cases.
Several of the other studies also reported upon the ability of PW-MRI to characterize perfusion–diffusion mismatches. Cho et al31 showed that among 52 patients with anterior circulation stroke, three had negative DW-MRI scans but marked perfusion abnormalities on PW-MRI, which represents a 100% mismatch and indicates the marked clinical utility of perfusion imaging. Similarly, Hakimelahi et al38 revealed a complete 100% diffusion–perfusion mismatch between the MRI protocols in 49 of 68 patients with lesion volumes <70 mL (p<0.001) in a retrospective cohort and 35 of 48 in a prospective cohort (p<0.001). An earlier study revealed that PW-MRI provided vital detail of regions of low perfusion and oligemia, and thus areas that were at risk of infarction could be potentially spared using thrombolytic therapy. Such areas of ischemic compromise were not evident when using DW-MRI, suggesting that this protocol would lead to underdiagnosis and undertreatment of some cases, particularly as diffusion–perfusion mismatch persisted >9 hours in 84% of cases. In one case, PW-MRI was able to elicit a 340% mismatch at almost 6 hours postonset of stroke symptoms, where DW-MRI would have missed the extent of ischemic tissue. Overall, PW-MRI was able to identify 20%–50% of mismatches, even when DW-MRI observed areas of cerebral hypoperfusion.
While PW-MRI has been consistently supported as a highly useful imaging modality for IE, the findings of Kucinski et al29 demonstrated the contrary, wherein this protocol was associated with overestimating the extent of hypoperfused brain tissue at risk of infarction. They found that the mean volume of cerebral blood that did not progress to infarction was 148 mL, but the sensitivity for predicting infarctive death was only 56%. Similarly, Motta et al35 showed that the degree of perfusion observed on PW-MRI did not always correlate with cognitive deficits in patients and that persistent hypoperfused areas of the brain did not always progress to ischemia and infarction, suggesting that the protocol is prone to overdiagnosis or overcharacterization of IE. However, the utility of PW-MRI for providing a measure of reliability for mismatching pre- and postthrombolytic therapy has been demonstrated to be desirable by Luby et al,34 who found sensitivity, specificity, and PPV of 82%, 80%, and 81%, respectively.
Discussion
The QUADAS-2 assessment revealed that 60% of the 10 studies had a high risk of bias for patient selection and index-test domains and 100% had a high risk of bias for the reference standard domain and patient flow and timing. Through this process of narrative description, the results revealed that in most cases, PW-MRI offered superior diagnostic capability to DW-MRI and MR angiography, with sensitivity as high as 100% and ability to detect significant diffusion–perfusion mismatches, which can significantly influence changes to management and in turn neurological outcomes. However, it was identified that PW-MRI may overestimate the degree of oligemia and ischemia in brain tissue, and indeed temporal observations showed that such aberrations did not always progress to overt infarction. As such, treatment in such cases would be unwarranted and expose patients to unnecessary effects. Despite this, it was demonstrated that PW-MRI substantially benefited care for a select number of cases, eg, better characterization of posterior circulation strokes, a limiting factor of DW-MRI. It also demonstrated consistency in detecting 20%–50% of mismatches, and in a proportion of cases mismatches in the order of 340%.
In view of the diagnostic accuracy of PW-MRI where high sensitivity for AIS (97.5%) was observed, the likelihood of correct diagnosis or classification of stroke, even when changes in brain tissue are markedly subtle, is high and the risk of false negatives low. However, the literature demonstrated that sensitivity varied somewhat, being as low as 78% for detecting perfusion–diffusion mismatches.32 Also, the small sample of this study may have resulted in an underestimation of the true accuracy of PW-MRI. Overall, PW-MRI showed specificity of 75%–88%. This appears to be largely due to difficulties in discriminating AIS from stroke mimics, eg, migraine and alcohol intoxication.36 Unfortunately, this detection and interpretation of perfusion–diffusion mismatches led to treatment of stroke mimics with unnecessary thrombolytic therapy only recognized following more stringent review of the clinical and image data. This problem may be difficult to overcome in practice, particularly given the narrow window of opportunity to instigate brain-sparing stroke therapy and clinical decision-making that likely favors treatment for AIS, even when a possibility of a stroke mimic exists, as a lack of treatment could be disabling or fatal in actual AIS cases.
Evidence to support the diagnostic accuracy indices of PW-MRI for AIS was limited by the lack of wider research reporting upon these outcome measures. Despite this, a recent meta-analysis of the accuracy of conventional MRI showed that it had similar sensitivity and specificity to that reported for PW-MRI here, but values that were superior to CT.41 These observations have also been supported in another meta-analysis of the accuracy of CT and MRI for AIS.42 Previous evidence has supported the superior ability of PW-MRI in characterizing diffusion–perfusion mismatches when compared to DW-MRI findings.10,43,44 Notably, such diffusion–perfusion deficits can persist >9 hours, exceeding the guideline-recommended treatment threshold by twofold. Therefore, for patients presenting with late-onset AIS, PW-MRI may offer diagnostic information that could signal suitability for reperfusion therapy.11 Copen et al11 believed that the ambiguity of AIS symptoms and thus time of presentation to clinicians was due to interpatient differences in the extent of collateral cerebral circulation. Those with extensive collateral supply and thus a large diffusion–perfusion deficits are likely to experience milder neurological deficits, while those with poorer collateral flow and thus small diffusion–perfusion deficits are likely to show more significant neurological impairment. As such, the former is likely to present late and the latter likely to present sooner after AIS onset. However, in either case, the ability of PW-MRI to more accurately characterize mismatches in diffusion and perfusion appears to represent the ideal imaging modality for informing AIS treatment.
In the hyperacute phases of AIS, imaging using CT is the first-line modality for imaging the brain, given the ready availability of scanners and fast acquisition times, which are desirable to inform reperfusion therapy, given that delays in treatment progressively increase the risk of poorer outcomes.45 However, some studies have shown that while conducting MRI can take additional time, the neurological outcomes of patients who subsequently receive reperfusion therapy are similar.46–49 The use of MRI has been shown to be associated with a significantly reduced rate of symptomatic stroke posttherapy,47 suggesting that the risks of delayed treatment do not outweigh the benefit of attaining additional imaging information to directly inform therapy. The National Clinical Guidelines for Stroke50 recommend that reperfusion therapy be commenced as soon as possible after the ascertainment of AIS, but within 3–4.5 hours of symptom onset, and thus opting to utilize MRI instead of CT for the purposes of informing therapy is a reasonable and achievable option.50
The findings of this review suggest that the use of PW-MRI should supersede that of DW-MRI, as this is likely to better inform therapeutic decision-making and in turn patient outcomes. The primary reason for the benefit of PW-MRI is its superior ability to characterize diffusion–perfusion mismatches. According to the mismatch hypothesis, the difference in diffusion and perfusion of blood to an area of brain tissue provides vital information concerning the reversibility of ischemia and thus the window of opportunity to intervene and optimize the likelihood of preventing irreversible infarctive damage.51 Indeed, the landmark DEFUSE and EPITHET trials52,53 showed that the profiling of diffusion–perfusion mismatching in patients with AIS and the instigation of early reperfusion therapy in these individuals resulted in significantly more favorable outcomes than in patients who did not receive PW-MRI to provide information about mismatching (p<0.01).
The findings of this review and other studies evaluating the modality in isolation54 indicate that DW-MRI is associated with a considerable false-negative rate. Specifically, this review showed that PW-MRI can increase the sensitivity of DW-MRI from 90% to 100%, and thus this reflects a 10% lower risk of false-negative diagnosis. At present, however, PW-MRI is not considered a routine MRI protocol, and thus DW-MRI remains the first-line approach. Where DW-MRI is negative, PW-MRI is employed to improve certainty in the exclusion or confirmation of stroke.50,55
Most studies in this review utilized magnetic field strengths of 1.5 T, with only Simonsen et al38 using a mix of 1.5 and 3.0 T. Rosso et al56 confirmed that 1.5 T offers greater sensitivity for stroke than 3.0 Tesla (99.1% v. 92.5%), as well as conferring a lower false-negative rate (0.6% vs 6.1%). As the magnetic field strength was the only considerable technical variant among the MRI protocols of studies included in this review, any recommendations for PW-MRI should consider the use of 1.5 T, although this requires evaluation in future research. However, the diagnostic utility of PW-MRI can also be affected by other limitations, including subjectivity and related bias regarding the interpretation of perfusion maps and the various techniques that can be used to generate quantitative measures of perfusion status, which have been known to confer significant variance in ischemia volume in some cases.12 PW-MRI can result in overestimation of diffusion–perfusion mismatches, albeit to a lesser extent than DW-MRI, though in other cases the extent of tissue at risk of infarction may be underestimated.9 Efforts to try and resolve this problem have focused upon defining thresholds of mismatching, but this has been compromised, due to interpatient variance and the course of AIS, which means that it is unlikely that universal thresholds for mismatching to inform treatment will emerge in future.30 As such, the images provided by PW-MRI for patients with AIS will probably have to be considered on a case-by-case basis if treatment decision-making is to be safely and optimally informed.
Overcoming the specific limitations of different MRI protocols may however be achieved through utilizing multimodal MRI strategies that comprise the simultaneous acquisition of DW, PW, gradient-echo, angiography, and FLAIR MRI, as has been suggested by Kim et al.25 Although there may be some concern that acquisition times of multimodal MRI may narrow the window of opportunity to instigate reperfusion therapy suggested in clinical guidelines, there is now sufficient evidence that MRI protocols can be acquired within a reasonable period that is not excessively disparate to CT. For some patients, MRI or gadolinium contrast agents are contraindicated, ie, those with metal implants or severe renal problems, but for those who are suitable, this could extend the treatment eligibility for patients with AIS, and in turn lead to improvements in neurological outcomes.25,57
Conclusion
This study revealed that PW-MRI offered beneficial diagnostic capability for diagnosing AIS compared to DW-MRI and MR angiography for AIS. Given that PW-MRI tends to provide superior accuracy for diagnosing and characterizing AIS, it is recommended that this protocol be used in place of or as an adjunct to DW-MRI. PW-MRI permits the calculation of diffusion–perfusion mismatches, which is essential for detecting penumbre and in turn predicting regions of brain ischemia at risk of infarction. This is fundamental to informing both acute and delayed reperfusion therapy. Current guidelines should seek to incorporate recommendations on the utility of PW-MRI for patients presenting with signs or symptoms of AIS within and beyond the 4.5-hour threshold for thrombolytic or thrombectomy treatment.
MRI in hyperacute and acute IS has been shown to have comparable diagnostic accuracy, and unlike CT or standard MRI protocols alone, is able to provide clinicians with additional data on the functional status of ischemic brain tissue to better inform their treatment plans and thus positively impact patient outcomes. In patients presenting early with AIS, standard MRI with PW-MRI should be considered a first-line imaging modality, as greater characterization of brain oligemia and ischemia may lead to more appropriate decision-making regarding reperfusion therapy.
Disclosure
The author reports no financial or other conflicts of interest in this work.
References
1. Gorelick PB. The global burden of stroke: persistent and disabling. Lancet Neurol. 2019;18(5):417–418. doi:10.1016/S1474-4422(19)30030-4
2. Campbell BCV, De Silva DA, MacLeod MR, et al. Ischaemic Stroke. Nat Rev Dis Primers. 2019;5(1):70–78. doi:10.1038/s41572-019-0118-8
3. World Health Organization. The top 10 causes of death [online]: World Health Organization. 2018.
4. Bal S, Bhatia R, Menon BK, et al. Time Dependence of Reliability of Noncontrast Computed Tomography in Comparison to Computed Tomography Angiography Source Image in Acute Ischemic Stroke. Int J Stroke. 2015;10(1):55–60. doi:10.1111/j.1747-4949.2012.00859.x
5. Kular S, Martin A. A primer in interpretation of head CT scans’. Br J Hosp Med. 2019;80(11):C156–c161. doi:10.12968/hmed.2019.80.11.C156
6. Chalela JA, Kidwell CS, Nentwich LM, et al. Magnetic resonance imaging and computed tomography in emergency assessment of patients with suspected acute stroke: a prospective comparison. Lancet. 2007;369(9558):293–298. doi:10.1016/S0140-6736(07)60151-2
7. Harris AD, Coutts SB, Frayne R. Diffusion and perfusion MR imaging of acute ischemic stroke. Magn Reson Imaging Clin N Am. 2009;17(2):291–313. doi:10.1016/j.mric.2009.02.001
8. Ryu WHA, Avery MB, Dharampal N, et al. Utility of perfusion imaging in acute stroke treatment: a systematic review and meta-analysis. J Neurointerv Surg. 2017;9(10):1012–1016. doi:10.1136/neurintsurg-2016-012751
9. Vymazal J, Rulseh AM, Keller J, et al. Comparison of CT and MR imaging in ischemic stroke. Insights Imaging. 2012;3(6):619–627. doi:10.1007/s13244-012-0185-9
10. Neumann-Haefelin T, Wittsack HJ, Wenserski F, et al. Diffusion- and perfusion-weighted MRI. The DWI/PWI mismatch region in acute stroke. Stroke. 1999;30(8):1591–1597. doi:10.1161/01.STR.30.8.1591
11. Copen WA, Schaefer PW, Wu O. MR perfusion imaging in acute ischemic stroke. Neuroimaging Clin N Am. 2011;21(2):259–267.
12. Kane I, Carpenter T, Chappell F, et al. Comparison of 10 different magnetic resonance perfusion imaging processing methods in acute ischemic stroke: effect on lesion size, proportion of patients with diffusion/perfusion mismatch, clinical scores, and radiologic outcomes. Stroke. 2007;38(12):3158–3164. doi:10.1161/STROKEAHA.107.483842
13. Campbell BC, Christensen S, Levi CR, et al. Comparison of computed tomography perfusion and magnetic resonance imaging perfusion-diffusion mismatch in ischemic stroke. Stroke. 2012;43(10):2648–2653. doi:10.1161/STROKEAHA.112.660548
14. Parsons MW, Brber PA, Chalk J, et al. Diffusion‐ and perfusion‐weighted MRI response to thrombolysis in stroke. Ann Neurol. 2002;51(1):28–37. doi:10.1002/ana.10067
15. NICE. Stroke and transient ischaemic attack in over 16s: Diagnosis and initial management: NICE. 2019.
16. White P, Nanpragasam A. What is new in stroke imaging and intervention? Clin Med (Northfield Il). 2018;18:13–16. doi:10.7861/clinmedicine.18-2-s13
17. Pollock A, Berge E. How to do a systematic review. Int J Stroke. 2018;13(2):138–156. doi:10.1177/1747493017743796
18. Farrugia P, Petrisor BA, Farrokhyar F, et al. Research questions, hypotheses and objectives. Canadian J Surgery. 2010;53(4):278.
19. Maltby J, Williams G, McGarry J, et al. Research Methods for Nursing and Healthcare. Routledge; 2014.
20. Bettany-Saltikov J. How to Do a Systematic Literature Review in Nursing: A Step-By-Step Guide. McGraw-Hill Education (UK); 2012.
21. Aromataris E, Riitano D. Constructing a search strategy and searching for evidence. A guide to the literature search for a systematic review. Am J Nurs. 2014;114(5):49–56. doi:10.1097/01.NAJ.0000446779.99522.f6
22. Simundic AM. Measures of Diagnostic Accuracy: basic Definitions. Ejifcc. 2009;19(4):203–211.
23. Leeflang MM, Deeks JJ, Gatsonis C, et al. Systematic reviews of diagnostic test accuracy. Ann Intern Med. 2008;149(12):889–897. doi:10.7326/0003-4819-149-12-200812160-00008
24. Schmidt RL, Factor RE. Understanding Sources of Bias in Diagnostic Accuracy Studies. Arch Pathol Lab Med. 2013;137(4):558–565. doi:10.5858/arpa.2012-0198-RA
25. Kim KW, Lee J, Choi SH, et al. Systematic Review and Meta-Analysis of Studies Evaluating Diagnostic Test Accuracy: a Practical Review for Clinical Researchers-Part I. General Guidance and Tips. Korean J Radiol. 2015;16(6):1175–1187. doi:10.3348/kjr.2015.16.6.1175
26. Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155(8):529–536. doi:10.7326/0003-4819-155-8-201110180-00009
27. Parikh R, Mathai A, Parikh S, et al. Understanding and using sensitivity, specificity and predictive values. Indian J Ophthalmol. 2008;56(1):45–50. doi:10.4103/0301-4738.37595
28. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi:10.1371/journal.pmed.1000097
29. Kucinski T, Naumann D, Knab R, et al. Tissue at Risk Is Overestimated in Perfusion-Weighted Imaging: MR Imaging in Acute Stroke Patients without Vessel Recanalization. Am J Neuroradiolog. 2005;26(4):815–820.
30. Butcher K, Passons M, Allport L, et al. Rapid assessment of perfusion-diffusion mismatch. Stroke. 2008;39(1):75–81. doi:10.1161/STROKEAHA.107.490524
31. Cho T, Hermier M, Alawneh JA, et al. Total mismatch: negative diffusion-weighted imaging but extensive perfusion defect in acute stroke. Stroke. 2009;40(10):3400–3402. doi:10.1161/STROKEAHA.109.563064
32. Copen WA, Rezai Gharai L, Barak ER, et al. Existence of the diffusion-perfusion mismatch within 24 hours after onset of acute stroke: dependence on proximal arterial occlusion. Radiology. 2009;250(3):878–886. doi:10.1148/radiol.2503080811
33. Straka M, Albers GW, Bammer R. Real-time diffusion-perfusion mismatch analysis in acute stroke. J Magn Reson Imaging. 2010;32(5):1024–1037. doi:10.1002/jmri.22338
34. Luby M, Ku KD, Latour LL, et al. Visual perfusion-diffusion mismatch is equivalent to quantitative mismatch. Stroke. 2011;42(4):1010–1014. doi:10.1161/STROKEAHA.110.603290
35. Motta M, Ramadan A, Hillis AE, et al. Diffusion-perfusion mismatch: an opportunity for improvement in cortical function. Front Neurol. 2015;5(1):280–290. doi:10.3389/fneur.2014.00280
36. Simonsen CZ, Madsen MH, Schmitz ML, et al. Sensitivity of Diffusion- and Perfusion-Weighted Imaging for Diagnosing Acute Ischemic Stroke Is 97.5. Stroke. 2015;46(1):98–101. doi:10.1161/STROKEAHA.114.007107
37. Wolman DN, Iv M, Wintermark M, et al. Can diffusion- and perfusion-weighted imaging alone accurately triage anterior circulation acute ischemic stroke patients to endovascular therapy? J Neurointerv Surg. 2018;10(12):1132–1136. doi:10.1136/neurintsurg-2018-013784
38. Hakimelahi R, Yoo AJ, He J, et al. Rapid identification of a major diffusion/perfusion mismatch in distal internal carotid artery or middle cerebral artery ischemic stroke. BMC Neurol. 2012;12(1):132. doi:10.1186/1471-2377-12-132
39. Nayak B. Understanding the relevance of sample size calculation. Indian J Ophthalmol. 2010;58(6):469–470. doi:10.4103/0301-4738.71673
40. Biesheuvel C, Irwig L, Bossuyt P. Observed Differences in Diagnostic Test Accuracy between Patient Subgroups: is It Real or Due to Reference Standard Misclassification? Clin Chem. 2007;53(10):1725–1729. doi:10.1373/clinchem.2007.087403
41. Zhang X, Liang H. Systematic review with network meta-analysis: diagnostic values of ultrasonography, computed tomography, and magnetic resonance imaging in patients with ischemic stroke. Medicine. 2019;98(30):16360. doi:10.1097/MD.0000000000016360
42. Xin Y, Han FG. Diagnostic accuracy of computed tomography perfusion in patients with acute stroke: a meta-analysis. J Neurol Sci. 2016;360(1):125–130. doi:10.1016/j.jns.2015.11.046
43. Sorensen AG, Copen WA, Ostergaard L, et al. Hyperacute stroke: simultaneous measurement of relative cerebral blood volume, relative cerebral blood flow, and mean tissue transit time. Radiology. 1999;210(2):519–527. doi:10.1148/radiology.210.2.r99fe06519
44. Darby DG, Barber PA, Gerraty RP, et al. Pathophysiological topography of acute ischemia by combined diffusion-weighted and perfusion MRI. Stroke. 1999;30(10):2043–2052. doi:10.1161/01.STR.30.10.2043
45. Kang D, Chalela JA, Dunn W, et al. MRI screening before standard tissue plasminogen activator therapy is feasible and safe. Stroke. 2005;36(9):1939–1943. doi:10.1161/01.STR.0000177539.72071.f0
46. Schellinger PD, Thomalla GÖ, Fiehler J, et al. MRI-based and CT-based thrombolytic therapy in acute stroke within and beyond established time windows: an analysis of 1210 patients. Stroke. 2007;38(10):2640–2645. doi:10.1161/STROKEAHA.107.483255
47. Köhrmann M, Jüttler E. MRI versus CT-based thrombolysis treatment within and beyond the 3 h time window after stroke onset: a cohort study. Lancet Neurol. 2006;5(8):661–667. doi:10.1016/S1474-4422(06)70499-9
48. Yoo S, Kwon SU, Lee D, et al. Comparison between MRI screening and CT-plus-MRI screening for thrombolysis within 3 h of ischemic stroke. J Neurol Sci. 2010;294(1):119–123. doi:10.1016/j.jns.2010.03.015
49. Royal College of Physicians. National clinical guideline for stroke [online]. 2016.
50. Bateman M, Slater L, Leslie-Mazwi T, et al. Diffusion and Perfusion MR Imaging in Acute Stroke: clinical Utility and Potential Limitations for Treatment Selection. Top Magn Reson Imaging. 2017;26(2):77–82. doi:10.1097/RMR.0000000000000124
51. Albers GW, Thijs VN, Wechsler L, et al. 2006 Magnetic resonance imaging profiles predict clinical response to early reperfusion: the diffusion and perfusion imaging evaluation for understanding stroke evolution (DEFUSE) study. Ann Neurol. 2006;60(5):508–517. doi:10.1002/ana.20976
52. Davis SM, Donnan GA, Parsons MW, et al. Effects of alteplase beyond 3 h after stroke in the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET): a placebo-controlled randomised trial. Lancet Neurol. 2008;7(4):299–309. doi:10.1016/S1474-4422(08)70044-9
53. Oppenheim C, Stanescu R, Dormont D, et al. False-negative diffusion-weighted MR findings in acute ischemic stroke. Am J Neuroradiol. 2000;21(8):1434–1440.
54. Powers WJ, Rabinstein A, Ackerson T, et al. 2018 Guidelines for the Early Management of Patients With Acute Ischemic Stroke: a Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2018;49(3):46–99. doi:10.1161/STR.0000000000000158
55. Rosso C, Drier A, Lacroix D, et al. Diffusion-weighted MRI in acute stroke within the first 6 hours: 1.5 or 3.0 Tesla? Neurology. 2010;74(24):1946–1953. doi:10.1212/WNL.0b013e3181e396d1
56. Nael K, Khan R, Choudhary G, et al. Six-Minute Magnetic Resonance Imaging Protocol for Evaluation of Acute Ischemic Stroke: pushing the Boundaries. Stroke. 2014;45(7):1985–1991. doi:10.1161/STROKEAHA.114.005305
57. Kim BJ, Kang HG, Kim H, et al. Magnetic resonance imaging in acute ischemic stroke treatment. J Stroke. 2014;16(3):131–145. doi:10.5853/jos.2014.16.3.131
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
