Back to Journals » Clinical Ophthalmology » Volume 13
Diagnostic and Management Strategies of Aspergillus Endophthalmitis: Current Insights
Authors Spadea L , Giannico MI
Received 22 September 2019
Accepted for publication 16 December 2019
Published 24 December 2019 Volume 2019:13 Pages 2573—2582
DOI https://doi.org/10.2147/OPTH.S219264
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Leopoldo Spadea, Maria Ilaria Giannico
University “La Sapienza”, Department of Sense Organs, Eye Clinic, Rome, Italy
Correspondence: Leopoldo Spadea
Head Eye Clinic, Policlinico Umberto 1, “Sapienza” University of Rome, Via Benozzo Gozzoli 34, Rome 00142, Italy
Tel +39 06 519 3220
Fax +39 06 8865 7818
Email [email protected]
Abstract: Fungal endophthalmitis is subsequent to endogenous or exogenous infection and represents an important complication of ocular surgery which may lead to significant visual loss and blindness. The prognosis is poor because of delayed diagnosis and limited availability of effective antifungal drugs with good ocular penetration. Furthermore, the critical issue in diagnosing fungal infection of the eye is microbiological identification of the etiologic agent in clinical samples. Aspergillus is among the most frequent isolated organisms in fungal endophthalmitis. Early diagnosis is essential to prevent severe complications and blindness. Treatments include local, systemic and surgical therapeutic strategies. The purpose of the present review is the analysis of the current procedures adopted to promptly diagnose and treat Aspergillus endophthalmitis.
Keywords: Aspergillus, endophthalmitis, fungal infection, cataract surgery, ocular mycoses
Introduction
Ocular fungal infections represent a significant cause of loss of vision due to the involvement of different segments of the eye: cornea, orbit, eyelid, lacrimal apparatus, conjunctiva, sclera and internal structures in case of endophthalmitis.1
Exogenous endophthalmitis may be consequent to postoperative or post-traumatic complications, whereas endogenous endophthalmitis derives from the hematogenous spread of pathogens into the eye and affects immunodepressed patients.2
The incidence of endophthalmitis ranges from 0.03% to 1.3% following cataract surgery and can be as high as 30% to 40% following open globe injuries.3 Fungal endophthalmitis accounts for 8.6% to 18.6% of culture-positive cases and has increased over the last 20 years, probably because of a larger amount of immunodepressed individuals.4–6
Aspergillus accounts for 56% to 74% of all cases of fungal endophthalmitis after cataract surgery.4,7 The rate varies between 4% and 14% of fungal endophthalmitis following bulbar trauma.8
Aspergillus is the second most common cause of endogenous fungal endophthalmitis after Candida; the infection involves most frequently immunocompromised patients affected by Human Immunodeficiency Virus (HIV), malignancy, diabetes mellitus, or in therapy with immunodepressive medications.9 Other associations include solid organ transplantation, drug use, lung disease and renal insufficiency. However, immunocompetent individuals may also be affected by Aspergillus endophthalmitis with no risk factors.10
Aspergillus causes reduction of visual capacity because of early macular involvement, retinal necrosis and choroidal damage.11 This severe and irreversible damage has to be prevented with an early diagnosis.
A PubMed search was performed using the search terms “Aspergillus, endophthalmitis, fungal infection, cataract surgery, ocular mycoses” and a full systematic review of the literature was conducted up until August 2019 to analyze current strategy to diagnose and treat Aspergillus endophthalmitis and discuss both pharmacological and surgical approach used for the management.
Ocular Mycoses: The Role of Aspergillus
Aspergillus is a harmless saprophyte fungus, and it is present everywhere.12 Infections due to Aspergillus commonly involve the lungs and the paranasal sinuses.13 Furthermore, infections of the tear ducts are very rare, with prolonged local therapy including antibiotics and corticosteroids being a very high-risk factor.14
Fungal etiology is responsible for about 5% of cases of lacrimal sac infection; the main pathogens include Aspergillus and Candida, but cases caused by Rhizopus have been reported as well.15
Fungal conjunctivitis is usually secondary to inflammation of the cornea, lacrimal sac and tear ducts. The etiologic agents include Candida, Aspergillus, Sporotrichum, Blastomyces, Coccidioides, Malassezia and dermatophytes that cause acute inflammation of the conjunctiva with mucopurulent discharge.1 Fungal scleral involvement has been reported after traumas caused by Aspergillus or Sporothrix schenckii.16
Fungal infection of the cornea manifests as purulent, ulcerative disease and accounts for 6% to 53% of cases of ulcerative keratitis.17 The most frequent risk factors include trauma, long-term use of corticosteroids and antibiotics, systemic diseases, especially diabetes, pre-existing eye surface infections and contact lenses.17,18 Fusarium and Aspergillus are the most frequent fungal agents affecting the cornea, particularly in young healthy men who work in the open air, such as farmers. Trauma factors include particles of mud or dust, tree branches, metal particles, plant-derived organic matter and the use of nylon-line brushcutters.1 Rosa et al stated that injuries of the eye favored infections caused by Fusarium (70%), Curvularia (11%) and Aspergillus (5%), whereas diabetes predisposed to infections caused by Fusarium (67%) and Candida (13%).19 Chronic use of topical ophthalmic drugs may lead to infections caused by Candida (44%) and Fusarium (38%); in contrast, in patients treated with topical corticosteroids, the most frequently isolated species are Candida, Aspergillus, Acremonium and Curvularia (22% each).19 Bloodstream infections are a common cause of inflammation of the uveal tract. The risk factors include catheters, Acquired Immune Deficiency Syndrome (AIDS), neutropenia, and immunosuppressive therapy. Most cases are caused by Candida, but other fungal pathogens are also involved: Cryptococcus, Sporothrix schenckii and Blastomyces. Multifocal uveitis and severe vitritis have been reported after fungal infections, as well as endophthalmitis.20 Endogenous infections may be due to complications of fungal inflammations located in other organs, such as the heart valves, the central nervous system and the genitourinary tract.21,22 Several cases of aspergillosis following aortic valve implantation have been described, presenting with fever of unknown origin or late embolic events,23 even if Rocco et al reported an unusual case of acute Aspergillus endophthalmitis from an aortic embolization with no systemic infectious symptoms.24 The mechanism of this infection was unclear, but probably the exposure likely occurred intra-operatively, through contaminated ventilation systems.24 Mortality range for Aspergillus aortitis varies from 93–100%.23–26 Clinicians must have a high index of suspicion in order to facilitate timely diagnosis and immediate treatment. Trans-esophageal echocardiography is essential in diagnosing the pseudoaneurysm, but computed tomography scan angiography of the chest is considered the gold standard for diagnosis.23–26 The serum biomarkers, such as the galactomannan (GM) enzyme immunoassay (EIA), in the diagnosis of invasive Aspergillosis, are still a discussion topic.
Endogenous Aspergillus endophthalmitis has been reported by Al Qahtani et al secondary to fungal endocarditis in an immunocompromised patient affected by Wegener’ granulomatosis.27 Prakash et al described a case of endophthalmitis due to Aspergillus fumigatus in a 40-year-old man with angioimmunoblastic T-cell lymphoma, who finally died for severe fungal sepsis.28
While Candidosis affects primarily people with weakened immune system, treated for sepsis with broad-spectrum antibiotics or nourished parenterally, or after organ transplantation, Aspergillus and Fusarium are most commonly involved in endogenous infections in drug addicts or people undergoing long-term corticosteroid therapy.21,22
Exogenous endophthalmitis occurs after trauma, intraocular surgery or as a complication of corneal inflammation, and Aspergillus infection has also been reported as a causal agent.29 Dogra et al described a rare bee sting-induced Aspergillus endophthalmitis complicated with necrotizing scleritis30 Gruener et al presented a case of necrotizing scleritis following Aspergillus fumigates endophthalmitis in a diabetic man, after pars plana vitrectomy for retinal detachment, so that the Authors suggest to suspect fungal infection after ocular surgery in the differential diagnosis, particularly in case of inadequate response to intravitreal antibiotics.31
Fungal infections of the eye socket are mostly a consequence of orbital inflammation in the surrounding tissues, such as the paranasal sinuses, skin and nasopharynx. Infections occur most frequently in immunocompromised patients. The etiologic factors may include Mucor, Rhizopus, Absidia, Aspergillus, Blastomyces and Sporothrix.32 Prompt diagnosis and effective treatment are extremely important for the future prognosis.
Diagnostics
Patients affected by Aspergillus ocular infection may suffer from pain, burning and foreign body sensation, tearing, hyperemia and blurred vision. A short flowchart of diagnostic approach in case of suspected ocular fungal infection is shown in Figure 1.
 |
Figure 1 Flowchart of diagnostic approach in suspected ocular fungal infection. |
The principal effects of ocular Aspergillus infection are summarized in Box 1.
Slit Lamp Examination
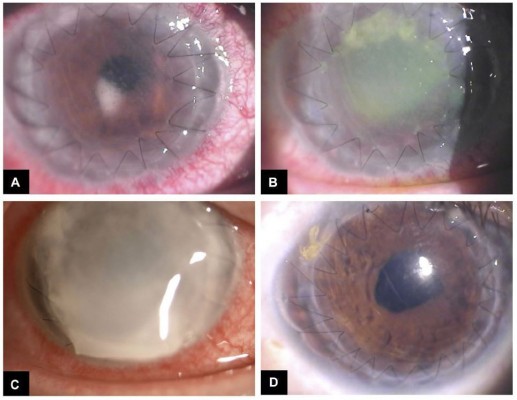
Slit lamp examination shows thickened eyelid margins and conjunctival injection, while corneal involvement often leads to grayish-white corneal stromal infiltration with fluffy margins located centrally and central corneal epithelial defects (Figure 2A). Corneal ulcer and melting with hypopyon can also be found, as well as corneal satellite lesions, flare and cells in the anterior chamber (Figure 2B–D).
Fundus Examination
When the infection spreads in the posterior segment, dense vitreous haze, vitritis or vitreous abscess may cause severe proliferative vitreoretinopathy, subretinal exudates and retinal detachment.
Fundus examination often shows fluffy white preretinal lesions with creamy white deep retinal lesions and intraretinal hemorrhages, large wedge-shaped quadrants of pigmented chorioretinal atrophy and scarring (Figures 3 and 4).
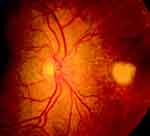 |
Figure 3 Fundus retinography of Aspergillus infection involving the posterior segment: subretinal exudates, intraretinal hemorrhages spread into the retina extended to the posterior pole. |
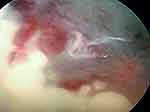 |
Figure 4 Fundus retinography of Aspergillus endophthalmitis showing vitritis, dense and wide white-yellow creamy lesions, diffuse intraretinal hemorrhages, partial retinal detachment. |
Fluorescein Angiography
Fluorescein angiography shows hyperfluorescence of the preretinal granuloma and the surrounding deep retinal lesions, and staining of the chorioretinal scars, focal vasculitis and leakage from the involved vessels.
Ultrasound Examination
Ultrasound examination is mandatory in case of suspected endophthalmitis and when the fundus is not explorable. Ultrasounds show dense opacities in the vitreous chamber for vitritis, thickening of the retinochoroidal layer due to subretinal exudative lesions, pre and intraretinal hemorrhages, preretinal layering of exudates that lead to epiretinal membranes and retinal detachment.
Cultural Investigations
The lacks of standardized serological tests, as well as the frequent absence of positive blood culture samples, make diagnosis very difficult. Vitreous culture, instead, shows a high percentage of positive result and may help in establishing diagnosis.33 The prognosis of fungal endophthalmitis depends upon the virulence of the organism, the timing of intervention, and the extent of intraocular involvement, but visual outcomes are often limited.34
Intraocular surgery, such as cataract surgery, may favor inoculation of Aspergillus into the eye, resulting in an acute-onset postoperative endophthalmitis, and leading to a delayed diagnosis and management. Haddock et al described two patients who presented endophthalmitis within the first two weeks after cataract surgery, suggesting the possibility of infection at the time of surgery or in the early postoperative period.35
Aspergillus is ubiquitary. It has been isolated from soil and decaying organic matter.36 Aspergillus fumigatus and Aspergillus flavus are the most common species implicated in intraocular infection. Other species detected in ophthalmic disease include Aspergillus niger, Aspergillus terreus, Aspergillus ustus, Aspergillus glaucus, Aspergillus versicolor and Aspergillus conicus.37–40
As suggested by Gao et al, fungal endophthalmitis may occur as a consequence of fungal keratitis.41 Xie et al reported fungal keratitis are due primarily to Fusarium infection; Aspergillus ranked second among all pathogens. In the culture-positive samples, cornea accounted for over half.42
Corneal scraping or corneal buttons might be used for cultural analysis, even if it is not reality to submit all keratitis corneas, hypopyon, or vitreous abscess samples for culture. Positive outcomes of corneal cultural samples are probably due to the spread of infection from the corneal tissue, so that they should be obtained before starting antifungal treatment.41
Although blood cultures, serological tests and pulmonary radiography may be negative in case od Aspergillus endophthalmitis, cultures of pars plana vitrectomy specimens and examination of Gram- or Giemsa-stained smears should be considered as diagnostic tests for deep retinitis and/or choroiditis.11,43,44 When scleral necrosis and perforation occur, confirmed diagnosis is possible after enucleation of the eye globe.43
Microbiological identification is essential for diagnosing fungal infections of the eye; samples may include swabs from the conjunctival sac, corneal scraping, vitreous or other material obtained during biopsy.1 Tissue material such as corneal scrapings can be lightened with dimethyl sulfoxide (DMSO), which allows better visualization of the fungus. In the culture methods, the samples are inoculated on Sabouraud medium and incubated at 37°C and 27°C for 7–10 days. The plates are examined daily followed by macroscopic and microscopic evaluation of the colonies.1 Other diagnostic methods include the detection of specific antigens, such as the galactomannan antigen of Aspergillus.1
Molecular Examinations
Molecular techniques, like fluorescence in situ hybridization (FISH), polymerase chain reaction (PCR) and reverse transcription-PCR (RT-PCR) for the detection of genetic material of the fungi in clinical specimens can also be used. PCR plays an important role since multiplex testing needs a small volume of fluid to be performed. Sterility of intraocular environment makes ocular fluids ideal for PCR analysis, although contamination by commensal microorganisms is still a potential confounding factor.40
Histopathology and Further Investigations
Histopathological examinations of tissue samples are always of great value, but not always possible. Rate of positivity of these tests is not specified in Literature.
High-resolution computed tomography (HRCT) and magnetic resonance (MR) imaging can also be very useful.1
In selected cases, when diagnostic pars plana vitrectomy and vitreous cultures are negative and ocular or systemic progression of the disease is confirmed, despite intravitreal or systemic treatment, retinochoroidal biopsy has proven to play a role in diagnosing fungal endophthalmitis and helping isolate the pathogen involved. Biopsy of chorioretinal tissue has to be reserved for selected cases because it carries significant risks of choroidal bleeding, vitreous loss and retinal detachment.45
Treatment
Antifungal Therapy
Intravitreal and/or systemic amphotericin B is commonly used to treat Aspergillus endophthalmitis, although the intraocular form may be toxic to retina.46 Intravitreal amphotericin B (5mg/0.1 mL) has been safely used. Intravitreal dexamethasone is administered to reduce intraocular inflammation, although the efficacy has not been tested in controlled, masked studies.9,36,47 Oral prednisone 1mg/kg/body weight should be used in tapering doses. Steroids should be administered in case of severe inflammation but with caution and close controls. Pars plana vitrectomy is necessary to confirm diagnosis and start correct treatment. Several species of Aspergillus can be highly resistant to amphotericin B and side effects often limit its systemic use. Alternative systemic antifungals include caspofungin and voriconazole. This last has good intraocular penetration allowing systemic treatment alone; when used intravitreally, it may be safer than amphotericin B.46
Voriconazole is a second-generation synthetic derivative of fluconazole with broad spectrum of activity.48 Intravitreal dose of voriconazole up to 100 microgram/mL is considered safe for the retina.49 Voriconazole should be considered as a first-line intravitreal agent to treat fungal endophthalmitis as well as amphotericin B.49
Itraconazole shows low MIC for many species of Aspergillosis, and selected reports reported a favorable response for the treatment of invasive aspergillosis.50,51 The vitreous penetration of itraconazole is better than amphotericin B but remains only a fraction of the serum level.52 Oral Itraconazole 200 mg twice-a-day has been positively described. Intravitreal and systemic voriconazole may represent a valid alternative treatment for Aspergillus terreus endophthalmitis, resistant to amphotericin B, even if the final visual outcome in the report mentioned was poor due to central macular scar.53
The duration of systemic antifungal therapy is variable but drugs should be administered for at least 1 month. The frequency of intravitreal injection changes depending on the clinical course, but published data report injections every 48 hrs on average. The appropriate treatment should be chosen together with an infectivologist, especially in case of immunocompromised patients.
Surgery
Common symptoms associated with fungal endophthalmitis are decreased vision, pain, hypopyon, blurring of the iris details. Initially, the infection involves the anterior segment, but later spreads to involve into the eye. Retinal detachment, cyclic membrane formation, and contraction of fibrous tissue may be consequent to inflammation due to Aspergillus.35 It is not always possible to identify fungal organisms in histological samples after pars plana vitrectomy with placement of silicone oil performed to repair retinal detachment, after several intravitreal injections.35 Previous reports suggested that using silicone oil could prevent the growth of microbes, including bacterial and fungal isolates.54 Furthermore, it helps reduce rates of complications, like retinal detachments or hypotony.55 Nevertheless, it was recently assessed that silicone oil might play a weaker role in antifungal therapy;56 furthermore, nontoxic concentrations of intravitreal drugs could create toxicity in a silicone oil-filled eye.57 Gao et al also did not recommend silicone oil tamponade in uncontrolled endophthalmitis eye.41
Vinekar et al presented a series of 6 eyes affected by fungal endophthalmitis (Aspergillus and Candida) following cataract surgery with a mean of 4.16 recurrences.58 The median interval after cataract surgery at presentation was 7 weeks. The eyes showed persistent infection despite a mean of two vitrectomies, systemic and intraocular use of antifungal agents, even newer agents like voriconazole. Following a final vitrectomy and IOL explantation with careful removal of the capsule, they were able to prevent further recurrences during a mean follow-up period of 36.5 months. The Authors suggested that the fungal spores are sequestered over the IOL surface and in the capsular bag and are responsible for the multiple recurrences.58 There is evidence to suggest that fungal filaments survive on the surface of IOL59 and in the capsular bag,60 even if prospective long-term studies in larger series are necessary to establish if IOL removal should be a standard of care.
Performing vitrectomy in case of fungal endophthalmitis is frequently discussed. Gao et al supported a prompt vitrectomy in case of a large vitreous abscess or lack of response to intravitreal injections.41 When complete vitrectomy is not allowed for unclear media, a core vitrectomy is indicated. Aggressive vitrectomy may increase the risk of retinal damage. A second vitrectomy should be performed when the media would get clearer and the infection controlled.41 On the other hand, surgical approach to fungal endophthalmitis following keratitis is controversial. Gao et al reported a penetrating keratoplasty (PKP) ratio of 81.5% in their series of patients affected by fungal endophthalmitis secondary to keratitis. They encountered two types of corneal opacity: the first was a large area of opacity resulting from corneal ulcer, melting or endothelial plaque; surgical treatment is always required in this case. The other type was a small scope of infiltration after injury with chestnut thorns. The Authors performed PKP after a mean time of 4.5 days from the beginning of the antifungal therapy, and PKP allowed to clear original infection focus for clinical examination and treatment of intraocular infection.41 PKP should be performed after one week treatment period in fungal keratitis, as reported in Literature.61,62 However, when combined with endophthalmitis, PKP should be performed earlier (PKP à chaud) (Figure 2D).
Fungal endophthalmitis may also occur after glaucoma drainage devices (GDD) for filtering surgery.33 Removal of GDD following endophthalmitis is still a discussion topic. If removal of GDD at the time of treatment could promote improvement in final visual acuity or not is unclear; some studies recommended the removal of GDD as it serves as reservoir of infection.33,63
Another relevant factor is represented by the state of latency of the tear ducts. In chronic dacryocystitis, superinfection of Aspergillus and Candida is possible, so that dacryocystorhinostomy (DCR) should be performed in case of epiphora, lacrimal sac mucocele and obstruction of the passage to prevent the risk of post-operative endophthalmitis caused by pathogens retained within the lacrimal system.43
Combined Antifungal Treatments
We discussed about medical and/or surgical treatment to manage Aspergillus endophthalmitis, but what about the possibility to combine antifungal drugs? The in vivo efficacy of a combined antifungal therapy in life-threatening systemic fungal infections has been proven,64 and good visual outcome has been reported after combination of intravitreal antifungals.65 Different types of drug interactions have been studied in vitro: synergism, indifference, or antagonism between these agents.66
Amphotericin B, a polyene, is firstly used for systemic mycosis and endophthalmitis caused by filamentous fungi.67 It acts by binding the surface sterols in the cell membrane of the fungi; this results in formation of pores and altered permeability.68 Voriconazole, an azole, acts chiefly by depleting ergosterol, the chief bioregulator of membrane integrity.67 As suggested by Steinbach et al azoles may inactivate amphotericin B which is adsorbed into the fungal cell surface, so that amphotericin B binding to fungal cell membrane is inhibited.69 On the contrary, amphotericin B when combined with lipophilic triazoles, like itraconazole, was found to be deleterious or ineffective, while amphotericin B when combined with voriconazole was found to be beneficial in a few but not all experimental studies.64,66,69 Mithal et al proposed a combination therapy with intravitreal amphotericin B and voriconazole for the treatment of filamentous endophthalmitis due to Aspergillus terreus, Aspergillus flavus and Fusarium solani. They also suggested to study the effect of combining these two drugs in vitro with regard to possible synergism and correlate it with the in vivo response.70
Conclusion
Aspergillus endophthalmitis represents a severe challenge for ophthalmologists. Early diagnosis is necessary to preserve visual function71 and eye globe anatomy, but often difficult. Ocular prognosis is variable and not infrequently necessitating enucleation of the painful blind eye. Multi-disciplinary approach may be essential for the diagnosis, for the management and treatment: it is critical for the ophthalmologist to work with infectious disease specialists, pharmacists, and any specialist can be involved particularly in case of systemic disease or unknown origin focus of infection. If the general condition of the patient permits a surgical procedure, a combined treatment of prompt vitrectomy and intravitreal antifungal agent injection may improve visual outcomes. Repeated injections and repeated vitrectomy are possible as well as the use of a combination of antifungal drugs. Removal of implanted IOL and PKP à chaud may be necessary to prevent recurrence and eliminate fungal growth colonies. Despite the efficacy of local and or systemic therapy, resistances to antifungal medicaments are more and more frequent and no standardized treatment protocols are still available.
 |
Box 1 Major Effects of Ocular Involvement by Aspergillus |
Disclosure
The authors report no conflicts of interest in this work.
References
1. Slowik M, Biernat MM, Urbaniak-Kujda D, Kapelko-Slowik K, Misiuk-Hojlo M. Mycotic infections of the eye. Adv Clin Med. 2015;24(6):1113–1117. doi:10.17219/acem/50572
2. Durand ML. Bacterial and fungal endophthalmitis. Clin Microbiol Rev. 2017;30:597–613. doi:10.1128/CMR.00113-16
3. Guest JM, Singh PK, Revankar SG, Chandrasekar PH, Kumar A. Isavuconazole for treatment of experimental fungal endophthalmitis caused by Aspergillus fumigatus. Antimicrob Agents Chemother. 2018;62:11. doi:10.1128/AAC.01537-18
4. Narang S, Gupta A, Gupta V, et al. Fungal endophthalmitis following cataract surgery: clinical presentation, microbiological spectrum, and outcome. Am J Ophthalmol. 2001;132:609–617. doi:10.1016/S0002-9394(01)01180-1
5. Williams MA, McMullan R, Hedderwick S, Mulholland DA, Best RM. Diagnosis and treatment of endogenous fungal endophthalmitis. Ophthalmologica. 2006;220:134–136. doi:10.1159/000090580
6. Vergoulidou M, Krause L, Foerster MH, Thiel E, Schwartz S. Endogenous filamentous fungal endophthalmitis-single-centre survey in patients with acute leukemia or post allogenic stem cell transplantation and review of the literature. Mycoses. 2011;54(6):. doi:10.1111/j.1439-0507.2010.02004.x
7. Wykoff CC, Flynn HW
8. Gupta A, Srinivasan R, Kaliaperumal S, Saha I. Post-traumatic fungal endophthalmitis – a prospective study. Eye (Lond). 2008;22:13–17. doi:10.1038/sj.eye.6702463
9. Riddell Iv J, McNeil SA, Johnson TM, Bradley SF, Kazanjian PH, Kauffmann CA. Endogenous Aspergillus endophthalmitis: report of 3 cases and review of the literature. Medicine (Baltimore). 2002;81(4):311–320. doi:10.1097/00005792-200207000-00007
10. Smith JR, Chee SP. Endogenous Aspergillus endophthalmitis occurring in a child with normal immune function. Eye (Lond). 2000;14(Pt 4):670–671. doi:10.1038/eye.2000.169
11. Rao NA, Hidayet AA. Endogenous mycotic endophthalmitis: variations in clinical and histopathologic changes in candidiasis compared with aspergillosis. Am J Ophthalmol. 2001;132:244–251. doi:10.1016/S0002-9394(01)00968-0
12. Glass RBJ, Hertzanu Y, Mendelsohn DB, Posen J Paranasal sinus aspergillosis: a case report with computed tomogram findings. J Laryngol Otol. 1984;98:199–205. doi:10.1017/S0022215100146420
13. Denning DW. Invasive aspergillosis. Clin Infect Dis. 1998;26:781–805. doi:10.1086/cid.1998.26.issue-4
14. Rozycki R, Rekas M, Wisniewski P. Mycotic lacrimal canaliculitis – case report. Klin Oczna. 2004;106:338–342.
15. Ghose S, Mahajan VM. Fungal flora in congenital dacryocystitis. Indian J Ophthalmol. 1990;38:189–190.
16. Fincher T, Fulcher SF. Diagnostic and therapeutic challenge of Aspergillus flavus scleritis. Cornea. 2007;26:618–620. doi:10.1097/ICO.0b013e318033de67
17. Ayse K, Sengul O. Ocular fungal infections. Curr Eye Res. 2011;36:179–189. doi:10.3109/02713683.2010.533810
18. Khater MM, Shehab NS, El-Badry AS. Comparison of mycotic keratitis with nonmycotic keratitis: and epidemiological study. J Ophthalmol. 2014;2014:254302.
19. Rosa RH, Miller D, Alfonso EC. The changing spectrum of fungal keratitis in South Florida. Ophthalmology. 1994;101:1005–1013. doi:10.1016/S0161-6420(94)31225-5
20. Kanski JJ, Bowling B. Okulistyka Kliniczna.
21. Lamaris GA, Esmaeli B, Chamilos G, et al. Fungal endophthalmitis in a tertiary care cancer center: a review of 23 cases. Eur J Clin Microbiol Infect Dis. 2008;27:343–347. doi:10.1007/s10096-007-0443-9
22. Mody KH, Ali MJ, Vemuganti GK, Nalamada S, Nail MN, Honavar SG. Orbital aspergillosis in immunocompetent patients. Br J Ophthalmol. 2014;98:1379–1384. doi:10.1136/bjophthalmol-2013-303763
23. Pasqualotto AC, Denning DW. Postoperative aspergillosis. Clin Microbiol Infect. 2006;12:1060–1076. doi:10.1111/j.1469-0691.2006.01512.x
24. Rocco JM, Benson MK. Aspergillus aortitis in an immunocompetent patient presenting with acute endophthalmitis. Infectious Dis Rep. 2018;10:7750.
25. Duygu H, Nalbantgil S, Ozerkan F, Kırılmaz B, Yağdı T. Aspergillus niger aortitis after aortic valve replacement diagnosed by trans esophageal echocardiography. Echocardiography. 2006;23(5):405–406. doi:10.1111/echo.2006.23.issue-5
26. Sanchez-Recalde A, Matè I, Merino JL, Simon RS, Sobrino JA. Aspergillus aortitis after cardiac surgery. JACC. 2003;41:152–156. doi:10.1016/S0735-1097(02)02606-2
27. AlQahtani GMS, AlSayed AAD, Gangadharan S, Adhi MI. Fungal endophthalmitis in a case of granulomatosis with polyangitis. Saudi J Ophthalmol. 2018;32:261–265. doi:10.1016/j.sjopt.2018.01.001
28. Prakash NP, Ano TM, Rakul N, Jaisankar P, Swapna B. Invasive Aspergillosis in refractive angioimmunoblastic T-cell lymphoma. Turk J Hematol. 2018;35:75–93.
29. Silva RA, Sridhar J, Miller D, Wykoff CC, Flynn HW
30. Dogra M, Narang S, Soos S, Gupta P. Successful management of bee sting induced Aspergillus fumigates endophthalmitis and scleritis. Indian J Ophthalmol. 2018;66:461–463. doi:10.4103/ijo.IJO_889_17
31. Gruener AM, Allen F, Stanford MR, Graham EM. Aspergillus fumigatus endophthalmitis with necrotizing scleritis following pars plana vitrectomy. Case Rep Ophthalmol Med. 2016;2016:9289532.
32. Mohamed MS, Abdel-Motaleb HY, Mobarak FA. Management of rhino-orbital mucormycosis. Saudi Med J. 2015;36:865–868. doi:10.15537/smj.2015.7.11859
33. Salim NL, Azhany Y, Abdul Rahman Z, Yusuf R, Liza-Sharmini AT. Infected Baerveldt glaucoma drainage by Aspergillus niger. Case Rep Ophthalmol Med. 2015;2015:249419.
34. Chhablani J. Fungal endophthalmitis. Expert Rev Anti Infect Ther. 2011;9(12):1191–1201. doi:10.1586/eri.11.139
35. Haddock LJ, Flynn HW
36. Weishaar PD, Flynn HW
37. Perri P, Campa C, Incorvaia C, et al. Endogenous Aspergillus versicolor endophthalmitis in an immunocompetent HIV-positive patient. Mycopathologia. 2005;160(3):259–261. doi:10.1007/s11046-005-6871-0
38. Paula JS, Bryk A
39. Chakrabarti A, Chatterjee SS, Das A, Shivaprakash MR. Invasive aspergillosis in developing countries. Med Mycol. 2011;49(Suppl 1):S35–S47. doi:10.3109/13693786.2010.505206
40. Smith WM, Fahle G, Nussenblatt RB, Sen HN. A rare case of endogenous Aspergillus conicus endophthalmitis in an immunocompromised patient. J Ophthalmic Inflamm Infect. 2013;3(1):37. doi:10.1186/1869-5760-3-37
41. Gao Y, Chen N, Dong X-G, Yuan G-Q, Tu B, Xie L-X. Surgical management of fungal endophthalmitis resulting from fungal keratitis. Int J Ophthalmol. 2016;9(6):848–853. doi:10.18240/ijo.2016.06.10
42. Xie L, Zhai H, Zhao J, Sun S, Shi W, Dong X. Antifungal susceptibility for common pathogens of fungal keratitis in Shandong Province, China. Am J Ophthalmol. 2008;146(2):260–265. doi:10.1016/j.ajo.2008.04.019
43. Comez AT, Komur B, Akcali A, Otkun MT. Ocular aspergillosis: obtaining a specimen is crucial for diagnosis. A report of three cases. Med Mycol Case Rep. 2012;1(1):39–41. doi:10.1016/j.mmcr.2012.06.002
44. Lance SE, Friberg TR, Kowalski RP. Aspergillus flavus endophthalmitis and retinitis in an intravenous drug abuser. A therapeutic success. Ophthalmology. 1988;95:947–949. doi:10.1016/S0161-6420(88)33087-3
45. Peddada K, Khan NM, Rubin J, et al. Diagnosis in vitreoretinal Aspergillosis with transvitreal retinochoroidal biopsy. Case Rep Ophthalmol Med. 2018;2018:8306163.
46. Riddell J
47. Mai AB, Jalali S, Das T, Gopinathan U. Role of intravitreal dexamethasone in exogenous fungal endophthalmitis. Eye (Lond). 1999;13(Pt 5):660–665. doi:10.1038/eye.1999.179
48. Walsh TJ, Dixon DM. Nosocomial aspergillosis: environmental microbiology, hospital epidemiology, diagnosis and treatment. Eur J Epidemiol. 1989;5:131–142. doi:10.1007/BF00156818
49. Gao H, Pennesi M, Shah K, et al. Safety of intravitreal voriconazole: electroretinographic and histopathologic studies. Trans Am Ophthalmol Soc. 2003;101:183–190.
50. Denning DW, Lee JY, Hostetler JS, et al. NIAID mycoses study group multi center trail of oral itraconazole therapy for invasive aspergillosis. Am J Med. 1994;97:135–144. doi:10.1016/0002-9343(94)90023-X
51. Breit SM, Hariprasad SM, Mieler WF, Shah GK, Mills MD, Grand MG. Management of endogenous endophthalmitis with voriconazole and caspofungin. Am J Ophthalmol. 2005;139:135–140. doi:10.1016/j.ajo.2004.08.077
52. Khan B, Vohra R, Kaur R, Singh SAV. Excellent outcome of Aspergillous endophthalmitis in a case of allergic bronchopulmonary aspergillosis. Indian J Ophthalmol. 2014;62:352–354. doi:10.4103/0301-4738.125552
53. Penigrahi PK, Roy R, Pal SS, Mukherjee A, Lobo A. Aspergillus terreus endogenous endophthalmitis: report of a case and review of literature. Indian J Ophthalmol. 2014;62:887–889. doi:10.4103/0301-4738.141065
54. Chrapek O, Vecerova R, Koukalova D, et al. The in vitro antimicrobial activity of silicone oil used in ophthalmic surgery. Biomed Pap Med Far Univ Palacky Olomouc Czech Repub. 2012;156(1):7–13. doi:10.5507/bp.2011.060
55. Aras C, Ozdamar A, Karacorlu M, Ozkan S. Silicone oil in the surgical treatment of endophthalmitis associated with retinal detachment. Int Ophthalmol. 2001;24(3):147–150. doi:10.1023/A:1021108907745
56. Ornek N, Apan T, Ourel R, Ornek K. Comparison of the antimicrobial effect of heavy silicone oil and conventional silicone oil against endophthalmitis-causing agents. Indian J Ophthalmol. 2014;62(4):388–391. doi:10.4103/0301-4738.126994
57. Hegazy HM, Kivilcim M, Peyman GA, et al. Evaluation of toxicity of intravitreal ceftazidime, vancomycin, and ganciclovir. In a silicone oil-filled eye. Retina. 1999;19(6):553–557. doi:10.1097/00006982-199911000-00013
58. Vinekar A, Dogra MR, Avadhani K, Gupta V, Gupta A, Chakrabarti A. Management of recurrent postoperative fungal endophthalmitis. Indian J Ophthalmol. 2014;62:136–140. doi:10.4103/0301-4738.128588
59. Biswas J, Kumar SK. Cytopathology of explanted intraocular lenses and the clinical correlation. J Cataract Refract Surg. 2002;28:538–543. doi:10.1016/S0886-3350(01)01159-2
60. Cusumano A, Busin M, Spitznas M. Mycotic infection of the capsular bag in postoperative endophthalmitis. J Cataract Refract Surg. 1991;17:503–505. doi:10.1016/S0886-3350(13)80859-0
61. Xie L, Dong X, Shi W. Treatment of fungal keratitis by penetrating keratoplasty. Br J Ophthalmol. 2001;85(9):1070–1074. doi:10.1136/bjo.85.9.1070
62. Sanders N. Penetrating keratoplasty in treatment of fungus keratitis. Am J Ophthalmol. 1970;70(1):24–30. doi:10.1016/0002-9394(70)90663-X
63. Al-Torbak AA, Al-Shahwan S, Al-Jadaan A, Al-Hommadi A, Edward DP. Endophthalmitis associated with the Ahmed glaucoma valve implant. Br J Ophthalmol. 2005;89(4):454–458. doi:10.1136/bjo.2004.049015
64. Ostrovsky-Zeichner L. Combination anti fungal therapy: a critical review of the evidence. Clin Microbiol Infect. 2008;14(Suppl 4):65–70. doi:10.1111/j.1469-0691.2008.01983.x
65. Sheyman AT, Cohen BZ, Friedman AH, Ackert JM. An outbreak of fungal endophthalmitis after intravitreal injection of compounded combined bevacizumab and triamcinolone. JAMA Ophthalmol. 2013;131:864–869. doi:10.1001/jamaophthalmol.2013.88
66. Clemons KV, Stevens DA. Animal models testing mono therapy versus combination anti fungal therapy: lessons learned and future directions. Curr Opin Infect Dis. 2006;19:360–364. doi:10.1097/01.qco.0000235163.70678.59
67. Ghannoum MA, Rice LB. Antifungal agents: mode of action, mechanism of resistance, and correlation of these mechanisms with bacterial resistance. Clin Microbiol Rev. 1999;12:501–517. doi:10.1128/CMR.12.4.501
68. Wingard LB, Zuravleff JJ, Doft BH, Berk L, Rinkoff J. Intraocular distribution of intravitreally administered amphotericin B in normal and vitrectomized eyes. Invest Ophthalmol Vis Sci. 1989;30(10):2184–2189.
69. Steinbach WJ, Stevens DA, Denning DW. Combination and sequential antifungal therapy for invasive aspergillosis: review of published in vitro and in vivo interactions and 6281 clinical cases from 1966 to 2001. Clin Infect Dis. 2003;37(Suppl 3):S188–S224. doi:10.1086/376524
70. Mithal K, Pathengay A, Bawdekar A, et al. Filamentous fungal endophthalmitis: results of combination therapy with intravitreal amphotericin B and voriconazole. Clin Ophthalmol. 2015;9:649–655.
71. Spadea L, Abbouda A, Abicca I, Paroli MP. Aspergillus flavus endophthalmitis after penetrating keratoplasty combined with cataract phacoemulsification and IOL implantation. Int Ophthalmol. 2015;35(1):145–148. doi:10.1007/s10792-014-0030-x
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.