Back to Journals » Clinical Ophthalmology » Volume 17
Diagnosis and Management Strategies in Sclerochoroidal Calcification: A Systematic Review
Received 11 April 2023
Accepted for publication 21 July 2023
Published 11 September 2023 Volume 2023:17 Pages 2665—2686
DOI https://doi.org/10.2147/OPTH.S399058
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Dr Scott Fraser
Ahmet Kaan Gündüz,1,2,* Diğdem Tetik1,*
1Department of Ophthalmology, Ankara University Faculty of Medicine, Ankara, Turkey; 2Private Eye Clinic, Farilya Business Center 8/38, Ankara, 06510, Turkey
*These authors contributed equally to this work
Correspondence: Ahmet Kaan Gündüz, Farilya Business Center 8/38, Ufuk Universitesi Cad, Çukurambar, Ankara, 06510, Turkey, Email [email protected]
Abstract: Sclerochoroidal calcification (SCC) is a rare disease which is characterized by calcium deposition in the sclera. The choroid is secondarily involved. Typical localization is in the midperipheral region, outside the vascular arcades. SCC is mostly located in the superotemporal quadrant. Often times, the patients are referred with the diagnosis of an amelanotic tumor. SCC may be dystrophic or metastatic. Metastatic SCC lesions are associated with conditions altering calcium and phosphate metabolism including primary and secondary hyperparathyroidism, vitamin D intoxication, renal failure, hyperphosphatemia, and destructive bony lesions. SCC lesions have a characteristic appearance and appear as distinct, ill-defined, yellow-white, elevated scleral/choroidal masses funduscopically. The purpose of this literature review is to review the current knowledge on SCC, highlight the imaging features, and discuss the differential diagnosis as well as management options.
Keywords: sclerochoroidal calcification, hyperparathyroidism, renal failure, ultrasonography, fluorescein angiography, optical coherence tomography
Introduction
Sclerochoroidal calcification (SCC) is a rare degenerative disorder which is characterized by calcium deposition in the sclera. As it is an uncommon condition, it is often misdiagnosed. Over the last two to three decades, there has been a growing interest in this subject leading to the publication of several papers.
In 1979, Wong et al reported calcification of sclera and choroid for the first time in a patient with hyperparathyroidism. Their case had keratopathy, cataracts, and SCC confirmed by histopathological examination.1 Until early 1980s, SCC was not a well-recognized clinical entity. In 1982, Goldstein et al published the first article on SCC in a patient with hyperparathyroidism with all its characteristic features on funduscopic examination, fluorescein angiography (FA), and ultrasonography (USG).2 The term “idiopathic sclerochoroidal calcification” was first mentioned by Lim et al in 1989. They reported a case with bilateral multiple calcific choroidal foci with no hypercalcemia or any other ocular diseases.3 In 1991, Sivalingam et al published the first case series comprising seven SCC patients, initially misdiagnosed with different ocular diseases.4
The exact pathogenesis of SCC is still not fully understood. Calcification in tissues occurs in two distinct mechanisms: dystrophic and metastatic.5 In dystrophic calcification, local deposition of crystalline calcium occurs in the damaged or necrotic tissues. In this situation, serum calcium and phosphorus levels are normal. Examples of dystrophic calcification in the eye include band keratopathy in cornea, senile scleral plaques anterior to the insertions of horizontal rectus muscles, optic nerve head drusen, and calcification in necrotic areas within retinoblastoma.6 In metastatic calcification, abnormal calcium and phosphorus metabolism leads to deposition of calcium in normal undamaged tissue. Common etiologies are primary and secondary hyperparathyroidism, vitamin D intoxication, renal failure, hyperphosphatemia, and neoplastic destructive bony lesions including multiple myeloma or metastases.5,6 In metastatic calcification, the localization of calcification includes the uveal tract, sclera, Bowman’s membrane, superficial stroma in the cornea, and subepithelium of the conjunctiva.7
The aims of this systematic review were to analyze the demographics, associated systemic findings, ancillary testing methods used, results of treatment, and follow-up results in patients with SCC.
Materials and Methods
We conducted a systematic literature review in PubMed, Web of Science and Scopus databases as of March 2023 using the keywords “sclerochoroidal calcification”, “sclerochoroidal calcification and related systemic diseases”, “idiopathic sclerochoroidal calcification”, “sclerochoroidal calcification and imaging modalities”, “sclerochoroidal calcification and treatment modalities”, “ocular calcification”, “calcium metabolism and eye”, “retinal pseudotumor”, “differential diagnosis of retinal tumors”. Also, reference lists of identified literatures were also screened for further information. Unpublished articles, photo essays, letters to journal, articles with insufficient data, and papers not published in English were excluded. This systematic review was registered with PROSPERO (International Prospective Register of Systematic Reviews) to validate its methodology (ID: CRD42023437352).
Statistical analysis of idiopathic SCC and SCC associated with systemic disease was made with respect to certain variables for which enough data was available in the literature search. The normality test and Pearson’s correlation test were used to reveal statistical differences.
Results
We retrieved 88 manuscripts upon our initial review process. Six manuscripts were excluded because they were review articles (n=3) or were irrelevant (n=3). Eighty-two manuscripts were further evaluated for screening. Twenty-one of them were excluded because they were not in English literature (n=13), had insufficient data (n=4), or were in incomplete case presentation formats including photo essays (n=2), letter to journal (n=1), and ahead of print (n=1). The flowchart for study design and manuscript selection is given in Figure 1.
 |
Figure 1 Flowchart for study design and manuscript selection. |
Sixty-one manuscripts were found to be suitable for inclusion in this review article. Of 61 articles, 50 were single-case reports and 11 were case series. The demographics, clinical features, and diagnostic methods used are summarized in Table 1.1–4,8–64
 |
Table 1 Demographics, Clinical Features, Treatment, and Follow-Up of Patients with Sclerochoroidal Calcification Included in This Review |
In this literature review, 477 eyes of 300 patients were found to have SCC. Among these, 169 (56.3%) patients were female, 125 (41.7%) patients were male, and information regarding 6 (2.0%) patients were not available. The mean patient age was 68.4 (26–88) years. One hundred and seventy-seven (59.0%) patients showed bilateral presentation while 113 (37.7%) had unilateral involvement. Laterality of 7 (2.3%) patients was not specified. Among 477 eyes, 147 eyes (30.8%) had multifocal lesions, 104 (21.8%) had unifocal lesions, and in the other 226 eyes (47.4%) multifocal vs unifocal involvement was not specified.
The Shields group had multiple papers on SCC, and there is a possibility that succeeding papers may have included the cases presented in the previous papers. Notwithstanding, all SCC cases reported by this group were included in the analysis. Therefore, the total number of patients included in our review may be more than the real sum total.
The reported imaging modalities used in the diagnosis of SCC were evaluated. Among 300 patients, data in 176 patients were available. In the remaining 124 patients, the number of patients and imaging modalities used were not specifically stated. The most common diagnostic method used in the evaluation of SCC was USG (66.0%) followed by optical coherence tomography (OCT) (47.7%), FA (20.5%), computed tomography (CT) (19.3%), fundus autofluorescence (FAF) (13.1%), indocyanine green angiography (ICGA) (6.3%), optical coherence tomography angiography (OCTA) (2.3%), visual field analysis (VF) (0.6%), and magnetic resonance imaging (MRI) (0.6%).
Systemic work-up was performed in 96 of 300 patients. Among these, 32 patients had no associated systemic disease and therefore classified as idiopathic SCC. In this group of 32 patients, the mean age was 69.6 (41–88) years. Bilateral presentation was seen in 26 patients (81.3%) and multifocal involvement in 19 (59.4%). Of the remaining 64 patients, primary hyperparathyroidism was found in 14 patients, Gitelman syndrome in 14, parathyroid adenoma in 9 patients, hypomagnesemia in 6, Bartter syndrome in 4, pseudogout in 3, hypercalcemia in 3, kidney failure in 3, familial chondrocalcinosis in 3, hypervitaminosis D in 1, hypovitaminosis D in 1, glomerulonephritis in 1, Albright’s hereditary osteodystrophy in 1, and calcium intoxication in 1. In 64 patients with systemic disease, precise information regarding demographic and ophthalmological findings could be found in 28. The mean age of patients with systemic associations was 58.4 (26–85) years. Of these patients, bilateral presentation was seen in 26 (92.9%) and multifocal involvement in 23 (82.1%).
There was not enough data to perform statistical correlation in many of the aspects the review addressed. We could only evaluate the differences between idiopathic SCC and those associated with systemic diseases with respect to age, laterality, and unifocal vs multifocal involvement in a limited number of patients. The normality test significance values were p=0.925 for the idiopathic group and p=0.594 for the systemic disease group with respect to age, laterality, and unifocal vs multifocal involvement. Subsequently, Pearson’s correlation test revealed no differences between the idiopathic and systemic disease groups with respect to these parameters (p=0.777). However, the correlation coefficient was r=0.343 indicating that there was a positive relationship between systemic involvement and parameters including younger patient age, bilaterality, and multifocality.
Among 300 patients, 161 patients had variable follow-ups ranging from 2 months to 20 years. A total of 150 patients (93.1%) remained stable during follow-up. Of the remaining 11 patients (6.8%), 9 developed choroidal neovascularization (CNV), 2 demonstrated lesion enlargement, and 1 showed increase in the surrounding atrophy area. Some patients had more than one mode of progression. Among eyes with CNV, 2 were treated with argon laser photocoagulation, 5 with intravitreal anti-VEGF (vascular endothelial growth factor) injections, and one with photodynamic therapy (PDT). Some eyes received more than one mode of treatment. Three eyes with CNV were observed without any intervention and remained stable thereafter. Bessette et al reported that CNV did not regress after 3 bevacizumab injections and PDT was administered as an adjunct.43 In the study by Goerlitz-Jessen, intravitreal anti-VEGF medications were injected with subtenon triamcinolone, but the number of anti-VEGF injections was not specified.47 In the case series of Battaglia Parodi, two patients complicated with CNV had intravitreal injections for several times.55 The last case reported by Sulavikova et al received intravitreal ranibizumab injections for 7 times together with laser photocoagulation.59
Discussion
Clinical Features of SCC
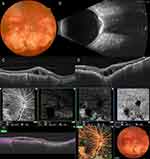
SCC is usually seen in elderly patients. There seems to be no gender predilection; however, a slight female predominance was noted in some series. Almost all the reported patients are whites.4 SCC is usually found as an incidental finding during routine eye examination and patients mostly tend to be asymptomatic. SCC has a characteristic appearance ophthalmoscopically. It manifests as a flat or minimally elevated yellow calcified mass (Figure 2A). It may be unilateral or bilateral and manifests as unifocal or multifocal discrete yellow placoid lesions with irregular borders. There is frequently a halo of retinal pigment epithelium (RPE) atrophy surrounding the lesion.6,20 The typical localization of the SCC lesions is usually in the superotemporal midperipheral fundus.11,20,39 SCC can also occur inferotemporally. It has been suggested that SCC develops at the site of the insertions of oblique muscles, presumably due to chronic tractional forces exerted on the adjacent sclera.4,18,20 However, SCC has been reported to occur in the macula, around the optic disc, and nasally as well. Visual acuity is usually not affected unless there is CNV or subretinal fluid. Additionally, in cases with macular localization or progression into the macula during follow-up, visual acuity may be altered.11,20,39,41,42
Systemic work-up is important in patients with SCC. Although most of the SCC cases are idiopathic, renal, endocrinologic, and skeletal pathologies leading to abnormal calcium and phosphorus metabolism should be excluded in all patients. While idiopathic cases tend to be seen in elderly patients, SCC associated with a systemic disorder is usually seen at younger ages. Both idiopathic SCC and those associated with systemic disorders usually appear as bilateral and multiple lesions.6,8,20 However, unilateral and unifocal involvement can also occur.
In the first case series regarding SCC conducted by Sivalingam et al in 1991, 14 eyes of 7 patients were included.4 Mean patient age was 69 years old and five patients (71.4%) were male. All the cases were asymptomatic and SCC was detected during routine ophthalmologic examination. All patients had bilateral multiple lesions and the average lesion count per eye was found to be 2.5. All SCC lesions were located near or above the superotemporal arcade. All patients had normal serum calcium, phosphate, and parathyroid hormone level; therefore, the cases were labelled as idiopathic SCC.4
Another case series by Honavar et al included 38 eyes of 27 patients with SCC.18 Mean patient age was found to be 70 years. Of 27 patients, 12 (44%) were male and 15 (56%) were female. None of the patients had visual symptoms at the time of diagnosis. While 16 (59%) patients had unilateral presentation, 11 (41%) had bilateral disease. Mean lesion count per eye was found to be 2. The most common localization harboring the lesion was found to be the superotemporal quadrant (56%). Systemic work-up revealed hypomagnesemia in 6 patients, Gitelman syndrome in 4, and primary hyperparathyroidism in one.20
Fung et al reported 13 eyes of nine patients with a mean age of 74 years.36 Five patients (55.5%) were male. All the patients were white. Mean visual acuity was found to be 20/33. Five (55.5%) patients showed unilateral presentation. The most common lesion localization was the superotemporal quadrant (70.5%). Systemic work up or blood calcium and phosphorus levels were not mentioned.39
Schachat et al published a case series composed of 19 patients with SCC. Mean patient age was 76 years. Eight patients (42.1%) were male and 11 (57.9%) were female. SCC was bilateral in 16 (84%) patients and unilateral in 3 (16%). The most common localization of the lesions was midperiphery and no quadrant was specified. Systemic work-up was done in nine patients of which one had hypercalcemia and the other had a history of surgical removal of parathyroid adenoma.11
In 2015, two consecutive papers on SCC were published by the Shields group.38,39 In the first paper, OCT findings were evaluated in 67 eyes of 46 patients.38 The mean age of the patients was 68 years and 57% were female. Almost all the patients were white (%98) and only one patient was of Hispanic origin. Twenty-one patients (45.7%) showed bilateral presentation and 25 patients (54.3%) were unilateral. Lesions were mostly located in the superotemporal quadrant (85%).41 In the second paper by the same group, 179 eyes of 118 patients were included.39 The mean age was 69 years and 60% were female. White patients comprised 98% of the whole cohort. SCC was unilateral in 57 patients (48%) and bilateral in 61 (52%) patients. Lesions were mostly found in the superotemporal quadrant (69%). Hyperparathyroidism was found in nine patients (27%), 5 of which had parathyroid adenoma. One patient (2%) had Bartter syndrome and six (11%) had Gitelman syndrome. Abnormal serum calcium was found in 21% of the patients, abnormal magnesium in 24%, and abnormal potassium in 7%.42
Associated Systemic Diseases
In this literature review, the most common systemic association seen with SCC was primary hyperparathyroidism. Primary hyperparathyroidism is characterized by the abnormally high amount of parathyroid hormone (PTH) production by the chief cells of the parathyroid gland. PTH is responsible for providing an equilibrium of serum calcium/phosphorus levels and when there is an imbalance due to excessive hormone production, serum calcium level increases resulting in metastatic calcification.46 Secondary hyperparathyroidism which is usually caused by chronic kidney disease occurs secondary to elevated serum calcium levels and compensatory increased PTH production.6 The second most common systemic association of SCC was found to be renal tubular metabolic alkalosis syndromes such as Gitelman and Bartter syndromes. These two distinct clinical conditions are associated with hypokalemia and metabolic alkalosis. While Bartter syndrome usually starts at an earlier age, Gitelman syndrome tends to be seen in late childhood. The classical clinical manifestations of Bartter syndrome are polyuria, polydipsia, failure to thrive, and tendency for dehydration. Gitelman syndrome is characterized by muscle weakness, fatigue, and carpopedal spasm. The relationship between these syndromes and SCC is not yet fully understood.20,46 Hypomagnesemia is also another important systemic association. Magnesium is known to have a role in the solubility of calcium pyrophosphate in the serum. When the serum magnesium level is low, calcium pyrophosphate deposition occurs in the joints as well as in the sclera and choroid. This mechanism is responsible for the tissue changes seen in calcium pyrophosphate deposition disease (pseudogout).6,14,46 Besides, hypervitaminosis D causes hypercalcemia and results in calcification in sclera and choroid. Interestingly, one SCC patient in the literature was diagnosed with hypovitaminosis D. The authors did not bring an explanation to the coexistence of hypovitaminosis D and SCC.44
Statistical analysis of patients with sufficient data revealed no differences between the idiopathic SCC and SCC occurring with systemic disease with respect to age, laterality, and unifocal vs multifocal involvement in this review. However, there was a positive correlation between systemic involvement and parameters including younger patient age, bilaterality, and multifocality.
Imaging Studies in SCC
In the majority of cases, the diagnosis of SCC can be made by indirect ophthalmoscopy. Ancillary testing methods used include A and B mode USG, FAF, FA, ICGA, OCT, OCTA, and CT. The common findings observed in each imaging modality are given in Table 2. There has been a paradigm shift in the use of these ancillary testing methods during the past 15 years. OCT and FAF have been increasingly used alongside the ubiquitous methods including USG, FA/ICGA, and CT. Currently, the most commonly used non-invasive tests include OCT, FAF, and USG.
 |
Table 2 Imaging Features in Sclerochoroidal Calcification |
A and B-Mode Ultrasonography
SCC causes acoustically solid, hyperechoic appearance with posterior shadowing in B-mode USG due to its high calcium content (Figure 2C). SCC may appear as plaque-like or nodular tumor-like (up to 4–6 mm in diameter) lesion on B-mode ultrasonography.17 On A-mode USG, the lesion displays high reflectivity.4
Fundus Autofluorescence
SCC gives rise to a mixed hyperautofluorescent and hypoautofluorescent appearance in FAF (Figure 2B). Calcific lesions display hyperautofluorescence; however, surrounding RPE atrophy may eventually lead to a hypoautofluorescence appearance.38,39 Total chorioretinal atrophy may lead to scleral hyperautofluorescence.
Fluorescein Angiography
The lesion usually shows hyperfluorescence in the early venous phase with increased hyperfluorescence in the late frames. In the late frames, there is usually no leakage (Figure 2D).4,10 FA also eloquently demonstrates CNV developing in the context of SCC. The degree of hyperfluorescence is usually less than that seen in common masquerading lesions including choroidal metastases and melanoma.
Indocyanine Green Angiography
There have been few papers on the ICGA findings of SCC. Lassandro et al reported hypocyanescence in early phases and mild hypercyanescence towards late phase.56 In another study, SCC lesions remained hypocyanescent during all the phases of ICGA.20 In two other papers, choroidal hyperpermeability giving rise to hypercyanescence with scattered hypocyanescent lesions due to impairment in choroidal circulation were noted in the macular area.52,54
Optical Coherence Tomography
SCC lesions are typically seen on OCT as hyperreflective, irregular lesions giving rise to posterior shadowing (Figure 2E). SCC starts in the sclera and gradually compresses the overlying choroid. This process causes attenuation and thinning of choroid on OCT. Outer retinal layers including outer nuclear layer, ellipsoid zone, and RPE are also found to be affected over some SCCs.28,32,34,39,52
With the developments in the OCT technology, enhanced depth imaging OCT (EDI-OCT) enabled the clinicians to visualize the internal tumor features down to sclera better. Hasanreisoglu et al classified the EDI-OCT appearances in SCC into 4 subtypes based on mountain-like patterns. Type 1 “flat” lesion was described as the thinnest subtype with no disturbance in the overlying RPE or sensory retina.41 Type 2 “rolling” lesions were thicker than type 1 and characterized by a dome shaped smooth lesion. Type 3 “rocky-rolling” lesions were thicker compared with type 2, had irregular and jutted surface characteristics and produced marked disruption of the overlying outer retina. Type 4 “table-mountain” subtype had abrupt elevation at both edges with a central plateau of relatively preserved central choroid and retina.41
Of these subtypes, type 3 was the thickest and type 4 was the largest in basal diameter. Type 3 had the most profound effect on the overlying choroid, RPE, and outer retinal layers. The retinal layers were undisturbed in type 1 lesions and outer retinal abnormalities were found in all other types. Type 4 showed the least outer retinal abnormalities compared to type 2 and 3 lesions. The choroid was thinned in all subtypes of SCC.41
Optical Coherence Tomography Angiography
There were 3 reports in the literature on OCTA findings in SCC at the time of this writing. In two studies, CNV associated with SCC was demonstrated using OCTA.51,55 In the other case report, OCTA features of SCC were described in detail. In this case, the outer retinal and choroidal slabs demonstrated hyporeflectivity in the lesion area probably because of the shadowing effect of calcium. The superficial and deep retinal plexi showed a mixed iso-hyporeflective appearance presumably due to compression of vascular structures by the lesion (Figure 2F). This case also featured a chorioretinal anastomosis best demonstrated on B-scan angio.60 The chorioretinal anastomosis may develop as a result of impediment of retinal venous outflow.
Computed Tomography
SCC lesions appear as hyperdense structures in the posterior eyewall consistent with the calcium content on CT.4,18,36 In some cases, there is chorioretinal atrophy along or superior to the arcade without typical SCC lesions. CT displayed eyewall calcium in these cases further providing support to the hypothesis that SCC starts in the sclera and progresses to invade the choroid.26,36
Differential Diagnosis
Sivalingam et al reported that SCC lesions were usually referred with the diagnoses of choroidal osteoma, choroidal metastasis, amelanotic choroidal nevus, and choroidal granuloma.4 In the series reported by Honavar et al,20 the most common referring diagnoses were choroidal metastases (26%), choroidal melanoma (21%), choroidal nevus (11%), and choroidal lymphoma (3%). Other conditions that may mimic SCC include chorioretinitis, choroidal hemangioma, regressed retinoblastoma, and retinal astrocytic hamartoma.6
Choroidal Metastases
Choroidal metastases are typically seen as yellow-colored lesions with fairly distinct margins and exudative retinal detachment (Figure 3). They are usually located in the macula and posterior pole and are rarely seen outside the vascular arcades.65 Choroidal metastatic lesions can be bilateral and multifocal and usually do not produce areas of RPE dropout. Patients usually present with vision loss, floaters, flashes, metamorphopsia, and pain. Some may also be asymptomatic. A history of malignancy is usually present but in 1/3 of the cases no primary cause is found at the time of detection of the choroidal mass.66 Metastatic lesions usually do not display calcium which is a distinguishing feature from SCC.
Choroidal Melanoma and Nevus
Choroidal melanoma and nevus are usually pigmented, but they can also be amelanotic in which case they may simulate SCC. Choroidal melanoma usually has distinct borders without RPE dropout, is associated with exudative retinal detachment, and lacks calcium on imaging studies (Figure 4). Choroidal nevus has similar features to choroidal melanoma, but it is usually smaller in size.67
Choroidal Lymphoma
The presence of multifocal creamy-yellow patches at the level of choroid is the most characteristic ophthalmoscopic finding. Also, obscuration of choroidal blood vessels by diffuse choroidal lymphoid aggregates is an important feature. Choroidal folds, lipofuscin deposits, optic disc swelling, and occasionally serous retinal detachments may be seen in choroidal lymphoma. All these features are not compatible with SCC.68
Choroidal Osteoma
Choroidal osteoma is usually seen in young individuals, mostly women. Ophthalmoscopically, choroidal osteoma appears as a yellow-white or orange-red mass depending on the pigmentary abnormalities on the surface. It presents as a unilateral, solitary, peripapillary oval or round lesion with distinct margins. The B-mode ultrasonogram shows calcification and associated posterior shadowing (Figure 5). Decalcification may occur in long standing osteomas. In contrast to SCC, many patients with choroidal osteoma show decrease in visual acuity from the posterior tumor location, decalcification, associated CNV, and hemorrhage associated with choroidal nonperfusion.69,70
Choroidal Hemangioma
Choroidal hemangioma is a benign vascular tumor of the choroid. It may manifest as circumscribed or diffuse choroidal hemangioma. Circumscribed choroidal hemangioma is sporadic and not associated with any systemic diseases. Diffuse choroidal hemangioma is usually seen in association with Sturge-Weber syndrome. Ophthalmoscopic examination of circumscribed choroidal hemangioma reveals unilateral, well-defined, non-pigmented, yellow or orange mass which may be accompanied by subretinal fluid and/or retinoschisis (Figure 6). B-mode USG shows an acoustically solid lesion. FA demonstrates early hyperfluorescence within the tumor in the arterial phase. Choroidal melanoma and metastasis usually manifest later onset hyperfluorescence.71
Management and Follow-Up
SCC is a benign condition which generally does not require treatment unless it is complicated. It is typically found in the mid-peripheral region outside of the vascular arcades and seldom affects the fovea. Correct diagnosis of SCC and evaluation for systemic diseases altering calcium metabolism such as hyperparathyroidism, renal failure, Bartter syndrome, Gitelman syndrome, and metastatic bony lesions are extremely important to prevent or reduce ocular and systemic morbidity.
SCC usually remains stable; however, in a minority of cases, it may demonstrate slow progression and enlargement on long-term follow-up. In a case series published by Schachat et al, 10 patients were followed ranging from 7 months to 10 years and no change was observed in any of the patients.11 Boutboul et al reported a case with familial chondrocalcinosis and SCC who was followed up for 24 years. The lesions were in the form of little placoid elevated lesions at the time of diagnosis but 24 years later, they appeared to evolve into tumorlike white choroidal lesions.23 In another case report on idiopathic SCC, no change or progression was noticed in the first year follow-up examination. Nevertheless, the lesion was found to be larger 10 years later.48
CNV associated with SCC is the most common cause of vision loss in SCC. CNV is associated with subretinal fluid, subretinal hemorrhages, exudates, and hemorrhagic RPE detachments. The treatment modalities used for CNV varies. There have been cases in which argon laser photocoagulation (ALPC) was used,15,17 while lately intravitreal anti-VEGF injections have become the other effective treatment option.43,47,55,59 However, if CNV is far from the macula, close follow-up of patients with CNV remains as another option.20,51,57
Conclusion
SCC is a rare disease often confused with other amelanotic fundus tumors. SCC lesions usually appear as calcific lesions superotemporally. SCC manifests as an acoustically solid mass on B-mode USG with posterior shadowing and displays calcification on CT. On FA, SCC shows early venous onset of fluorescence and late hyperfluorescence. On OCT, the lesion has either a smooth or jutted appearance with scleral hyperreflectivity consistent with calcium content. There is posterior shadowing from the calcium content. On OCTA, SCC displays hyporeflectivity in the outer retina and choriocapillaris from posterior shadowing. Associated CNV and retinochoroidal anastomosis are also nicely demonstrated using OCTA. There is no treatment available to ameliorate the degenerative effects of SCC on the sclera and choroid. Secondary CNV can be observed or treated using laser photocoagulation, PDT, and/or intravitreal anti-VEGF injections if vision is affected. Although not statistically proven in this systematic review, unilateral cases in elderly patients usually do not require systemic work-up. However, bilateral SCC seen in younger patients generally warrant systemic investigation.
As a limitation, our review had a low threshold for risk of bias assessment for inclusions of manuscripts if they met the initial eligibility criteria. With respect to different papers on the same subject from the same research group, there was no way to exclude patients included concurrently in these papers. We acknowledge this shortcoming but exclusion of some of these papers might have resulted in loss of important data.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Wong S, Zakov ZN, Albert DM. Scleral and choroidal calcifications in a patient with pseudohypoparathyroidism. Br J Ophthalmol. 1979;63(3):177–180. doi:10.1136/bjo.63.3.177
2. Goldstein BG, Miller J. Metastatic calcification of the choroid in a patient with primary hyperparathyroidism. Retina. 1982;2(2):76–79. doi:10.1097/00006982-198200220-00003
3. Lim JI, Goldberg MF. Idiopathic sclerochoroidal calcification. Case report. Arch Ophthalmol. 1989;107(8):1122–1123. doi:10.1001/archopht.1989.01070020188015
4. Sivalingam A, Shields CL, Shields JA, et al. Idiopathic sclerochoroidal calcification. Ophthalmology. 1991;98(5):720–724. doi:10.1016/S0161-6420(91)32228-0
5. Kumar V, Abbas AK, Aster JC. Robbins Basic Pathology. Philadelphia, WB Saunders: Elsevier Health Sciences; 2017:25–26.
6. Shields JA, Shields CL. CME review: sclerochoroidal calcification: the 2001 Harold Gifford Lecture. Retina. 2002;22(3):251–261. doi:10.1097/00006982-200206000-00001
7. Berkow JW, Fine BS, Zimmerman LE. Unusual ocular calcification in hyperparathyroidism. Am J Ophthalmol. 1968;66(5):812–824. doi:10.1016/0002-9394(68)92795-5
8. Munier F, Zografos L, Schnyder P. Idiopathic sclerochoroidal calcification: new observations. Eur J Ophthalmol. 1991;1(4):167–172. doi:10.1177/112067219100100402
9. Kobune M, Neda H, Mogi Y, et al. Ectopic choroidal calcification of the eyes of a patient with parathyroid adenoma. Intern Med. 1993;32(1):57–59. doi:10.2169/internalmedicine.32.57
10. Suzuki J, Takeda M, Sekine N, et al. Bilateral metastatic sclerochoroidal calcification in a patient with hyperparathyroidism. Ophthalmologica. 1992;205(1):10–14. doi:10.1159/000310304
11. Schachat AP, Robertson DM, Mieler WF, et al. Sclerochoroidal calcification. Arch Ophthalmol. 1992;110(2):196–199. doi:10.1001/archopht.1992.01080140052025
12. Saatci AO, Kaynak S, Kazanci L, Durak I, Tunc M. Calcification at the posterior pole in scleritis. A case report. Int Ophthalmol. 1996;20(5):285–287. doi:10.1007/BF00131925
13. McCabe CM, Mieler WF, Postel EA. Idiopathic sclerochoroidal calcification in a 41-year-old woman. Arch Ophthalmol. 1997;115(8):1082–1083. doi:10.1001/archopht.1997.01100160252023
14. Shields JA. Sclerochoroidal calcification in calcium pyrophosphate dihydrate deposition disease (pseudogout). Arch Ophthalmol. 1997;115(8):1077–1079. doi:10.1001/archopht.1997.01100160247021
15. Cohen SY, Guyot-Sionnest M, Puech M. Choroidal neovascularization as a late complication of hyperparathyroidism. Am J Ophthalmol. 1998;126(2):320–322. doi:10.1016/S0002-9394(98)00160-3
16. Bourcier T, Blain P, Massin P, Grünfeld JP, Gaudric A. Sclerochoroidal calcification associated with Gitelman syndrome. Am J Ophthalmol. 1999;128(6):767–768. doi:10.1016/S0002-9394(99)00277-9
17. Leys A, Stalmans P, Blanckaert J. Sclerochoroidal calcification with choroidal neovascularization. Arch Ophthalmol. 2000;118(6):854–857.
18. Zaheen M, Sellar W, Mucci B. Idiopathic sclerochoroidal calcification. Eye. 2000;14(4):681–684. doi:10.1038/eye.2000.176
19. Vezzoli G, Soldati L, Jansen A, Pierro L. Choroidal calcifications in patients with Gitelman’s syndrome. Am J Kidney Dis. 2000;36(4):855–858. doi:10.1053/ajkd.2000.17622
20. Honavar SG, Shields CL, Demirci H, Shields JA. Sclerochoroidal calcification: clinical manifestations and systemic associations. Arch Ophthalmol. 2001;119(6):833–840. doi:10.1001/archopht.119.6.833
21. Floegel I, Klein A, Langmann G. Metastatic sclerochoroidal calcification involving the fovea. Retina. 2002;22(4):503–505. doi:10.1097/00006982-200208000-00021
22. Cooke CA, McAvoy C, Best R. Idiopathic sclerochoroidal calcification. Br J Ophthalmol. 2003;87(2):245–246. doi:10.1136/bjo.87.2.245
23. Boutboul S, Bourcier T, Heligon JP, et al. Familial pseudotumoral sclerochoroidal calcification associated with chondrocalcinosis. Br J Ophthalmol. 2004;88(8):1094–1095. doi:10.1136/bjo.2003.039925
24. Kim M, Pian D, Ferrucci S. Idiopathic sclerochoroidal calcification. Optometry. 2004;75(8):487–495. doi:10.1016/S1529-1839(04)70173-6
25. Shields JA, Karatza EC, Shields CL, Cohen SB. Drusenlike deposits and sclerochoroidal calcification in a patient with glomerulonephritis. Retina. 2004;24(2):304–306. doi:10.1097/00006982-200404000-00020
26. Garuti S, Klais CM, Fisher YL, Peiretti E, Yannuzzi LA. Unusual clinical manifestation of sclerochoroidal calcifications. Retina. 2005;25(4):517–519. doi:10.1097/00006982-200506000-00020
27. Gupta R, Hu V, Reynolds T, Harrison R. Sclerochoroidal calcification associated with Gitelman syndrome and calcium pyrophosphate dihydrate deposition. J Clin Pathol. 2005;58(12):1334–1335. doi:10.1136/jcp.2005.027300
28. Sun H, Demirci H, Shields CL, Shields JA. Sclerochoroidal calcification in a patient with classic Bartter’s syndrome. Am J Ophthalmol. 2005;139(2):365–366. doi:10.1016/j.ajo.2004.07.054
29. Pakrou N, Craig JE. Idiopathic sclerochoroidal calcification in a 79-year-old woman. Clin Exp Ophthalmol. 2006;34(1):76–78. doi:10.1111/j.1442-9071.2006.01118.x
30. Lindstedt EW, van den Born LI, Veckeneer M, Baarsma GS. Sclerochoroidal calcification: idiopathic or associated with systemic disease? Retin Cases Brief Rep. 2007;1(3):141–144. doi:10.1097/01.ICB.0000279643.99324.93
31. Choi JY, Bianciotto C, Shields JA, Shields CL. Sclerochoroidal calcification in a patient with chronic hypercalcemia from undiagnosed parathyroid adenoma. Retin Cases Brief Rep. 2009;3(4):431–433. doi:10.1097/ICB.0b013e31818ba944
32. Miller KV, Eller AW. Sclerochoroidal calcifications: wide-field imaging. Semin Ophthalmol. 2009;24(1):5–8. doi:10.1080/08820530802508595
33. Lee H, Kumar P, Deane J. Sclerochoroidal calcification associated with Albright’s hereditary osteodystrophy. BMJ Case Rep. 2012;2012:bcr0320126022. doi:10.1136/bcr-03-2012-6022
34. Yohannan J, Channa R, Dibernardo CW, et al. Sclerochoroidal calcifications imaged using enhanced depth imaging optical coherence tomography. Ocul Immunol Inflamm. 2012;20(3):190–192. doi:10.3109/09273948.2012.670358
35. Rao RC, Choudhry N, Gragoudas ES. Enhanced depth imaging spectral-domain optical coherence tomography findings in sclerochoroidal calcification. Retina. 2012;32(6):1226–1227. doi:10.1097/IAE.0b013e3182576e50
36. Hara K, Tanito M, Kodama T, Ohira A. A case of chorioretinal atrophy due to sclerochoroidal calcification. Acta Ophthalmol. 2013;91(2):e167–8. doi:10.1111/j.1755-3768.2012.02470.x
37. Wong CM, Kawasaki BS. Idiopathic sclerochoroidal calcification. Optom Vis Sci. 2014;91(2):e32–7. doi:10.1097/OPX.0000000000000125
38. Caminal-Mitjana JM, Padrón-Pérez N, Arias-Barquet L, Rubio-Caso MJ, Català-Mora J. Correlation between spectral-domain optical coherence tomography and autofluorescence findings in sclerochoroidal calcification. Can J Ophthalmol. 2013;48(4):331–334. doi:10.1016/j.jcjo.2013.01.024
39. Fung AT, Arias JD, Shields CL, Shields JA. Sclerochoroidal calcification is primarily a scleral condition based on enhanced depth imaging optical coherence tomography. JAMA Ophthalmol. 2013;131(7):960–963. doi:10.1001/jamaophthalmol.2013.67
40. Zhang J, Davis AS, Spitze A, Lee AG. Bilateral Adie’s tonic pupil and hyperopic shift in idiopathic sclerochoroidal calcification. Neuroophthalmology. 2015;39(2):96–99. doi:10.3109/01658107.2015.1004724
41. Hasanreisoglu M, Saktanasate J, Shields PW, Shields CL. Classification of sclerochoroidal calcification based on enhanced depth imaging optical coherence tomography “mountain-like” features. Retina. 2015;35(7):1407–1414. doi:10.1097/IAE.0000000000000468
42. Shields CL, Hasanreisoglu M, Saktanasate J, Shields PW, Seibel I, Shields JA. Sclerochoroidal calcification: clinical features, outcomes, and relationship with hypercalcemia and parathyroid adenoma in 179 eyes. Retina. 2015;35(3):547–554. doi:10.1097/IAE.0000000000000450
43. Bessette AP, Singh AD. Multimodal imaging of choroidal neovascularization associated with sclerochoroidal calcification. Ocul Oncol Pathol. 2016;2(4):234–238. doi:10.1159/000446214
44. Ali ZC, David VP. Sclerochoroidal calcification associated with hypovitaminosis D. Can J Ophthalmol. 2017;52(4):e121–e122. doi:10.1016/j.jcjo.2017.02.001
45. Brahma VL, Shah SP, Chaudhry NA, Prenner JL. Bilateral idiopathic sclerochoroidal calcifications. Open Ophthalmol J. 2017;11:76–79. doi:10.2174/1874364101711010076
46. Sugarman JA, Douglass AM, Say EA, Shields CL. Stones, bones, groans, thrones, and psychiatric overtones: systemic associations of sclerochoroidal calcification. Oman J Ophthalmol. 2017;10(1):47–49. doi:10.4103/0974-620X.200693
47. Goerlitz-Jessen M, Ali MH, Grewal DS. Rare complication of sclerochoroidal calcifications. JAMA Ophthalmol. 2019;137(1):111–112. doi:10.1001/jamaophthalmol.2018.2457
48. Slean GR, Kalevar A, Chen J, Johnson R. Enlargement of sclerochoroidal calcifications: multimodal imaging update. Retin Cases Brief Rep. 2018;12(1):S122–S124. doi:10.1097/ICB.0000000000000644
49. Abouzaid M, Al-Sharefi A, Artham S, Masri I, Kotagiri A, Joshi A. A rare ophthalmic condition associated with primary hyperparathyroidism (PHPT): sclerochoroidal calcification (SC). Endocrinol Diabetes Metab Case Rep. 2019;2019(1):19. doi:10.1530/EDM-19-0003
50. Ciaffi J, Borlandelli E, Mancarella L, Brusi V, Meliconi R, Ursini F. Sclerochoroidal calcifications associated with early-onset calcium pyrophosphate deposition disease. Clin Rheumatol. 2020;39(9):2825–2826. doi:10.1007/s10067-020-05148-7
51. Fortes BH, Dalvin LA, Bakri SJ. Multimodal imaging of sclerochoroidal calcification associated with choroidal neovascular membrane. Can J Ophthalmol. 2021;56(3):e98–e101. doi:10.1016/j.jcjo.2020.11.007
52. Mitamura M, Kase S, Ishida S. Multimodal imaging in sclerochoroidal calcification: a case report and literature review. BMC Ophthalmol. 2020;20(1):248. doi:10.1186/s12886-020-01520-y
53. Sharifi M, Yousefi BT. Idiopathic dural optic nerve sheath calcification associated with sclerochoroidal calcification: case report and review of literatures. Case Rep Ophthalmol. 2021;12(2):402–406. doi:10.1159/000511339
54. Thomson AC, Brown GT, Dolores-Rodriguez A, Hunter AA. A case of extensive bilateral idiopathic sclerochoroidal calcification and review of literature. Int Med Case Rep J. 2021;14:749–755. doi:10.2147/IMCRJ.S336237
55. Battaglia Parodi M, Arrigo A, Pina A, et al. Choroidal neovascularization complicating sclerochoroidal calcifications. Am J Ophthalmol Case Rep. 2021;24:101235. doi:10.1016/j.ajoc.2021.101235
56. Lassandro NV, Danieli L, Nicolai M, Pirani V, Pelliccioni P, Mariotti C. Sclerochoroidal calcification as an incidental finding during oncological staging of a patient with parotid malignancy: a case report. Eur J Ophthalmol. 2022;32(4):NP67–NP70. doi:10.1177/1120672121999629
57. Dedina L, Chen TS, Durkin S, Little M. Choroidal neovascularization: a rare complication of sclerochoroidal calcification. Asia Pac J Ophthalmol. 2022;11(3):297. doi:10.1097/APO.0000000000000442
58. Nabih O, Hamdani H, El Maaloum L, Allali B, El Kettani A. Sclerochoroidal calcification associated with chondrocalcinosis: a clinical case. Ann Med Surg. 2022;74:103275. doi:10.1016/j.amsu.2022.103275
59. Šulavíková Z, Káčerik M, Šustykevičová Z, Krásnik V. Idiopathic sclerochoroidal calcifications. A case report. Cesk Slov Oftalmol. 2022;78(2):86–92. doi:10.31348/2022/12
60. Tetik D, Gündüz AK. Multimodal imaging including swept source optical coherence tomography angiography in a case with sclerochoroidal calcification. Photodiagnosis Photodyn Ther. 2022;40:103054. doi:10.1016/j.pdpdt.2022.103054
61. Nieves-Martínez IM, Villegas VM, Schwartz SG. Optical coherence tomography angiography findings in sclerochoroidal calcification. P R Health Sci J. 2022;41(1):41–44.
62. Stevenson M, Pagnamenta AT, Mack HG, et al. The Bartter-Gitelman spectrum: 50-year follow-up with revision of diagnosis after whole-genome sequencing. J Endocr Soc. 2022;6(7):bvac079. doi:10.1210/jendso/bvac079
63. Yildirim TD, Mammadov T, Saatci AO, Sari I. Bilateral sclerochoroidal calcification in a patient with calcium pyrophosphate deposition disease. Rheumatology. 2023;62(6):e207–e208. doi:10.1093/rheumatology/keac633
64. Mani X, Johnson R. A case of sclerochoroidal calcification masquerading as a retained intraocular foreign body. Radiol Case Rep. 2023;18(5):2034–2038. doi:10.1016/j.radcr.2023.02.049
65. Shields CL, Kalafatis NE, Gad M, et al. Metastatic tumours to the eye. Review of metastasis to the iris, ciliary body, choroid, retina, optic disc, vitreous, and/or lens capsule. Eye. 2023;37(5):809–814. doi:10.1038/s41433-022-02015-4
66. Arepalli S, Kaliki S, Shields CL. Choroidal metastases: origin, features, and therapy. Indian J Ophthalmol. 2015;63(2):122–127. doi:10.4103/0301-4738.154380
67. Shields JA, Shields CL. Posterior uveal melanoma: clinical features. In: Shields JA, Shields CL, editors. Intraocular Tumors. An Atlas and Textbook. Philadelphia: Wolters Kluwer; 2008:80–615.
68. Mashayekhi A, Shukla SY, Shields JA, Shields CL. Choroidal lymphoma: clinical features and association with systemic lymphoma. Ophthalmology. 2014;121(1):342–351. doi:10.1016/j.ophtha.2013.06.046
69. Shields CL, Shields JA, Augsburger JJ. Choroidal osteoma. Surv Ophthalmol. 1988;33(1):17–27. doi:10.1016/0039-6257(88)90069-0
70. Shields CL, Sun H, Demirci H, Shields JA. Factors predictive of tumor growth, tumor decalcification, choroidal neovascularization, and visual outcome in 74 eyes with choroidal osteoma. Arch Ophthalmol. 2005;123(12):1658–1666. doi:10.1001/archopht.123.12.1658
71. Shields CL, Honavar SG, Shields JA, Cater J, Demirci H. Circumscribed choroidal hemangioma: clinical manifestations and factors predictive of visual outcome in 200 consecutive cases. Ophthalmology. 2001;108(12):2237–2248. doi:10.1016/S0161-6420(01)00812-0
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.