Back to Journals » Eye and Brain » Volume 15
Diagnosis and Management of Pineal Germinoma: From Eye to Brain
Authors Cohen D, Litofsky NS
Received 27 December 2022
Accepted for publication 3 April 2023
Published 13 April 2023 Volume 2023:15 Pages 45—61
DOI https://doi.org/10.2147/EB.S389631
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Margaret Wong-Riley
David Cohen,1 N Scott Litofsky2
1Department of Neurology, University of Missouri School of Medicine, Columbia, MO, USA; 2Department of Neurosurgery, University of Missouri School of Medicine, Columbia, MO, USA
Correspondence: N Scott Litofsky, Department of Neurosurgery, One Hospital Drive, MC, 321, Columbia, MO, 65212, USA, Tel +1-573-882-4908, Fax +1-573-884-5184, Email [email protected]
Abstract: Pineal germinomas can be very complex in terms of presentation, diagnosis, and management. This review attempts to simplify this complexity in an organized manner, addressing the anatomic relationships that provide the basis for the uniqueness of pineal germinoma. Ocular findings and signs and symptoms of elevated intracranial pressure are the keys to suspecting the diagnosis and obtaining the necessary imaging and cerebrospinal fluid studies. Other symptoms can suggest spread beyond the pineal region. Surgery may only be needed to obtain tissue for a definitive diagnosis, as germinoma is highly responsive to chemotherapy and focused radiation therapy. Hydrocephalus, usually related to tumor obstruction of the cerebral aqueduct, may also need to be addressed. Outcome for pineal germinoma is usually excellent, but relapse can occur and may require additional intervention. These issues are detailed in this review.
Keywords: pineal germinoma, signs and symptoms, ocular findings, management, anatomy
Introduction
Pineal germinomas belong to a class of tumors called germ cell tumors, which resemble tumors associated with gonads. Other germ cell tumors include teratomas (mature and immature), choriocarcinomas, yolk sac tumors, and endodermal sinus tumors.1 Two theories explain how these types of tumors occur in the brain. According to the “Germ Cell Theory”, germinomas arise from germ cells, which become transformed and misplaced in the bilaminar embryonal disc of the primitive streak and become enfolded in the brain with neural tube development; other germ cell tumors develop along another oncogenic line originating from transformed germ cells.2 In contrast, the “Embryonic Theory” suggests that primordial germ cells may migrate abnormally, survive despite their abnormal location, and undergo malignant transformation for neoplastic totipotentiality.3
Germ cell tumors are uncommon in the central nervous system, representing 0.6% of brain neoplasms in the United States.4 In Japan, germ cell tumors may occur with a similar frequency or somewhat more frequently – up to 2.8% of intracranial tumors – depending on epidemiological methodology.5,6 Germinomas are the most common pediatric intracranial neoplasm in Japan.1 With a predilection for midline structures, germinomas occur in the pineal gland (the most common site) followed by the supra-sellar compartment.7 The majority of germinomas occur in males;8 however, prevalence of supra-sellar germinomas is slightly higher in females.9
Because the pineal gland is located adjacent to the corpus callosum, the quadrigeminal plate, the midbrain, the cerebral aqueduct, and the third ventricle,10 pineal tumors can be in contact with cerebrospinal fluid (CSF) pathways and neural structures easily susceptible to compressive effects. Tumors arising in this region are commonly associated with hydrocephalus and elevated intracranial pressure (ICP),10 neurological symptoms and signs, including visual compromise, endocrine dysfunction, and dissemination of tumor cells and protein markers into cerebrospinal fluid or other neural tissues. This review will focus on pineal germinoma and address the role of tumor location in its phenotypic behavior, diagnosis, and management.
Anatomy
The name of the pineal gland, also referred to as epiphysis cerebri, comes from the Latin “pinealis” where pinea means pine cone, owing to its pine cone shape.11 A distinctive feature initially pointed out by Descartes is that the pineal is the only organ that does not have bilateral symmetry in the sagittal plane of the brain.11 Because of this unique characteristic, as well as some local mobility of the structure, Descartes claimed that the pineal was the seat of the soul.12
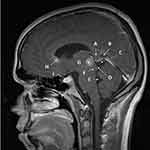
The pineal region (Figure 1) is composed of the pineal body within the quadrigeminal arachnoidal cistern.13 The pineal gland is attached to the posterior aspect of the roof of the third ventricle and located at the center of the posterior diencephalon where the pineal floats in the posterior basal cisterns,14 hence its mobility. The pineal gland is in contact with the pineal recess and dorsal supraspinal recess containing the choroid plexus of the third ventricle.15 While Vollrath in 1981 describes 6 anatomical variants of pineal gland have been identified in mammals based on shape, spatial relationship to the third ventricle, and parenchymal continuity,16 variants Type A – in which the pineal gland is round or oval, proximal to the posterior edge of the diencephalon, and often in the vicinity of the third ventricle in contact with cerebrospinal fluid – and Type ABC – in which the pineal gland is elongated into a rod-shape and extending more towards the cerebellum – are most common in humans.15,16 The pineal has a ventro-caudal relationship to the upper aperture of the cerebral aqueduct, where anterior extension of the germinoma can result in hydrocephalus by blocking the aqueduct.11 Proximity to the third ventricle can facilitate subependymal spread to the suprasellar area or spread into the cerebrospinal fluid itself with resulting drop metastases into the cerebral aqueduct and, more inferiorly, CSF dissemination. Tumor growth with compression of the tectum leads to Parinaud’s syndrome.17,18
Pathways originating in the retina innervate the pineal gland. Visual projections from the eye relay information via the retino-hypothalamic tract to the suprachiasmatic nucleus (SCN) of the hypothalamus, also known as the circadian pacemaker. The SCN then projects a circadian signal to the paraventricular nucleus (PVN). From the PVN, the signal is projected to the intermediolateral cell column, next to the superior cervical ganglion, and finally to the pineal gland via post-ganglionic sympathetic fibers from the superior cervical ganglion.19 When stimulated by sympathetic input, the pineal gland secretes melatonin, where light inhibits secretion and darkness stimulates it.15 The rate of melatonin secretion involves several components based on sleep-wake cycle. These pathways can provide potential corridors for tumor spread into the suprasellar area.17,18 Other potential neural structures in the area, which could serve as routes for tumor spread include the trochlear nerves, which originate from the posterior aspect of the tectum on the quadrigeminal plate. Such involvement has been observed with astrocytoma, causing an isolated superior oblique muscle deficit.20 Isolated superior oblique deficit is unlikely in germinoma due to broader involvement of other tectal structures.
The medial posterior choroidal arteries are the main vasculature supply to the pineal gland. The posterior cerebral arteries, which are located lateral to the brain stem on each side, provide the branches of medial posterior choroidal arteries. Typically, 1 to 3 small arteries supply the lateral walls of the pineal gland on each side. In about 1/3 of cases, only one side will have arterial supply.11 Venous structures in the area include the two internal cerebral veins anteriorly and inferiorly, the Veins of Rosenthal lateral to the pineal coursing superiorly to join the Vein of Galen, and the straight sinus running from the pineal area superiorly to the torcula. These structures are often displaced or engulfed by tumor growth. They can serve as conduits for hematogenous spread.21,22
Germinomas, as well as other pineal tumors, are thought to have three phases of disease, which relate to anatomic relationships of the tumors.23 Vomiting occurs in the first phase, as early hydrocephalus and elevated ICP affect the area postrema and the vomiting center in the fourth ventricle. In the second phase, accompanied by compression of the tectum and further increases in ICP, patients experience blurred vision, diplopia, change in mental outlook, ataxia, dizziness, and Parinaud’s syndrome. The third phase, with worsening hydrocephalus and further compression of the brain stem and corticospinal tracts, is manifested by papilledema, marked weakness, and varying levels of spasticity.
Diagnosis
Signs and Symptoms
Ocular Findings
Because of their proximity to the tectum and CSF pathways, pineal germinomas frequently present with ocular signs and symptoms. The associated symptoms and signs are dependent on which anatomic structures are compromised, and the manner of such compromise. These24 neuroophthalmological symptoms tend to manifest in early stages of disease and require ophthalmological exam at all stages of diagnosis and treatment.25
Three types of optic neuropathies can occur, including 1) compressive, in which blood supply to optic nerve is compromised, 2) papilledematous, in which blockage of axonal flow occurs, and 3) infiltrative.26 Blurred vision can occur because of optic nerve compromise by papilledema, infiltration of an optic nerve, or edema of an optic nerve. Papilledema is usually caused by elevated ICP,18 and is found on fundoscopic examination in 60% of pineal region tumors.24 A very large tumor could cause elevated intracranial pressure, but most of the time, papilledema is caused by hydrocephalus from blockage of the cerebral aqueduct by tumor in the third ventricle or compression of the tectal plate and compression of the aqueduct. Symptoms which suggest hydrocephalus include headaches, nausea, projectile vomiting, and lethargy.27 But because patients with papilledema can be asymptomatic, a fundoscopic exam is essential, regardless of symptoms.
Visual blurriness secondary to intracranial hypertension from a pineal germinoma (even if such elevated ICP is prolonged) is rarely associated with permanent visual loss, in contrast to optic nerve compression from the suprasellar component of a bifocal tumor,26 where a delay in diagnosis and treatment can lead to permanent visual loss.18
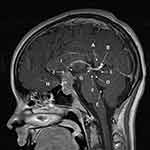
Visual field deficits can also occur if the pineal germinoma is a bifocal tumor with the suprasellar component impinging on the optic chiasm (Figure 2). Both bedside and formal visual fields should be performed as part of evaluation. These field deficits are often asymmetric.25,28 Unilateral blindness and loss of the papillomacular bundle from tumor cells seeding the optic nerve sheath and/or infiltrating the optic nerve can occur.29 This seeding may occur in a delayed manner and has been observed to occur as long as 12 years after initial diagnosis.30
Compression of the tectum, specifically the superior colliculus of the quadrigeminal plate, results in Parinaud’s Syndrome.27,31 Up to 75% patients may have Parinaud’s Syndrome.25 The most characteristic sign is supranuclear palsy of conjugate upward gaze when eyes are straight in primary position, as seen in 69.7% of patients.32 Compared to other pineal tumors, up-gaze paresis is more common in germinoma because the pattern by which germinoma grows near the midbrain compresses or infiltrates the vertical gaze centers near the superior colliculus, including the interstitial nucleus of Cajal and the rostral interstitial nucleus of the medial longitudinal fasciculus (riMFL).27 The superior rectus and inferior oblique muscles are responsible for upward elevation of the eyes. The riMFL sends information directly to the superior rectus and inferior oblique muscles.33 Usually, downward gaze is not affected. Dysfunction of the bilaterally innervated superior rectus and inferior oblique muscles of the oculomotor nerve from the riMLF may be responsible.33 Other components of Parinaud’s Syndrome include pupils semi-dilated with light-near dissociation (in which the pupils react to accommodation but not to light), convergence-retraction nystagmus, and upper eyelid retraction (also known as Collier’s sign).8 Takami et al observe 37.3% of patients have light-near dissociation (Argyll-Robertson pupil),32 but that these patients are less likely to have germinoma than other non-germinomatous germ cell tumors. Diplopia has been seen in 74.3% of patients and is usually caused by trochlear nerve involvement.32,33 Bugueno-Montanes et al also report a Parinaud “plus” syndrome, in which Parinaud’s Syndrome occurs in conjunction with diplopia,8 possibly due to additional invasion of surrounding structures of the midbrain tectum. Tumor size itself does not correlate with ocular manifestations.32 Loco-regional extensions of pineal germ cells with extension of tumor into the midbrain are not uncommon.17 Perhaps, the loco-regional extensions could contribute to this Parinaud “plus” syndrome. These ocular manifestations are key for suspecting a pineal mass.34 Notably, ocular manifestations tend to persist, even after treatment, in over 50% of cases; in those with upgaze paresis, 80% have persistent signs.32 Persistence of ocular symptoms and signs occurs even with surgery,35 including those requiring additional surgery after chemotherapy.36
Other ocular findings have been observed with pineal germinoma. Exercise-induced diplopia, similar to manifestations of myasthenia gravis, can occur.23 Bilateral uveitis, differentiated from sarcoidosis and tuberculosis, has been identified in a patient with germinoma who was experiencing “floaters.”37 The most common symptom is blurry vision. Other symptoms include eye pain and photophobia. In some situations, the patient may be asymptomatic.38 Optic neuritis with cerebrospinal fluid oligoclonal bands, classic findings of multiple sclerosis, have been reported in patients with germinoma alone.39 A paraneoplastic syndrome can occur from collapsin response- mediating protein antibodies from pineal germinoma and cause autoimmune optic neuropathy and associated subacute vision loss with bilateral disc edema, visual field defects, vitreous cells, and vascular leakage.40 Retinal phlebitis can accompany paraneoplastic syndrome, presenting with loss of visual acuity,41 diplopia,42 and red, painful eye.37 While the classic symptom of retinal phlebitis is a painless decrease in vision, other symptoms include blind spots, metamorphopsia (changes in shape of object), or changes in color vision. Patients with retinal phlebitis are most commonly asymptomatic.43
Brain-Related Non-Ocular, Non-Endocrinologic Findings
Headache is a hallmark symptom of elevated ICP. Headache occurs commonly in patients with germinoma (88.3% of patients in 1 series),44 usually related to hydrocephalus. If not treated, disease progression can lead to “hydrocephalic fits”, associated with posturing and coma.45 Prompt intervention with ventriculostomy can sometimes resuscitate the patient. Unfortunately, these events can be confused with seizures, resulting in delay in intervention and subsequent patient death.
Hemiparesis can result from loco-regional spread into the thalamus or basal ganglia,46,47 or from infiltration or compression of the internal capsule, cortico-spinal tract in the cerebral peduncle,48 or medulla.49 Additional symptoms can include cognitive impairment, involuntary movements, fever of unknown origin, mental disturbances and abnormal behavior, character changes, and convulsions.43 Difficulties with thermoregulation have also been observed.47 Germinoma in these locations is generally uncommon, and occurs more often in Asian patients.47 MRI may not show connections of the mass to the pineal area. On MRI, these tumors are often associated with cysts, hemorrhage, calcifications, edema, and brainstem hemiatrophy, thought to be related to Wallerian degeneration of the cortico-spinal tract.47
Spine-Related Findings
The most common type of metastasis associated with germinomas are spinal “drop” metastasis.27 Occurrence of these drop metastases synchronously with pineal germinoma is unusual,50 although Lo et al find an incidence of synchronous presentation in 11% of pineal germinoma.51 When these spinal metastases occur, symptoms may consist of low back pain,52 monoparesis,53 paraparesis, or quadriparesis or tetraparesis,54 paresthesia,55 numbness with a sensory level, spasticity,54 and urinary retention.56 What symptoms and signs are present is dependent on the level of the spine involved, the size of the lesion, and the location within the spinal canal. Tumor can be extramedullary, compressing the spinal cord, or intramedullary, expanding the cord.
Imaging
Computed Tomography
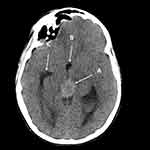
Patients with the above clinical signs or symptoms warrant imaging of the brain. Computed tomography (CT) scan of the head is usually the primary imaging modality when the patient presents with rapid neurological decline or progressively worsening headaches. CT is sufficient to detect pineal region tumors as well as associated hydrocephalus secondary to obstruction of the aqueduct. On CT, pineal germinomas appear as homogenous, hyperdense masses compared to adjacent structures (Figure 3).57 CT is also helpful to characterize calcifications, hemorrhage, and cysts (present 20–52% of cases).57
Magnetic Resonance Imaging
Magnetic resonance imaging (MRI) with and without gadolinium is the gold standard for imaging. Pineal masses and hydrocephalus are readily identified on MRI. MRI can help differentiate pineal neoplasms from other benign masses in the region, identify presence of leptomeningeal spread, and characterize lesion enhancement patterns. MRI shows strong contrast enhancement of pineal germinomas. T1- and T2-weighted images are isointense to grey matter, and diffusion weighted imaging (DWI) is comparatively hyperintense.57 Apparent diffusion coefficient (ADC) values have been inconsistently reported as typically higher than pineoblastomas,57 lower than pineoblastoma,58 or no different than pineoblastoma.59 MRI spectroscopy can be helpful to differential pineal germinoma from other pineal tumors as germinomas exhibit a high lipid profile.58
Loco-regional extension has been identified in all patients with careful imaging review on MRI. Duron et al identify extension to the third ventricle in 88% of patients, optic nerve and chiasm in 19%, thalamus in 47%, midbrain in 42%, distant sub-ependymal areas in 19%, and fourth ventricle in 3%.17 Between 5% and 10% of germinomas may arise in basal ganglia or thalamus, at least in Japan.60 Enhancing lesions in these locations, therefore, may not represent metastases.61 Because disseminated disease and subependymal tumor spread are common at diagnosis (13%), neural axis screening with spinal MRI is recommended.57
Other Imaging Modalities
While CT and/or MRI have a predictive value of 85–90% for germ cell tumors, they are unable to differentiate completely between CNS germinomas and lymphomas from other neoplasms.62 Use of combined 201TL and 67Ga brain spectroscopy can be helpful in this regard. Positive readings of 67Ga and 201Tl with more uptake of 67Ga than 201Tl tend to occur in patients with CNS lymphoma or germinomas. Brain spectroscopy (SPECT) can also be used to monitor for relapses, thus avoiding second-look operations.62 FDG-PET, however, has not been found to be helpful in differentiating germinoma from pineal parenchymal tumors.59
Imaging can also play a role in target section for stereotactic biopsy. MET-PET can be used to select the best target in thalamic or basal ganglia components of lesions, where prognosis is worse compared to pineal and suprasellar regions, partly due to difficulty evaluating efficacy of treatment with conventional neuroimaging.60 Kawai et al use (11) C-methionine (MET) Positron Emission Tomography (PET) to assess treatment response in germinoma of the basal ganglia and thalamus.60 After initial treatment, a transient increase in MET uptake occurred in germinoma lesions. After more intense treatment, the MET decreased, suggesting possible improvement. Kawai et al caution that further studies are needed which include histologic confirmation.60
Because drop metastases may occur in up to 30% of pineal tumors and 11% of germinoma at initial presentation,51,63 MRI of the spine without and with contrast is indicated prior to any definitive intervention or biopsy. Preferably, this MRI should be performed prior to lumbar puncture for CSF sampling to avoid artifactual dural enhancement, which can be misinterpreted as dural metastasis or leptomeningeal dissemination.64 However, Mark et al found such dural enhancement in only 3.9% of patients who had MRI after lumbar puncture.65
Cerebrospinal Fluid Profile
Laboratory Tests
While histopathology is the gold standard for diagnosis,10 the invasive nature of surgical procedures is of some risk.1 Tumor markers can theoretically circumvent the need for surgery for diagnosis. These markers are best obtained from cerebrospinal fluid from lumbar puncture or ventriculostomy. Germinoma can secrete small amounts of Beta Human Chorionic Gonadotropin (b-HCG) through syncytiotrophoblastic components.9,27,66 Although a strict universal strict cutoff has not been established, usually the amount of b-HCG is less than 50 mUI/mL to distinguish the tumor from choriocarcinoma.1,27 In addition, Placental Alkaline Phosphatase (PLAP) CSF levels in germinomas were higher than non-germinomas using Chemiluminescent Enzyme Immunoassay (CLEIA), suggesting that PLAP levels have a high sensitivity and specificity for germinomas when correlated with tumor biopsy. Alpha feto-protein (AFP) should also be assessed, as AFP is elevated with yolk sac tumors,67,68 which can be used to differentiate a pineal mass from germinoma. Teratomas – mature or immature – do not secrete tumor markers, so a maker-negative tumor could be a germinoma, a teratoma, or a non-germ cell tumor.
Cytology
Cytology can also be obtained for diagnosis.69,70 Coordination with the laboratory is essential, as the specimen must be processed within 2 h of obtaining the sample. Unfortunately, tumor cells are present in the spinal fluid in only 38% of germinomas (25/66), so positive cytology is not a dependable diagnostic test.71 Furthermore, positive CSF cytology was not correlated with presence of spinal lesions on MRI.71 Even as a prognostic test, cytology has limitations as non-cranio-spinal irradiation achieved excellent progression-free survival (PFS) even in patients with positive CSF cytology without spinal lesion on MRI. Finally, CSF pleocytosis, presence of CSF oligoclonal bands, and abnormalities of CSF IgG can occur in germinoma,40,55 so CSF suggestive of other disease processes, like multiple sclerosis, does not necessarily exclude germinoma.
Cytology does play a key role in staging germinoma. Modified Cheng criteria can be used in which CSF cytology and spinal MR imaging are routinely examined for metastatic disease before treatment with positive cytology indicating metastatic disease.71 Ilcus et al suggested staging as M0 if disease is localized on MRI with no evidence of metastasis and CSF cytology is negative.27 If either intra-cranial or spinal metastasis is identified or positive cytology is present, then germinoma should be classified as disseminated disease M+.27 Alternatively, if intracranial metastases are present, the tumor should be designated M2, and spinal metastasis warrants M3 designation.72
Endocrine Dysfunction
Endocrine dysfunction can occur with pineal germinoma, particularly if the tumor presents as a bifocal tumor with a suprasellar component adjacent to the hypothalamic–pituitary axis.1 Endocrinopathy can also occur after radiation therapy,73 although only 5% of patients developed any new endocrinopathy; pre-operative endocrinopathy persisted in 55% of patients after radiation.74 Symptoms observed include delay or regression of sexual development, hypopituitarism, growth failure, precocious puberty, fluctuations in blood pressure, heart rate, respiration, and temperature, gastric motility, and alterations in mood, memory, appetite, and emotional behavior.75 Precocious puberty may result from secretion of b-HCG,9 so precocious puberty is possible even in the absence of a suprasellar mass.28 Diabetes insipidus (DI), associated with polydipsia and polyuria, occurs in up to 41% of pineal germinomas and 93% of suprasellar germinomas.75 Water deprivation testing is considered gold standard.76 Prolactin may be increased due to compression of the pituitary stalk. Sklar et al find that 5 of 8 patients (62.5%) with suprasellar germinomas had hyperprolactinema.77 Germinoma should be suspected in any child with visual complaints and endocrinological findings.78 Furthermore, any pediatric patient with central DI of unknown cause should have germinoma at least in the differential.79 When suprasellar lesions are identified, endocrinological testing is appropriate, even in the absence of symptoms.57
The above-described diagnostic findings are summarized in Table 1.
 |
Table 1 Components in Diagnosis of Pineal Germinoma |
Management
Principles in management of pineal germinomas have been outlined by Allen et al as follows:
- Biopsy
- Staging by CT, myelography, CSF markers, and MRI
- Chemotherapy
- Radiation75
An alternative way to develop a management plan has been proposed by Frappaz et al, who suggest answering the following questions:
- Does the tumor originate from the pineal or an adjacent structure?
- Does the tumor only involve the pineal or also the suprasellar area?
- Is the tumor only found in the pineal or has it also metastasized?
- How old is the patient and what is his/her ethnicity?
- Can a definitive diagnosis be reached without a biopsy?
- Is surgical resection indicated, or will biopsy only suffice?
- What is the best treatment for the diagnosis?31
The following section attempts to address these points (Figure 4).
Surgery
Indications for Surgery
Pineal germinoma generally does not require surgical resection. These tumors are very radio-sensitive and chemo-sensitive, so surgery is not usually performed primarily to obtain a specific diagnosis. Endoscopic biopsy or stereotactic biopsy are preferred to open resection. Importantly, germ cell tumors can be mixed, consisting of multiple tumor types. The most common combination is germinoma and teratoma.9 Since teratomas are resistant to radiotherapy and chemotherapy, surgical removal must be considered. Generally, surgical resection of germinoma is then usually reserved for when residual mass after initial treatment remains,73 which suggests a mixed tumor.
Shibamoto et al feel that typical germinoma can be diagnosed based on typical clinical or radiologic findings, together with slight elevation of b-HCG levels in serum and/or CSF and quick response to radiation or chemotherapy.80 This approach may be reasonable in Japan, where the incidence of germ cell tumors is higher.6 This management plan, however, is not felt to be satisfactory elsewhere.
Surgical Resection
If surgical resection is needed, several approaches are available, depending upon a particular tumor’s extensions.57 If the tumor extends superiorly, displacing vein of Galen inferiorly, a suboccipital trans-tentorial approach is preferred. An infratentorial supra-cerebellar approach is advised if the tumor is growing inferiorly to the vein of Galen. When the tumor extends laterally, a trans-cortical trans-parietal approach is probably best.81–84 Finally, a trans-ventricular approach is recommended when the tumor extends anteriorly into the third ventricle.81–84 These procedures carry risk of post-operative complications, including cerebellar injury (infra-tentorial supra-cerebellar), memory disturbances (trans-ventricular), and visual field dysfunction (suboccipital trans-tentorial and trans-cortical trans-parietal).57,81–84
Neuro-Endoscopy
Due to heterogeneity in tumors and advancements in surgical procedures, tissue diagnosis of pineal germinomas is the standard of care.73 Best options in a patient with a pineal mass with β-HCG less than 50 mIU/mL are endoscopic biopsy or stereotactic biopsy. With neuro-endoscopy, key objectives of management can be achieved by one-step surgery in pineal region, including:
- CSF sampling for analysis of tumor markers
- Treatment of hydrocephalus by third ventriculostomy
- Tumor biopsy for histologic diagnosis85
Hayashi et al show a diagnostic accuracy with endoscopy for germ cell tumors of 96.6% (169 of 175 tumors).85 Misdiagnosis is possible,57 particularly because granulomatous reactions can be seen, often adjacent to third ventricle.6,86 Furthermore, endoscopic biopsy may miss the teratomatous component of mixed tumor,6 although stereotactic biopsy has a similar, though perhaps slightly less likely, risk. Complications can also occur, the most significant of which is bleeding into the ventricle that can obscure visualization, be difficult to control, and lead to abortion of the procedure.85,87 Prolonged external ventricular drainage may then be required.88,89 Another concern of endoscopic biopsy is the potential for tract recurrence and CSF.90 This risk is generally low, as Luther et al show with 0 out of 10 low-risk patients (0%) and only 2 out of 22 high-risk patients (9.1%) experiencing such seeding.91
Stereotactic Biopsy
Stereotactic brain biopsy is another option for obtaining diagnostic tissue. Classically, this technique has included use of a stereotactic frame.10,92–94 Precision is key, given the adjacent vascular structures that must be avoided, and such precision is provided with the frame. Frameless techniques have been used, but morbidity and mortality are higher compared to framed biopsy.95 Diagnostic accuracy is thought to be superior to endoscopic biopsy.10,87 Popovic and Kelly obtain tissue diagnosis in 33 of 34 patients (97.0%).96 From their review of the literature, Zacharia and Bruce report an accuracy rate of 94.4%.95 Large series of patients or direct comparisons of stereotactic biopsy and endoscopic biopsy are not forthcoming, so one can conclude that techniques are probably relatively equivalent for diagnosis. Hemorrhage from adjacent vascular structures is a major complication of concern.10,87 One solution to avoid this complication is use of a lateral temporal approach. This approach traverses either superior or middle temporal gyrus. The trajectory passes parallel to Sylvian fissure sloping ventrally and anteriorly, rather than in an orthogonal plane, to enter the lateral surface of pineal tumor. No specific tumor size prohibits use of this approach. The nonorthogonal temporal approach avoids major deep venous structures, lateral ventricle, and critical motor pathways, ensuring safety of the procedure.87
In addition to routine histopathological examination of the biopsied tissue, additional immunohistochemistry and molecular tests can be performed to determine if the tumor is germinoma. While chromosomal instability is a characteristic of all pineal germ cell tumors, global DNA hypomethylation is exclusively present on germinomas.9,27
Germinomas are further characterized by abundant expression of genes linked to immune response – CCL8, CD72, and IL6R – which are consistent with lymphocyte infiltration.8 Lastly, germinomas highly expresses PD-21.9 Despite these advances, molecular characterization of germ cell tumors plays a very limited role clinically.97
Chemotherapy
Because of the radiosensitivity of germinomas, historically the treatment of choice has been radiotherapy with potential for cure.98 However, because of the potential for complications from associated radiation exposure, including neurocognitive and neuroendocrine dysfunction,74 neoadjuvant chemotherapy has become a key part of the treatment to help lower the radiation dosage while maintaining high cure rates. CNS germinoma patients should be completely cured with minimum morbidity by using appropriate doses of chemotherapy and intense modulation radiation therapy in future.80 Even pathologically pure germinomas with a significantly elevated b-HCG (>50 mIU/mL) can be managed with a reduced dose of radiotherapy if effective chemotherapy is provided.99 Patients with bifocal germinomas should be treated with neoadjuvant chemotherapy and radiation just like uni-focal tumors.31 Several chemotherapy regimens have been used, usually based on platinum drugs.73 One regime consists of four cycles of a regimen consisting of carboplatin on day 1 at 600 mg/m2 /day and etoposide (VP16) at 150 mg/m2 /day on days 1–3.72 Another regime is carboplatin 600 mg/m2 on day 1 and VP16 90mg/m2 on days 1–3 alternating with ifosfamide 1800 mg/m2 on days 1–5 and VP16 90 mg/m2 on days 1–5 (“PE/IE” protocol) prior to irradiation; this protocol is associated with 5-year PFS of 88% and a 5-year OS of 96%.57
Radiation Therapy
Loco-regional treatment is preferred over craniospinal irradiation (CSI) if disease is not disseminated. Blakely et al compared 50 Gray (Gy) to 30 Gy radiation dosage with chemotherapy, finding no significant difference in relapse rate between the two groups,73 so reduced radiation dosing with chemotherapy is preferred.74 For limited disease, treatment with whole-ventricular irradiation (WVI) 24 Gy and tumor boost of 16 Gy. Patients with a complete response to chemotherapy can be followed with WVI 18 Gy and a. boost 12 Gy in 1.6–1.8 Gy fractions. Patients with metastatic disease can be treated with CSI of 24 Gy and tumor boost 16 Gy in 1.8 Gy fractions.57 Stereotactic radiosurgery can also be used for focal disease.100,101 Alternatively, for small lesions, radiation can be withheld altogether.74 Some might have concerns with these approaches due to the loco-regional spread so often observed. Patients with bifocal germinomas should be treated with neoadjuvant chemotherapy and radiation just like uni-focal germinoma.31 For bifocal germinoma, a broader radiation field (whole brain) may be required, as disease free survival is better than with focal radiation (66.6% versus 96.6%).47 Some recommend WVI field as the best practice for cases of non-metastatic germinomas.57,72
Hydrocephalus
Pineal germinomas are frequently associated with obstructive hydrocephalus due to the compression or blockage of the aqueduct of Sylvius.9 Cerebrospinal fluid diversion procedures are often needed.73 Perhaps 90% of patients have concurrent hydrocephalus at presentation.102 Likely symptoms include headache, nausea, vomiting, and dizziness.73 For those acute presentation, typical management of pineal tumors with hydrocephalus that are rapidly deteriorating, treatment with mannitol, glucocorticoids, and external ventricular drainage via ventriculostomy can be life-saving and stabilize the patient prior to completion of necessary work-up and avoid the need to rush into decompressive surgery.10,45 Cerebrospinal fluid can be obtained and additional imaging can be performed. If definitive diagnosis can be made via CSF profile, early initiation of chemotherapy, while the ventriculostomy is in place can lead to resolution of the hydrocephalus and subsequent removal of the ventriculostomy,103 which suggests benefit with germinomas with an approach to defer biopsy for diagnosis when β-HCG ≤50 mIU/mL in serum and/or CSF with characteristic neuroimaging features of germinoma and typical clinical findings (such as evidence of diabetes insipidus).103 Median ventriculostomy duration in this setting has been 7 days.103
An endoscopic third ventriculostomy (ETV) with or without biopsy can then be performed for a more definitive treatment of hydrocephalus and obtaining a tissue diagnosis (Figure 5).9,32,90,102 Treatment of hydrocephalus is a key consideration as Takami et al show that 43 of 56 cases of pineal germ cell tumors (81%) presented with radiographically confirmed hydrocephalus.32 Tumor size is significantly associated with hydrocephalus on presentation, as 93% of patients with tumors <25 mm did not present with hydrocephalus. Twenty mm is the optimal predictive threshold for hydrocephalus.32 Endoscopic surgery is less invasive and associated with fewer complications than open craniotomy.73
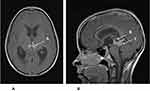 |
Figure 5 (A) Axial MRI with gadolinium of the same 14-year-old from Figure 3. The enhancing tumor (A) is extending towards the thalamus. (B). Sagittal MRI with gadolinium of the same 14-year-old showing the enhancing tumor (B) and a cyst within the tumor (C). The patient had an endoscopic third ventriculostomy with biopsy to confirm the diagnosis of germinoma and treat his hydrocephalus. He was then treated with chemotherapy, followed by local radiation therapy. |
Generally, ventriculoperitoneal shunting (VPS) is avoided because of its potential complications including shunt failure, infections, and intracranial hypotension.73 Furthermore, peritoneal metastases have been reported after VPS.104 Exceptions to this principle include large supra- or para-sellar masses causing compartmentalization hydrocephalus, where simultaneous VPS and septal fenestrations are advised.9 Because small ventricular spaces secondary to CSF diversion procedures can affect tumor access during surgery, such CSF diversion procedures should be deferred until after tumor debulking in the cases of mixed germinomas with residual after chemotherapy and radiation.73
Metastases
Drop metastases are the most common type of metastasis associated with germinomas due to proximity of germinoma to CSF pathways.27 These drop metastases are associated with spinal symptoms and signs, as previously discussed. If present, craniospinal radiation is recommended. Focal hypofractionated radiotherapy has previously been used for a single spinal metastasis of a pineal region tumor,105 but other reports are lacking. Hematogenous spread is less common but can occur.21 Theoretically, pineal germinomas should be more susceptible to hematogenous spread because the pineal gland is outside of the blood–brain barrier.62 Proximity of the tumor to large draining veins could also be a factor, as hematogenous spread occurs through adjacent blood vessels.21 The major routes of spread are along CSF pathways, on the ventricular surface, and/or via subependymal routes.106 In 5–10% of patients at diagnosis, metastatic disease is found in the CSF.106 Wang et al demonstrate increased likelihood of developing distant metastases after VP shunting is in place, leading to a worse prognosis.107 Extra-neural metastases are rare.106 Fast-developing leptomeningeal dissemination can occur, but rarely so.108
Follow-Up
Follow-up for patients with germinoma is recommended for every 4–6 months for the first 3 years and then yearly thereafter.72 Such follow-up should include MRI of the brain. Spinal MRI is indicated if spinal lesions have been previously identified, or the patient has developed symptoms and signs referrable to the spine. Vigilant long-term follow-up is required, if metastatic germinoma has occurred,73 even as long as 15-year post-treatment.54 Because long-term outcomes remain unclear, continual follow-up is recommended even after complete remission has been achieved,54 perhaps even for life.30
When relapse occurs, tumors tend to occur outside of the focal radiation field related to subependymal spread or spread via CSF.31,108 Fast-developing diffuse subarachnoid/leptomeningeal dissemination is extremely rare but can occur.102 Cases of relapse, thiotepa-based, or melphalan-based HDC regimens followed by autologous stem cell rescue can be used.57,70 For instance, Modak et al report that 7 of 9 patients (77.8%) remained in remission for 48months with this approach.70
Outcomes
Calaminus et al report patients with intracranial germinomas receiving either CSI (125 patients) or 2 courses of carboplatin and etoposide alternating with etoposide and ifosfamide followed by local radiotherapy (65 patients) had 5-year overall survival, progression-free survival, and event-free survival for the combined treatment was 96%, 88%, and 88%, respectively.109 However, only progression-free survival showed a significant decrease when compared to the radiotherapy only group. During the 60-month period, 11 patients (4 CSI and 7 combination therapy) had a relapse in median 10 months. Bartels et al report a 94% progression-free survival using carboplatin and VP16 chemotherapy in combination with radiation.110 Similar results were found in the ACNS0122 Phase II clinical trial and ACNS1123 phase II clinical trial that used the same chemotherapy regimen but with non-germinoma tumors.57
Survival is not the only outcome measure. Takami et al examine ophthalmologic signs and symptoms after surgery, finding a significant increase in the number of patients developing ophthalmic symptoms with second look surgery compared to no second look surgery.32 Similarly, Bruce et al show one-third of patients undergoing pineal tumor resection with at least 1 year of follow-up develop ophthalmic dysfunction.35 For example, in a series of 80 patients who had interhemispheric occipital trans-tentorial surgical resection for a variety of pineal region tumors, Tomita et al demonstrate that 24 patients (30%) had Parinaud syndrome, of which the findings resolved in 6 months in 16 patients and persistent double vision remained in 7.5% after surgery (2.5% vertical, 2.5% horizonal, and 2.5% exotropia).111 Pre-operative evaluation is not reported, so one cannot know if these were new findings or not. Patients have significantly more ophthalmic symptoms when undergoing resection after chemotherapy as opposed to not undergoing surgery.36 Patients should receive appropriate counseling for significant risk of new ophthalmic abnormalities if pineal tumor resection is being considered.32
Future Directions and Challenges
Great strides have been made over the years in the diagnosis and management of pineal germinoma, with survival in the range of 90%. Further improvement in diagnosis could be possible with more sensitive and specific cerebrospinal fluid analysis, including the use of molecular markers present in fluid or on examination of cytological specimens. Additional neuro-imaging techniques with current imaging modalities could also aid in non-invasive differentiation of germinoma from other tumors in the pineal region. These improvements could reduce the need for invasive procedures and identify the need for resection early in the case of mixed tumors.
Management can be advanced with improvements in surgical technology. Targeted chemotherapy could become an option if appropriate markers are identified. Radiation techniques to reduce toxicity could be helpful. Standardized surveillance protocols could help address patients with incomplete follow-up.
The major challenge to achieving these improvements is number of patients with germinoma. Many ophthalmologists, neurologists, neurosurgeons, neuro-oncologists, or other care providers may only see a few patients with germinoma in their careers. Recognizing the implications of subtle physical findings or keeping up-to-date with new developments for an uncommon disease can be difficult for many. Having enough patients for quality clinical trials is also problematic. Coordination of multiple centers could provide some solution to this challenge.
Conclusion
Pineal germinoma is frequently associated with ocular signs and symptoms related to its location. Signs and symptoms from effects in other locations of the brain or spinal cord can also occur. Surgical resection is not usually required, but endoscopic or stereotactic biopsy is often necessary if diagnosis cannot be made on the basis of cerebrospinal fluid examination. Chemotherapy with some form of radiation therapy can often lead to excellent outcomes. Long-term follow-up is recommended.
Acknowledgments
The authors wish to acknowledge Douglas C. Miller, MD, PhD, for his assistance in obtaining the images used in this paper.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Aihara Y, Watanabe S, Amoro K, et al. Placental alkaline phosphatase levels in cerebrospinal fluid could have a decisive role in the differential diagnosis of germ cell tumors. J Neurosurg. 2019;131:687–694. doi:10.3171/2018.3.JNS172520
2. Teilum G, Albrechtsen R, Norgaard-Pedersen B. The histogenetic-embryologic basis for reappearance of alpha-fetoprotein in endodermal sinus tumors (yolk sac tumors) and teratomas. Acta Pathol Microbiol Scand A. 1975;83(1):80–86. doi:10.1111/j.1699-0463.1975.tb01360.x
3. Sano K, Matsutani M, Seto T. So-called intracranial germ cell tumours: personal experiences and a theory of their pathogenesis. Neurol Res. 1989;11(2):118–126. doi:10.1080/01616412.1989.11739874
4. Vuong HG, Ngo TNM, Dunn IF. Incidence, prognostic factors, and survival trend in pineal gland tumors: a population-based analysis. Front Oncol. 2021;11:780173. doi:10.3389/fonc.2021.780173
5. McCarthy BJ, Shibui S, Kayama T, et al. Primary CNS germ cell tumors in Japan and the United States: an analysis of 4 tumor registries. Neuro Oncol. 2012;14(9):1194–1200. doi:10.1093/neuonc/nos155
6. Kinoshita Y, Yawasaki F, Tominaga A, et al. Pitfalls of neuroendoscopic biopsy of intraventricular germ cell tumors. World Neurosurg. 2017;106:430–434. doi:10.1016/j.wneu.2017.07.013
7. Srinivasan N, Pakala A, Mukkamalla C, Oswal A. Pineal germinoma. South Med J. 2010;103(10):1031–1037. doi:10.1097/SMJ.0b013e3181ebeeff
8. Burgueno-Montanes C, Santalla-Castro C, Pena-Suarez J. Parinaude “plus” syndrome in a patient with dysgerminoma. Arch Soc Esp Oftalmol. 2016;91(7):341–345. doi:10.1016/j.oftal.2016.01.003
9. Frappaz D, Dhall G, Murray M, et al. EANO SNO and Euracan consensus review on the current management and future development of intracranial germ cell tumors in adolescents and young adults. Neuro-Oncology. 2022;24(4):516–527. doi:10.1093/neuonc/noab252
10. Quick-Weller J, Lescher S, Baumgarten P, et al. Stereotactic biopsy of pineal lesions. World Neurosurg. 2016;96:124–128. doi:10.1016/j.wneu.2016.04.130
11. Simon E, Afit A, M’baye M, Mertens P. Anatomy of the pineal region applied to its surgical approach. Neurochirurgie. 2015;61(2–3):70–76. doi:10.1016/j.neuchi.2013.11.008
12. Abhyanker R. On the seat of the soul: Descartes’ pineal gland (914). Neurology. 2020;94(15 Supplement):S914.
13. Ringertz N, Nordenstam H, Flyger G. Tumors of the pineal region. J Neuropathol Exp Neurol. 1954;13(4):540–561. doi:10.1097/00005072-195410000-00004
14. Gonzalez-Lopez P, Abarca-Olivas J, Luma E, Martonell-Llobregat C, De Los Santos V. Pineal region anatomy. In: Florian I, editor. Pineal Region Lesions. Switzerland: Springer-Nature; 2020:7–19.
15. Gheban A, Rosca I, Crisan M. The morphological and functional characteristics of the pineal gland. Med Pharm Rep. 2019;92(3):226–234. doi:10.15386/mpr-1235
16. Lewczuk B, Przybylska-Gornowicz B. Gross anatomy of the pineal complex in animals. In: Turgut M, editor. Step by Step Experimental Pinealectomy Techniques in Animals for Researchers. Hauppauge, New York: Nova Science Publishers, Inc; 2013:1–32.
17. Duron L, Sanders F, Thiesse F, et al. Loco-regional extensions of central nervous system germ cell tumors: a retrospective radiological analysis of 100 patients. Neuroradiology. 2018;60(1):27–34. doi:10.1007/s00234-017-1928-6
18. Frappaz D, Pedone C, Thiesse P, et al. Visual complaints in intracranial germinomas. Pediatr Blood Cancer. 2017;64(10):e26543. doi:10.1002/pbc.26543
19. Moore R. Neural control of the pineal gland. Behav Brain Res. 1995;73(1–2):125–130. doi:10.1016/0166-4328(96)00083-6
20. Taguchi H, Kashii S, Kikuchi M, Yasuyoshi H, Honda Y. Superior oblique paresis with contralateral relative afferent pupillary defect. Graefes Arch Clin Exp Ophthalmol. 2000;238(11):927–929. doi:10.1007/s004170000206
21. Balsitis M, Rothwell I, Pigott T, Davidson N, Veitch Y, Lowe J. Systemic metastasis from primary intracranial germinoma: a case report and literature review. Br J Neurosurg. 1989;3(6):717–723. doi:10.3109/02688698908992697
22. Gay J, Janco R, Lukens J. Systemic metastasis in primary intracranial germinoma: case report and literature review. Cancer. 1985;55(11):2688–2690. doi:10.1002/1097-0142(19850601)55:11<2688::AID-CNCR2820551125>3.0.CO;2-H
23. Fierro B, Croce G, Filusto L, Carbone N, Lupo I. Ocular pseudomyasthenia: report of a case with a pineal region tumor. Ital J Neurol Sci. 1991;12(6):593–596. doi:10.1007/BF02336957
24. Chang CG, Kageyama N, Kobayashi T, Yashida J, Negoro M. Pineal tumors: clinical diagnosis, with special emphasis on the significance of pineal calcification. Neurosurgery. 1981;8(6):656–668. doi:10.1227/00006123-198106000-00004
25. Serova N, Grigoreva N, Khavboshina A, Butenko E. Neuro-ophthalmological symptoms in patients with pineal and suprasellar germinomas. Vestn Oftalmol. 2020;136(4):39–46. doi:10.17116/oftalma202013604139
26. Goldenberg-Cohen N, Ehrenberg M, Toledano H, et al. Preoperative visual loss is the main cause of irreversible poor vision in children with a brain tumor. Front Neurol. 2011;62(2):1–6.
27. Ilcus C, Silaghi H, Georgescu C, et al. Molecular pathology and targeted therapies for personalized management of central nervous system germinoma. J Pers Med. 2021;11(7):661. doi:10.3390/jpm11070661
28. Saitoh M, Tamaki M, Kokunai T, Matsumoto S. Clinico-biological behavior of germ-cell tumors. Childs Nerv Syst. 1991;7(5):246–250. doi:10.1007/BF00299006
29. Manor R, Bar-Ziv J, Tadimor R, Eisbruch A, Rechavi G. Pineal germinoma with unilateral blindness. seeding of germinoma cells in the optic nerve sheath. J Clin Neuroophthalmol. 1990;10(4):239–243.
30. Pereira L, Green A, Hwang T, McCulley T. Suprasellar germinoma and late perioptic seeding. Eur J Ophthalmol. 2008;18(1):159–161. doi:10.1177/112067210801800130
31. Frappaz D, Conter C, Szathmari A, Vasijevic A, Mottolese C. The management of pineal tumors as a model for a multidisciplinary approach in neuro-oncology. Neurochirugie. 2014;61(2–3):208–211. doi:10.1016/j.neuchi.2014.03.003
32. Takami H, Grafffeo C, Perry A, Giannini C, Daniels D. The third eye sees double: cohort study of clinical presentation, histology, surgical approaches, and ophthalmic outcomes in pineal region germ cell tumors. World Neurosurg. 2021;150:482–490. doi:10.1016/j.wneu.2021.03.030
33. Ortiz JF, Eissa-Garces A, Ruxmohan S, et al. Understanding parinaud’s syndrome. Brain Sci. 2021;11:1469. doi:10.3390/brainsci11111469
34. Dong Y, Wei S, Pi Y, Yan R. Ocular manifestations of brainstem tumor. Zhonghua Yan Ke Za Zhi. 2009;45(11):999–1003.
35. Bruce IN, Stein BM. Surgical management of pineal region tumors. Acta Neurochir. 1995;134(3–4):130–135. doi:10.1007/BF01417679
36. Hankinson EV, Lyons CJ, Hukin J, Cochrane DD. Ophthalmological outcomes of patients treated for pineal region tumors. J Neurosurg Pediatr. 2016;17(5):558–563. doi:10.3171/2015.10.PEDS15415
37. Zako M, Banno S, Numanami H, Watanabe T, Tsuzuki T, Sashajima H. A case of bilateral uveitis associated with seminoma/germinoma in thymus and pineal glands. two primary lesions. Am J Ophthalmol Case Rep. 2022;27:101589. doi:10.1016/j.ajoc.2022.101589
38. Harthan J, Opitz D, Franstein S, Morettin C. Diagnosis and treatment of anterior uveitis: optometric management. Clin Optom. 2016;8:23–25. doi:10.2147/OPTO.S72079
39. Shukla S, Pula J, Khari S, Lee J. Paraneoplastic optic neuropathy and pineal germinoma with collapsin response-mediating protein antibodies. J Neuro Ophthalmol. 2018;38(2):198–199. doi:10.1097/WNO.0000000000000619
40. Miki A, Fugitiara M, Yosida A, Nakamura M, Azumi A. A case of bilateral vasculitis associated with pineal germinoma. Am J Ophthalmol Case Rep. 2018;11:142–145. doi:10.1016/j.ajoc.2018.07.003
41. Zhou Y, Vickers A, Chan N, et al. Atypical presentations of intracranial dysgerminoma mimicking central nervous system inflammatory or demyelinating disease. Can J Ophthalmol. 2020;55(2):159–166. doi:10.1016/j.jcjo.2019.07.018
42. Uludag G, Onay A, Onal S. onay A, Onal S. Unilateral paraneoplastic optic disc edema and retinal periphlebitis in pineal germinoma. Am J Ophthalmol Case Rep. 2018;10:236–239. doi:10.1016/j.ajoc.2018.03.013
43. Palestine A. Retinal vasculitis. Whitcup Nussenblatts Uveitis Fund Clin Pract. 2020;2020:350–367.
44. Shabo E, Czech T, Nicholson J, et al. Evaluation of the perioperative and postoperative course of surgery for pineal germinoma in SIOP CNS GCT 96 Trial. Cancers. 2022;14(14):3555. doi:10.3390/cancers14143555
45. Phi JH, Kim S-K, Kang TH, Wang KC. Hydrocephalic fits: forgotten but not gone. Childs Nerv Syst. 2012;28(11):1863–1868. doi:10.1007/s00381-012-1832-7
46. Kobayashi T, Kageyawa N, Kida Y, Yoshida J, Shibuya N, Okamura K. Unilateral germinomas involving the basal ganglia and thalamus. J Neurosurg. 1981;55(1):55–58. doi:10.3171/jns.1981.55.1.0055
47. Huang Z, Dong Q, Song E, et al. Germinomas of the basal ganglia and thalamus: four case reports. World J Clin Cases. 2020;8(19):4558–4564. doi:10.12998/wjcc.v8.i19.4558
48. Cohen D, Bhatti M, Giannini C, Eckel L, Garrity J, Chen J. Intracranial pure germinoma with optic nerve infiltration. J Neuro Ophthalmol. 2020;40:112–116. doi:10.1097/WNO.0000000000000893
49. Yasuhara T, Ichikawa T, Miyoshi Y, et al. Primary germinoma in the medulla oblongata -case report-. Neural Med Chir. 2011;51(4):326–329. doi:10.2176/nmc.51.326
50. Nagishi M, Suzuki R, Tanaka YH, et al. Pure germinoma of the pineal gland with synchronous spinal dissemination. Neurol Med Chir. 2010;50(6):505–508. doi:10.2176/nmc.50.505
51. Lo AC, Hodgson D, Dang J, et al. Intracranial germ cell tumors in adolescents and young adults: a 40-year multi-institutional review of outcomes. Int J Rad Onc Biol Phys. 2020;106(2):269–278. doi:10.1016/j.ijrobp.2019.10.020
52. Morrison AL, Smith AB, Benjamin V, Allen JC, Rushing EJ. Late spinal metastases from an isolated pineal region germinoma mimicking a schwannoma. J Clin Neurosci. 2011;18(8):1126–1127. doi:10.1016/j.jocn.2010.11.030
53. Utsuki S, Oka H, Sagiuchi T, Fujii K. Late intrathoracic relapse of pineal germinoma connected to the intraspinal canal. Neurol India. 2007;55(2):151–153. doi:10.4103/0028-3886.32788
54. Hanakita S, Takenobu A, Kambe A, Watanabe T, Shin M, Teraoka A. Intramedullary recurrence of germinoma in the spinal cord 15 years after complete remission of a pineal lesion. J Neurosurg Spine. 2012;16(5):513–515. doi:10.3171/2012.2.SPINE11499
55. Kageyama H, Suzuki J, Ohara Y. Intramedullary spinal cord germinoma clinically mimicking multiple sclerosis: a case report. Surg Neurol Int. 2019;10(21):1–4. doi:10.25259/SNI_466_2019
56. Aoyama T, Hida K, Ishii N, Seki T, Ikeda J, Iwasaki Y. Intramedullary spinal cord germinoma – 2 case reports. Surg Neurol. 2007;67(2):177–83; discussion 183. doi:10.1016/j.surneu.2006.05.062
57. Lombardi G, Poliani P, Manara R. Diagnosis and treatment of pineal region tumors in adults: a EURACAN, overview. Cancers. 2022;14(15):3646. doi:10.3390/cancers14153646
58. Yamasaki F, Kinoshita Y, Takayasu T, et al. Proton magnetic resonance spectroscopy detection of high lipid levels and low apparent diffusion coefficient is characteristic of germinomas. World Neurosurg. 2018;112:e84–e94. doi:10.1016/j.wneu.2017.12.078
59. Kakigi T, Okada T, Kanagaki M, et al. Quantitative imaging values of CT, MR, and FDG-PET to differentiate pineal parenchymal tumors and germinomas: are they useful. Neuroradiology. 2014;56(4):297–303. doi:10.1007/s00234-014-1334-2
60. Kawai N, Myake K, Yamamoto Y, et al. Use of 11C-methionine positron emission tomography in basal germinoma: assessment of treatment response and residual tumor. Childs Nerv Syst. 2009;25(7):845–853. doi:10.1007/s00381-009-0841-7
61. Zhang H, Qi S, Fan J, et al. Bifocal germinomas in the pineal region and hypothalamus- neurohypophyseal axis: primary or metastasis? J Clin Neurosci. 2016;34:151–157. doi:10.1016/j.jocn.2016.06.009
62. Kosuda S, Kusano S, Ishihara S, et al. Combined 201Tl and 67Ga brain spect in patients with suspected central nervous system lymphoma or germinoma: clinical and economic value. Ann Nucl Med. 2003;17(5):359–367. doi:10.1007/BF03006602
63. Gurusamy S, Yaakub A, Hitan W, Maglana S, Ibrahim W. Pineal gland tumor with drop metastasis: a case report. Cureus. 2022;14(10):e29855. doi:10.7759/cureus.29855
64. Ghariabeh K, Perez H, Al-Chalabi M, Sheikh A, Mahfooz N. Spinal epidural venous engorgement-potential imaging confounder after diagnostic lumbar puncture. Radiol Case Rep. 2022;17(12):4752–4755. doi:10.1016/j.radcr.2022.09.015
65. Mark I, Dillon W, Richie M, Villanueva-Meyer J. MRI findings after recent image-guided lumbar puncture: the rate of dual enhancement and subdural collections. Am J Neuroradiol. 2022;43(5):784–788. doi:10.3174/ajnr.A7496
66. Allen J, Chacko J, Donahue B, et al. Diagnostic sensitivity of serum and lumbar CSF bHCG in newly diagnosed CNS germinoma. Pediatr Blood Cancer. 2012;59(7):1180–1182. doi:10.1002/pbc.24097
67. Allen J, Nisselbaum J, Epstein F, Rosen G, Schwartz M. Alphafetoprotein and human chorionic gonadotropin determination in cerebrospinal fluid. J Neurosurg. 1979;51(3):368–374. doi:10.3171/jns.1979.51.3.0368
68. Carr C, O’neill B, Huchhalter C, Stong M, Ware M. Biomarkers of pineal region tumors: a review. Ochsner J. 2019;19(1):26–31. doi:10.31486/toj.18.0110
69. Gindhart T, Tsukahara Y. Cytologic diagnosis of pineal germinoma in cerebrospinal fluid and sputum. Acta Cytol. 1979;23(4):341–346.
70. Torzewski M, Lackner K. Cerebrospinal fluid cytology: a highly diagnostic method for the detection of diseases of the central nervous system. J Lab Med. 2016;40:S1.
71. Kanamari M, Takamari H, Suzuki T, et al. Necessity for craniospinal irradiation of germinoma with positive cytology without spinal lesion on MR imaging- a controversy. Neuro Onc Adv. 2021;3(1):1.
72. Osorio D, Allen J. Management of CNS germinomas. Clin Oncol. 2015;4(4):273–279.
73. Blakely J, Grossman S. Management of Pineal Region tumors. Curr Treat Options Oncol. 2006;7(6):505–516. doi:10.1007/s11864-006-0025-6
74. Fouladi M, Grant R, Barachel S, et al. Comparison of survival outcomes in patients with intracranial germinomas treated with radiation alone versus reduced-dose radiation and chemotherapy. Childs Nerv Syst. 1998;14(10):596–601. doi:10.1007/s003810050279
75. Horowitz M, Hall W. Central nervous system germinomas. A review. Arch Neurol. 1991;48(6):652–657. doi:10.1001/archneur.1991.00530180110026
76. Diederich S, Eckmanns T, Exner P, Al-Saadi N, Bahr V, Oelkers W. Differential diagnosis of polyuric/polydipsia syndromes with the aid of urinary vasopressin measurements in adults. Clin Endocrinol. 2001;54(5):665–671. doi:10.1046/j.1365-2265.2001.01270.x
77. Sklar C, Grumbach M, Kaplan S, Conte F. Hormonal and metabolic abnormalities associated with central nervous system germinoma in children and adolescents and the effect of therapy: report of 10 patients. JCEM. 1981;52(1):9–16. doi:10.1210/jcem-52-1-9
78. Huang W, Zhang X, Wang W, Day Y, Qui H, Wei S. Ocular manifestations of intracranial germinomas: three case reports and literature review. Chin Med J. 2012;125(15):2790–2793.
79. Ramelli G, Van der Weid N, Stanga Z, Mullis P, Buergi U. Suprasellar germinomas in childhood and adolescence: diagnostic pitfalls. J Pediatr Endocrinol Metab. 1998;11(6):693–697. doi:10.1515/JPEM.1998.11.6.693
80. Shibamoto Y. Management of central nervous system germinoma: proposal for a modern strategy. Prog Neurol Surg. 2009;23:119–129.
81. Sonabend AM, Bowden Bruce JN, Bruce JN. Microsurgical resection of pineal region tumors. J. Neurooncol. 2016;130(2):351–366. doi:10.1007/s11060-016-2138-5
82. Berhouma M, Jemel H, Ksira I, Khaldi M. Transcortical approach to a huge pineal mature teratoma. Pediatr Neurosurg. 2008;44(1):52–54. doi:10.1159/000110663
83. Berhouma M, Ni H, Vallee B. The endoscopic intraventricular management of pineal cysts: a minimally invasive modus operandi. Acta Neurochir. 2013;155(10):1901–1905. doi:10.1007/s00701-013-1849-z
84. Little KM, Friedman AH, Fukushima T. Surgical approaches to pineal region tumors. J Neurooncol. 2001;54(3):287–299. doi:10.1023/A:1012766902431
85. Hayashi N, Murai H, Ishihara S, et al. Nationwide investigation of the current status of therapeutic neuroendoscopy for ventricular and paraventricular tumors in Japan. J Neurosurg. 2011;115(6):1147–1157. doi:10.3171/2011.7.JNS101976
86. Mueller W, Schneider G, Huffman K, Zschenderlein R, von Dierling A. Granulomatous tissue response in germinoma, a diagnostic pitfall in endoscopic biopsy. Neuropathology. 2007;27(2):127–132. doi:10.1111/j.1440-1789.2006.00749.x
87. O’Connor T, Fabiano A, Prasad D, Morin N, Feastermaker R. Lateral temporal approach for image-guided stereotactic biopsy of pineal region tumors. World Neurosurg. 2021;147:144–149. doi:10.1016/j.wneu.2020.11.128
88. Sumer M, Acikgoz B, Akpinar G. External ventricular drainage for acute obstructive hydrocephalus developing following spontaneous intracerebral hemorrhages. Neurol Sci. 2002;23(1):29–33. doi:10.1007/s100720200020
89. Al-Saiari S, Asiri FA, Farag AA, et al. Multiple lessons learned from a single case: complications from pineal germinoma management. Surg Neurol Int. 2022;13:29. doi:10.25259/SNI_944_2021
90. Haw C, Steinbok P. Ventriculoscope Tract recurrence after endoscopic biopsy of pineal germinoma. Pediatr Neurosurg. 2001;34(4):215–217. doi:10.1159/000056022
91. Luther N, Stetler J, Dunkel I, Christos P, Wellons JC, Souweidane MM. Subarachnoid dissemination of intraventricular tumors, following simultaneous endoscopic biopsy and third ventriculostomy. J Neurosurg Pediatrics. 2010;5(1):61–67. doi:10.3171/2009.7.PEDS0971
92. Smith J, Quinones-Hinojosa A, Barbaro N, Mcdermott M. Frame-based stereotactic biopsy remains an important diagnostic tool with distinct advantages over frameless stereotactic biopsy. J Neurooncol. 2005;73(2):173–179. doi:10.1007/s11060-004-4208-3
93. Wen C, Linskey M. Frame-based stereotaxy in frameless era; current capabilities, relative role, and the positive- and negative predictive values of blood through the needle. J Neurooncol. 2009;93(1):139–149. doi:10.1007/s11060-009-9871-y
94. Neumann J, Campos B, Younes B, et al. Frame-based stereotactic biopsies using an intraoperative MR-scanner are as safe and effective as conventional stereotactic procedures. PLoS One. 2018;13(10):e0205772. doi:10.1371/journal.pone.0205772
95. Zacharia BE, Bruce JN. Stereotactic biopsy considerations for pineal tumors. Neurosurg Clin N Am. 2011;22(3):359–66, viii. doi:10.1016/j.nec.2011.05.008
96. Popovic EA, Kell PJ. Stereotactic procedures for lesions of the pineal region. Mayo Clin Proc. 1993;68(10):965–970. doi:10.1016/s0025-6196(12)62268-x
97. Marker DF, Pearce TM. Germ cell tumors of the central nervous system: a brief review and site-specific considerations. Semin Diagn Pathol. 2022;40:47–51. doi:10.1053/j.semdp.2022.07.002
98. Konovalov A, Pitskhelavri D. Principles of treatment of the pineal region tumors. Surg Neurol. 2003;59(4):250–268. doi:10.1016/S0090-3019(03)00080-6
99. Lim D, Yoo K, Lee N, et al. Intensive chemotherapy followed by reduced-dose radiotherapy for biopsy-proven CNS germinoma with elevated beta-human chorionic gonadotropin. J Neurooncol. 2014;117(2):279–285. doi:10.1007/s11060-014-1381-x
100. Endo H, Kumabe T, Jokura H, Tuminga T. Stereotactic radiosurgery followed by whole ventricular irradiation for primary intracranial germinoma of the pineal region. Min Minimal Inv Neurosurg. 2005;48(3):186–190. doi:10.1055/s-2004-830263
101. Mori Y, Kobayashi T, Hasegawa T, Yoshida K, Kuda Y. stereotactic radiosurgery for pineal and related tumors. Prog Neurol Surg. 2009;23:106–108.
102. Morgenstern P, Osbun N, Schwartz T, Greenfield J, Tsiouris A, Souweidane M. Pineal region tumors: an optimal approach for simultaneous endoscopic third ventriculostomy and biopsy. Neurosurg Focus. 2011;30(4):E3. doi:10.3171/2011.2.FOCUS10301
103. Ronsley R, Bouffett E, Dirks P, Drake J, Kulkami A, Bartels U. Successful management of symptomatic hydrocephalus using a temporary external ventricular drain with or without endoscopic third ventriculostomy in pediatric patients with germinoma. J Neurosurg. 2022;137:807–812. doi:10.3171/2021.8.JNS211443
104. Xu K, Khine K, Ooi Y, Quinsey C. A systemic review of shunt-related extraneural metastases of primary central nervous system tumors. Clin Neurol Neurosurg. 2018;174:239–243. doi:10.1016/j.clineuro.2018.09.038
105. [Application of navigation system ExacTrac in radiation therapy of a female patient with disseminated pineoblastoma]. Zh Vopr Neirokhir Im N N Burdenko. 2010;2010:43–46. Russian.
106. Aridgides P, Janssens G, Braunstein S, et al. Gliomas, germ cell tumors, and craniopharyngiomas. Pediatr Blood Cancer. 2021;68(Suppl 2):e28401. doi:10.1002/pbc.28401
107. Wang P, Li Y, Qiu X. The external metastases of the central nerve system germ cell tumors: case review and report of the literature. Chin Neurosurg J. 2021;7(1):29. doi:10.1186/s41016-021-00246-0
108. Chen R, Tao C, You C, Ju Y. Fast-developing fatal diffuse leptomeningeal dissemination of a pineal germinoma in a young child: a case report and literature review. Br J Neurosurg. 2022;36(2):262–269. doi:10.1080/02688697.2018.1520804
109. Calaminus G, Kortmann R, Worch J, et al. SIOP CNS GCT 96: final report of outcome of a prospective multinational nonrandomized trial for children and adults with intracranial germinoma comparing craniospinal irradiation alone with chemotherapy followed by focal primary site irradiation for patients with intracranial germinomas. Neurooncology. 2013;15(6):788–796. doi:10.1093/neuonc/not019
110. Bartels U, Onar-Thomas A, Patel SK, et al. Phase II trial of response-based radiation therapy for patients with localized germinoma: a children’s oncology group study. Neuro-Oncology. 2022;24(6):974–983. doi:10.1093/neuonc/noab270
111. Tomita T, Alden T, Dipatri A. Pediatric pineal region tumors: institutional experience of surgical managements with posterior interhemispheric transtentorial approach. Childs Nerv Syst. 2022. doi:10.1007/s00381-022-05595-4
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.