Back to Journals » Therapeutics and Clinical Risk Management » Volume 18
Diagnosis and Emerging Treatment Strategies for Mucopolysaccharidosis VII (Sly Syndrome)
Authors Poswar FDO, Henriques Nehm J, Kubaski F , Poletto E , Giugliani R
Received 31 July 2022
Accepted for publication 27 November 2022
Published 22 December 2022 Volume 2022:18 Pages 1143—1155
DOI https://doi.org/10.2147/TCRM.S351300
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Garry Walsh
Fabiano de Oliveira Poswar,1– 4 Johanna Henriques Nehm,1 Francyne Kubaski,5 Edina Poletto,6 Roberto Giugliani1– 4,7– 9
1Clinical Research Group in Medical Genetics, Clinical Research Center, Hospital de Clínicas de Porto Alegre, Porto Alegre, RS, Brazil; 2Medical Genetics Service, Hospital de Clinicas de Porto Alegre, Porto Alegre, RS, Brazil; 3Postgraduate Program in Genetics and Molecular Biology, UFRGS, Porto Alegre, RS, Brazil; 4DR Brasil Research Group, HCPA, Porto Alegre, RS, Brazil; 5Greenwood Genetic Center, Biochemical Genetics Laboratory, Greenwood, SC, USA; 6Department of Pediatrics, Stanford University School of Medicine, Stanford, CA, USA; 7Department of Genetics, UFRGS, Porto Alegre, RS, Brazil; 8DASA Genômica, São Paulo, SP, Brazil; 9Casa dos Raros, Porto Alegre, RS, Brazil
Correspondence: Roberto Giugliani, Medical Genetics Service- HCPA / Dep Genet UFRGS, 2350 Ramiro Barcelos, Porto Alegre, RS, 90035-903, Brazil, Tel +55 51 3359 6338, Email [email protected]
Abstract: Mucopolysaccharidosis VII (MPS VII, Sly syndrome) is an ultra-rare lysosomal disease caused by a deficiency of the enzyme β-glucuronidase (GUS). The diagnosis is suspected based on a range of symptoms that are common to many other MPS types, and it is confirmed through biochemical and molecular studies. Besides supportive treatment, current and emerging treatments include enzyme replacement therapy, hematopoietic stem cell transplantation, and gene therapy. This review summarizes the clinical manifestations, diagnosis, and emerging treatments for MPS VII.
Keywords: lysosomal disorders, mucopolysaccharidosis type VII, Sly syndrome, enzyme replacement therapy, hematopoietic stem cell transplantation, gene therapy
Introduction
Mucopolysaccharidoses (MPSs) form a heterogeneous group of disorders caused by the deficiency of one of the enzymes involved in the breakdown of glycosaminoglycans (GAGs), which takes place in the lysosome. There were 11 enzyme deficiencies classically recognized,1 but recently arylsulfatase K deficiency was described and added to this group.2 Although the disease seems to originate from abnormal storage of GAGs, it is now accepted that the primary GAGs storage triggers a pathogenic cascade, with many other factors involved, including inflammation.3
Patients with MPS may present severe manifestations that could include non-immune hydrops fetalis (NIHF)4 and/or early neurodegeneration, or have a more attenuated phenotype that could be marked by corneal clouding and mild bone and joint abnormalities.5 This heterogeneity is clear not only across the different MPS types but also within the same type, with different variants and levels of residual enzyme activity. Several contributions aiming to establish genotype–phenotype correlations were already published.6
Mucopolysaccharidosis type VII (MPS VII, Sly syndrome) was first described in 1973 when the deficiency of the enzyme β-glucuronidase (GUS, EC 3.2.1.31) was found in a patient with MPS-like clinical and radiological findings.7 Thereafter, the GUSB gene that codifies beta-glucuronidase was cloned and mapped.
Soon it was recognized that MPS VII is an ultra-rare condition, with an estimated incidence of less than one case per 1,000,000 individuals, being responsible for less than 2% of MPS cases in most series.8,9
This paper will review the clinical manifestations of MPS VII, the approach to diagnosis, the current treatment measures, and the emerging therapeutic strategies.
Clinical Manifestations
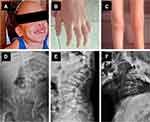
Patients with MPS VII have a wide range of manifestations, including cognitive impairment, hepatosplenomegaly, coarse facial features, cardiac valve disease, recurrent upper respiratory infections, short stature and bone dysplasia (Figure 1). These signs and symptoms are very similar to those described in other types of MPS, particularly the MPS types I and II.10 As in other MPS types, the age of onset of the signs and symptoms may be different according to the disease severity, and in the milder end of the severity spectrum, preservation of cognition may occur.
A distinctive feature of MPS VII, however, is the high proportion of patients that present with severe antenatal disease including NIHF. Complications related to NIHF are also a major cause of death for patients with MPS VII, and about half of the patients do not survive beyond 1 year of life.11 Since the first description of NIHF as a form of presentation in 1982, it has been proposed to include MPS VII in the diagnostic workup for NIHF.12 It is now recognized that MPS VII is among the most common lysosomal disorders identified in this context.4,13 Besides, more than 10 cases of prenatal diagnosis of MPS VII due to suggestive features were reported.14 However, as this investigation is not always performed, some patients with MPS VII die from NIHF without being properly diagnosed, and a lysosomal disorder is only considered after familial recurrence.15
The circumstance in which the diagnosis is established is associated with clinical outcomes. Patients who are diagnosed prenatally, even when pregnancy is not terminated, usually have a very limited survival. Most of those patients die at late pregnancy or soon after birth due to heart, kidney, or respiratory failure.11,14,15 In the cases diagnosed postnatally, MPS VII remains an early-onset and severe condition with the median disease onset being the first day of life, and the median survival being 42 months.14 However, the clinical course is variable, and it is not entirely predicted by the presence of NIHF by itself. For instance, in a case series including 23 patients with a history of NIHF, 13 survived the infancy period with a mild to intermediate phenotype.11
The knowledge about the natural history and the range of clinical manifestations associated with MPS VII is important to recognize high-risk groups of patients for selective screening.10 Table 1 summarizes the clinical manifestations of MPS VII, their age of onset, and frequencies.
 |
Table 1 MPS VII Manifestations |
Diagnosis
Biochemical diagnosis
The biochemical diagnosis of MPS VII consists of the quantification of the activity of GUS, which is required for the stepwise degradation of glucuronic acid-containing GAGs: chondroitin sulfate (CS), dermatan sulfate (DS), and heparan sulfate (HS).1,7,11,16,17
The enzyme deficiency can be demonstrated in serum, leukocytes, cultured fibroblasts, or dried blood spots (DBS) using fluorimetry with 4-methylumbelliferyl (4-MU) derived substrate,11,17,18 and in DBS using liquid chromatography tandem mass spectrometry.19,20
The enzyme assay is usually performed when clinical suspicion is raised in symptomatic patients, or in asymptomatic individuals who are considered at risk due to family history. In the prenatal period, the measurement of enzyme activity can be performed in chorionic villus,21–23 cultured amniocytes,15 or leukocytes from blood from the umbilical cord.15
GAG quantification can aid biochemical diagnosis. Several methods can be employed for GAG analysis, the most used methods are dimethylmethylene blue (DMMB),24 electrophoresis,25 and quantification by liquid chromatography–tandem mass spectrometry (LC-MS/MS).25 LC-MS/MS offers several advantages compared to the conventional colorimetric methods: precise and accurate quantification, discrimination of GAG subclasses or GAG-derived oligosaccharides, and it can be employed in a variety of sample types (urine, plasma/serum, DBS, cerebrospinal fluid, cells, tissues, synovial fluid).25–31 GAG quantification can also be performed in the supernatant of amniotic fluid and it is very helpful to support the prenatal investigation.32 Additionally, it can be used for therapeutic monitoring.25–31
Saville and colleagues reported a novel method for the quantification of disease-specific signatures from GAGs in which urine samples can be derivatized, allowing the identification of GAG fragments unique to specific MPS subtypes; this allows the identification of MPS VII by the elevation of the nonreducing end fragment UA-HN-UA (1S) [uronic acid–hexosamine–uronic acid] which is exclusively elevated in the urine of MPS VII patients.30
As pseudodeficiency of beta-glucuronidase has been reported, GAG analysis combined with molecular analysis can discriminate true MPS VII-positive results from reduced in vitro enzyme activity due to pseudodeficiency.11,33–35
Molecular Diagnosis
Molecular genetics testing is usually recommended for the confirmation of the biochemical diagnosis. Moreover, it allows identification of carriers, appropriate genetic counseling for families, and prenatal genetic testing for additional pregnancies.
The enzyme GUS is encoded by the 20 kb-long glucuronidase beta gene (GUSB, OMIM# 253220), located in the long arm of chromosome 7 (7q11.21–7q11.22). It contains 12 exons that encode for a 651-amino acid precursor and a mature 629-residue protein and displays significant genetic heterogeneity.34 Multiple pseudogenes or fragments containing GUSB sequences were identified in different chromosomes across the human genome, hampering the initial sequencing of the gene.36 A few pseudodeficiency alleles have also been described.35,37
There are currently 80 disease-causing variants described in the GUSB gene; most of them (74%) are missense variants, others are nonsense (11%), splicing (5%), or small deletions and indel variants (7%) (HGMD Professional, accessed on 06/14/2022). Some cohorts of MPS VII patients have more heterogeneous genotypes, with novel variants constantly being described and frequent compound heterozygosity observed.34,38 Because it is an ultra-rare disease, only dozens of patients were reported worldwide, with the higher incidence in the region of British Columbia and in The Netherlands, with a prevalence of 0.29 and 0.24 cases per 100,000 live births, respectively.8
The most common MPS VII-causing variant is p.Leu176Phe, found in patients from different cohorts worldwide.34,38,39 In Brazil, for example, this variant accounts for 96% of the alleles identified in MPS VII patients.39 Due to its presence in homozygosis in attenuated MPS VII patients and to the prediction from in vitro and in silico studies, this variant was originally associated with the attenuated phenotype of the disease.34 However, more recent reports of p.Leu176Phe homozygotes showed patients with variable clinical manifestations, including in the severe spectrum,11,39 suggesting the genotype–phenotype correlation is not as straightforward as previously thought and other factors might be influencing it. The other most frequent pathogenic variants are also exonic point mutations – p.Arg357Ter, p.Pro408Ser, p.Pro415Leu, and p.Ala619Val.34
The attenuated phenotype is traditionally thought to be associated with residual enzyme activity, as this is generally true for other lysosomal disorders. However, some MPS VII patients have attenuated phenotypes despite the very low GUS activity,39,40 indicating that the residual activity alone is not predictive of the clinical course.
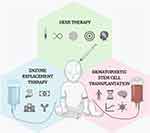
Treatment
Enzyme Replacement Therapy
Enzyme replacement therapy (ERT) is a treatment available for several LSDs. It was initially approved in 1990 for Gaucher disease, with great results. From 2003 onwards, it began to be used for mucopolysaccharidoses, with positive results across many disease manifestations. Vestronidase alfa is the first ERT developed to treat patients with mucopolysaccharidosis VII.41 It is a formulation of recombinant human GUS (rhGUS) that has previously been successfully treated in an animal model.42 It is produced using a genetically modified Chinese hamster ovary cell line, similar to laronidase, the enzyme used to treat MPS I. However, vestronidase alfa has a longer enzyme half-life after absorption in fibroblasts, when compared to laronidase (40 days vs 3 to 4 days, respectively).43 Through mannose-6 phosphate (M6P) residues present in oligosaccharide chains, the enzyme can bind to M6P receptors on the surface of cells. Subsequently, vestronidase alfa is internalized into cellular lysosomes, and it degrades GAGs accumulated in affected tissues.44
Due to the rarity and clinical variability of mucopolysaccharidosis VII, the development of a specific treatment for this disease was considered very challenging, making animal models that present a disease similar to humans of paramount importance. Mice with MPS VII provide a good model for LSDs, as the effectiveness of treatments can be phenotypically confirmed. Animal models of dogs with MPS VII have also been reported.45 Preclinical studies with the MPS VII murine model using rhGUS purified with sodium metaperiodate and sodium borohydride to inactivate the M6P recognition markers revealed reduced GAG accumulation in lysosomes, improvements in various soft and connective tissues such as bone, improved animal survival, and decreased accumulation of GAGs in the brain.46 Other preclinical studies in adult mice tagged a short peptide consisting of fatty acids to rhGUS showed that 4 mg/kg intravenously reduced neuronal and glial storage in the brain after 12 weeks of treatment.47 In neonatal dogs with MPSVII, ERT demonstrated resolved mitral valve regurgitation.48
Three clinical trials were conducted to assess the efficacy and safety of vestronidase alfa (see Table 2). In an open-label Phase I/II clinical trial to determine the dose, 4 mg/kg intravenous vestronidase alpha was administered every 2 weeks (QOW) without any significant safety concerns (ClinicalTrials.gov: NCT02418455). A Phase II clinical trial in subjects <5 years of age was performed to determine additional evidence for the long-term safety and efficacy of vestronidase alfa. Lastly, a placebo-controlled Phase III clinical trial evaluated the use of recombinant human beta-glucuronidase (alphavestronidase) in 12 patients with MPS VII49 The sample was composed of 8 females and 4 males, with an age range of 8–25 years, the majority of them being white (75%) and of Hispanic or Latino ethnicity (50%). In order to account for a heterogeneous sample, a novel blind start study design with a variable placebo run-in period was used. While urinary GAG (uGAG) excretion was the primary endpoint, clinical response was also assessed by using a multi-domain responder index (MDRI). The MDRI consists of the following clinical domains: 6-minute walk test, forced vital capacity, shoulder flexion, visual acuity, and Bruininks–Oseretsky Test of Motor Proficiency. After 24 weeks of treatment, uGAG excretion levels were significantly reduced in all subjects. Furthermore, 10 in 12 patients had an improvement in at least one MDRI domain. In all three studies, vestronidase alfa was administered with antihistamine premedication, infusion rate titration, and careful patient monitoring, so no significant safety concerns were identified.50
 |
Table 2 Clinical Trials for MPS VII |
On November 15, 2017, after nearly two decades of success with studies in animal models and other mucopolysaccharidoses, vestronidase alfa was finally approved for children and adults with MPS VII by the FDA in the United States.39 As of 2018, vestronidase alfa was also authorized in the European Union (EU)/European Economic Area (EEA), UK, Brazil, Chile, and Mexico.51,52 For the treatment of mucopolysaccharidosis VII, 4mg/kg of vestronidase alfa is given as a slow intravenous (IV) infusion once every 2 weeks. An antihistamine with or without a sedative action, with or without an antipyretic drug, should be administered 30–60 minutes before the infusion to reduce the risk of hypersensitivity.53
Although ERT treatment has shown efficacy, improving survival and quality of life of patients with MPS VII, treatment can be limited mainly because it does not modify the sequelae of the disease that are present until the time of treatment. In addition, postnatally applied vestronidase alfa does not cross the blood–brain barrier. Many patients can produce anti-enzyme antibodies and need immunomodulation to tolerate treatment.54
A study with ERT in utero in mice with MPS VII was performed, and 20mg/kg were administered to fetuses in the litter at embryonic day 14.5 by intrahepatic injection, while control mice received injections of vehicle or phosphate-buffered saline.54 ERT in utero prevented the development of anti-enzyme antibodies, demonstrated an improvement in the survival of the animals, penetrated the brain microglia decreasing inflammation, and improved neurological tests such as grip strength, compared to mice treated only postnatally.55 In utero therapy for MPS VII and other LSDs is being investigated through a Phase I clinical trial that started in 2021 in order to determine the maternal and fetal safety and the feasibility of in utero fetal enzyme replacement therapy in fetuses (Clinical Trials.gov: NCT04532047).
The prospect of a second generation of ERT with the use of brain penetrating enzymes, already approved in Japan for MPS II56 and in development for MPS I, may be interesting for MPS VII also due to the high proportion of patients who present central nervous system (CNS) involvement.
Hematopoietic Stem Cell Transplantation
Hematopoietic stem cell transplantation (HSCT) aims to correct the clinical manifestations of the disease by providing an active enzyme from the transplanted cells that can lead to substrate reduction.57 Because of its incidence, there are not that many published cases of HSCT performed in MPS VII patients (n=9). Yamada and colleagues reported a case of a patient whose diagnosis was performed at 1 month of age, but only received an allogeneic bone marrow transplantation (BMT) when she was 12 years of age (already presenting neurological impairment, skeletal deformities, and wheelchair bound). Ten months post-transplant, the patient presented almost normal GUS levels and a decrease in uGAG excretion, as well as improvement in the clinical course, and shortness of breath in which she was able to walk, ride a bicycle, and take a bath alone. As expected, because of the age at transplant, her neurological impairment was not reversed.58
Montaño and colleagues reported the results of HSCT/BMT in five MPS VII patients. Two out of five patients had a positive outcome; the fifth patient had a BMT at 7 months of age, and the patient did not have any clinical manifestations at 15 months of age reaching normal development milestones (started walking at 1 year of age) highlighting the impact of early treatment. Nonetheless, the patient still has some cardiomyopathy with moderate atrial enlargement and a prominent forehead. The fourth patient underwent BMT at 3 years of age; 12 years post-transplant, the patient shows moderate clinical symptoms suggesting that the BMT might have somewhat slowed down disease progression. However, at the last exam, the patient still presents clinical symptoms, skeletal deformities, neurological impairment, and restrictive and obstructive respiratory disease. The first patient had a BMT at 2 years of age, which failed, and another BMT at 4 years of age. There is no long-term follow-up data in this case. The second patient underwent BMT at 7 years of age and died from transplant-related complications. The third patient had a very severe phenotype, there are no data reporting the age at the transplant, and the patient died years after the procedure.11
Sisinni and colleagues reported a 2-year-old MPS VII patient with a mild phenotype that underwent an allogeneic HSCT. The patient was submitted to HSCT twice due to the rejection of the graft in the first transplant. After a myeloablative regimen was employed, there was engraftment after the second transplant with matched unrelated cord blood. Six years post-transplant, the patient showed normal GUS activity, reduction in total GAG levels (but still higher than age-matched controls), normal motor function, improvement of coarse facial features, resolution of organomegaly, and stabilization of skeletal deformities. Since the patient had a moderate phenotype, no scoliosis or neurological impairment was present pre- or post-transplantation.57
Another patient was treated by HSCT with unrelated human leukocyte antigen (HLA)-matched cord blood at 14 months of age. Besides the conditioning regimen employed, the patient had several infections: rotavirus gut, Staphylococcus aureus, cytomegalovirus reactivation; and graft-versus-host disease (GVHD) grade III that resolved, and the patient achieved full chimerism. The patient improved post-transplant and started walking at 20 months of age. However, the patient developed chronic pulmonary insufficiency in his second year of life and died at 25 months of age.59
Dubot and colleagues have reported a case in which the patient was diagnosed at 2 weeks of age, started ERT at 4 months of age, and received an HSCT at 13 months of age. The patient had developed severe skin and gut-GVHD in which ERT was stopped 6 months post-transplantation. At 4 years of age, the patient has normal psychomotor development, stabilized growth curve, and no organomegaly. This report highlights the need for an early diagnosis followed by early treatment.60 Despite transplant-related complications, as long as the transplant is performed before the development of irreversible damage (mainly neurological and skeletal) HSCT can be considered a treatment option for MPS VII. For that purpose, it is also required that appropriate conditioning regimen is used and the patient reaches full engraftment. The outcome can be improved in cases where HSCT is combined with other therapeutic approaches such as ERT and gene therapy because HCST has limited benefit in tissues such as bone, cartilage, eye, and the CNS considering the time of engraftment.11,60
Gene Therapy and Genome Editing
Gene therapy uses recombinant nucleic acids to modify genetic sequences for therapeutic purposes. It can be done in vivo – when the product is administered directly to the patient – or ex vivo – when cells are modified in vitro and then transferred to the patient. MPS VII is a good candidate for gene therapy since a) it is a monogenic disorder, b) the deficient enzyme is soluble and can transit from an enzyme-producing cell to an enzyme-deficient cell, and c) restoring low levels of enzyme presumably is sufficient to lessen disease burden.
The availability of naturally occurring MPS VII animal models61–63 propelled the development of many pre-clinical gene therapy studies in the 2000s. The MPS VII dog model, particularly, was extremely useful to evaluate the long-term efficacy of gene therapy, with some animals being followed up to 11 years post-treatment.64,65 Most of the research done for MPSs used in vivo administration of viral vectors, due to their high efficiency in transducing cells and delivering the GUSB cDNA.
MPS VII mice and dogs treated intravenously with vectors based on lentivirus,66,67 adeno-associated virus,68 or retrovirus69,70 showed increased enzyme activity and reduced GAG storage in visceral tissues, including hard-to-treat cardiovascular tissues, improving heart function.65,69–71 Some improvements in the brain tissue as well as in behavior tests were reported in mice treated from birth66 and in mice with the attenuated phenotype.72 The skeletal disease was partially ameliorated in both animal models, resulting in a decrease in bone mineral volume, surface density, and thickness.66,72–76 However, the treatment could not prevent lumbar spine disease,77 and cartilaginous tissues were not much responsive to either vector. Although the therapy was administered systemically, uGAG levels were not reduced with gene therapy.67,73 Other strategies such as plasmid vector78 or ex vivo gene therapy of hematopoietic stem cells79 were also tested in MPS VII mice with, however, low therapeutic efficacy.
Systemic administrations of viral vectors rose concern about the potential insertional mutagenesis these vectors can cause, as demonstrated by the high incidence of hepatocellular carcinomas developed in MPS VII-treated mice.80,81 Thus, in situ gene therapy was pursued, as local injections require reduced viral titers and provoke fewer immunogenic responses.82 Intracranial and intrathecal administrations of GUSB-expressing viral vectors showed reversion of the phenotype in the tissue, with increased enzyme activity and correction of storage lesions.67,83–86 Treated mice also improved performance in behavioral studies87,88 and had longer lifespans, with visceral tissues being corrected by drainage of the vector to the bloodstream.85 Interestingly, intravitreal administration also led to some biochemical correction in brain regions through diffusion and trans-synaptic transfer.89
Collective data with both mouse and large animal models pointed to in situ administration of viral vectors as the most efficient strategy so far to target the CNS. By that route of administration, normal or even supraphysiological levels of the enzyme can be achieved in the brain,90,91 while intravenous injections failed. However, new strategies should address skeletal disease, which is one of the major disease burdens faced by patients.
Meanwhile, new gene therapy techniques – like genome editing – hold great promise for mucopolysaccharidoses.92 Clinical trials for MPS I and MPS II already took place, while different approaches have been tested in the pre-clinical setting for these diseases, such as ex vivo,93 and intravenous94,95 or nasal96 delivery of CRISPR-encoding plasmids. There are no complete studies targeting MPS VII with genome editing, though a couple of reports suggest this technique may be beneficial for the eye manifestations of the disease.97 A comparison between ERT, HSCT, and gene therapy as therapeutic approaches for MPS VII is provided in Figure 2.
Supportive Treatment
Despite advances in the development of specific therapies, patients with MPS VII remain with several health issues that require multidisciplinary care. These include not only sequelae already established before starting the therapy but also late complications that may eventually not be prevented by ERT, HSCT, or gene therapy. Adequate surveillance of the patients will ensure that disease complications are detected in the early stages and allow for better outcomes of supportive interventions. Patients may benefit from regular clinical consultations every 6 months, with laboratory, imaging, and functional tests done annually or as indicated.98
Developmental delay and neurological regression are very common in MPS VII.11 A standard test for developmental quotient from a developmental specialist may be useful for monitoring the disease progression and guiding learning support. Neurological examination, brain and spine MRI, and nerve conduction velocities may also identify the presence of cord compression, hydrocephalus and carpal tunnel syndrome. Surgical interventions may be needed in those cases.23
Clinical examination may detect the presence and progression of joint contractures, scoliosis, genu valgum and other osteoarticular abnormalities. A radiological examination may clarify the presence of complications including hip dysplasia, and a referral to an orthopedic surgeon will be needed. Interventions applied in this context may include the use of nonsteroidal anti-inflammatory drugs, physical therapy, orthosis, and surgical procedures.11,23
Echocardiogram, pulmonary function tests and endurance tests, including 6-minute-walk test, should be performed to detect and monitor cardiopulmonary involvement. When exertional intolerance is present, NT-pro-BNP may be helpful to distinguish a cardiac cause from pulmonary and musculoskeletal involvement.99 In advanced cases, heart failure may be managed by standard guidelines, and valve replacement should be considered.99 A severe respiratory disease, on the other hand, may require oxygen therapy or the use of non-invasive ventilation.38
Regular surveillance for audiological, ophthalmological and dental manifestations are also required. When indicated, patients may benefit from different procedures including ear tube placement, hearing aids, keratoplasty and orthodontic treatment.23
While surgical procedures may be necessary for many of the MPS complications, it is important to emphasize that special caution is needed, since patients with MPS VII are at high anesthetic risk, due to difficult airway management, respiratory complications, and, more rarely, cardiac complications.100 A summary of supportive treatments is presented in Table 1.
Conclusion and Prospects
Along the almost 50 years after its initial report, much progress has been accomplished regarding the understanding and management of MPS VII. Its perinatal presentation with NIHF, observed in a large percentage of cases, is now well recognized, and screening for MPS VII in these patients became a common practice. Despite being an ultrarare disease and one of the less frequent MPSs, intensive pre-clinical and clinical research led to the development of specific enzyme replacement therapy. The fact that the rhGUS produced for MPS VII treatment (vestronidase alfa) has a longer half-life compared to other ERTs enables its administration every 2 weeks, a convenient advantage compared to the ERTs for the other treatable MPSs. The prospect of prenatal ERT is particularly interesting for this type of MPS, considering the high proportion of NIHF in affected patients. A new generation of ERT with brain penetrating enzymes may be interesting for this condition, considering the high proportion of patients who present CNS involvement. Still considering the CNS manifestations, HSCT seems to be a therapeutic alternative, but the risks related to this procedure should be weighted with the potential benefits, being the age of the procedure an important component of the decision. Emerging genetic therapies, including gene therapy and genome editing, may become available in the future, especially considering that robust preclinical work has been already performed in this area. Despite the progress in the treatment of MPS VII, several unmet needs still persist, and supportive therapy continues to play a major role in the management of this challenging disease.
Abbreviations
4-MU, 4-methylumbelliferyl; BMT, bone marrow transplantation; CNS, central nervous system; CS, chondroitin sulfate; DMMB, dimethylmethylene blue; DS, dermatan sulfate; ERT, enzyme replacement therapy; GAG, glycosaminoglycan; GUS, β-glucuronidase; GVHD, graft-versus-host disease; HLA, human leukocyte antigen; HS, heparan sulfate; HSCT, hematopoietic stem cell transplantation; IV, intravenous; LC-MS/MS, liquid chromatography–tandem mass spectrometry; M6P, mannose-6 phosphate; MDRI, multi-domain responder index; MPS, mucopolysaccharidosis; NIHF, non-immune hydrops fetalis; rhGUS, recombinant human β-glucuronidase; uGAG, urinary glycosaminoglycan.
Disclosure
The authors report no conflicts of interest related to this work.
References
1. Neufeld EF, Muenzer J. The mucopolysaccharidoses. In: Valle DL, Antonarakis S, Ballabio A, Beaudet AL, Michell GA, editors. The Online Metabolic and Molecular Bases of Inherited Disease. McGraw Hill; 2007.
2. Verheyen S, Blatterer J, Speicher MR, et al. Novel subtype of mucopolysaccharidosis caused by arylsulfatase K (ARSK) deficiency. J Med Genet. 2021;jmedgenet-2021–108061. doi:10.1136/JMEDGENET-2021-108061
3. Wiesinger AM, Bigger B, Giugliani R, et al. The inflammation in the cytopathology of patients with mucopolysaccharidoses- immunomodulatory drugs as an approach to therapy. Front Pharmacol. 2022;1797. doi:10.3389/FPHAR.2022.863667
4. Vianey-Saban C, Acquaviva C, Cheillan D, et al. Antenatal manifestations of inborn errors of metabolism: biological diagnosis. J Inherit Metab Dis. 2016;39(5):611–624. doi:10.1007/s10545-016-9947-8
5. Vijay S, Wraith JE. Clinical presentation and follow-up of patients with the attenuated phenotype of mucopolysaccharidosis type I. Acta Paediatr. 2005;94(7):872–877. doi:10.1080/08035250510031584
6. Clarke LA, Giugliani R, Guffon N, et al. Genotype-phenotype relationships in mucopolysaccharidosis type I (MPS I): insights from the international MPS I registry. Clin Genet. 2019;96(4):281–289. doi:10.1111/CGE.13583/
7. Sly WS, Quinton BA, McAlister WH, Rimoin DL. Beta glucuronidase deficiency: report of clinical, radiologic, and biochemical features of a new mucopolysaccharidosis. J Pediatr. 1973;82(2):249–257. doi:10.1016/S0022-3476(73)80162-3
8. Khan SA, Peracha H, Ballhausen D, et al. Epidemiology of mucopolysaccharidoses. Mol Genet Metab. 2017;121(3):227–240. doi:10.1016/j.ymgme.2017.05.016
9. Federhen A, Pasqualim G, de Freitas TF, et al. Estimated birth prevalence of mucopolysaccharidoses in Brazil. Am J Med Genet A. 2020;182(3):469–483. doi:10.1002/AJMG.A.61456
10. Kubaski F, de Oliveira Poswar F, Michelin-Tirelli K, et al. Diagnosis of mucopolysaccharidoses. Diagnostics. 2020;10(3):172. doi:10.3390/diagnostics10030172
11. Montaño AM, Lock-Hock N, Steiner RD, et al. Clinical course of sly syndrome (mucopolysaccharidosis type VII). J Med Genet. 2016;53(6):403–418. doi:10.1136/jmedgenet-2015-103322
12. Nelson A, Peterson LA, Frampton B, Sly WS. Mucopolysaccharidosis VII (β-glucuronidase deficiency) presenting as nonimmune hydrops fetalis. J Pediatr. 1982;101(4):574–576. doi:10.1016/S0022-3476(82)80707-5
13. Al-Kouatly HB, Felder L, Makhamreh MM, et al. Lysosomal storage disease spectrum in nonimmune hydrops fetalis: a retrospective case control study. Prenat Diagn. 2020;40(6):738–745. doi:10.1002/pd.5678
14. Zielonka M, Garbade SF, Kölker S, Hoffmann GF, Ries M. Quantitative clinical characteristics of 53 patients with MPS VII: a cross-sectional analysis. Genet Med. 2017;19(9):983–988. doi:10.1038/gim.2017.10
15. Poyatos-Andújar AM, García-Linares S, Carretero P, et al. Prenatal mucopolysaccharidosis VII: a novel pathogenic variant identified in GUSB gene. Clin Case Rep. 2021;9(2):790–795. doi:10.1002/ccr3.3644
16. Holtz M, Montaño AM, Sly WS. Association between mucopolysaccharidosis type VII and hydrops fetalis. Ultrasound Obstet Gynecol. 2020;55(3):416–417. doi:10.1002/UOG.20371
17. Glaser JH, Sly WS. Beta-glucuronidase deficiency mucopolysaccharidosis: methods for enzymatic diagnosis. J Lab Clin Med. 1973;82(6):969–977.
18. Civallero G, Michelin K, de Mari J, et al. Twelve different enzyme assays on dried-blood filter paper samples for detection of patients with selected inherited lysosomal storage diseases. Clin Chim Acta. 2006;372(1–2):98–102. doi:10.1016/J.CCA.2006.03.029
19. Liu Y, Yi F, Kumar AB, et al. Multiplex tandem mass spectrometry enzymatic activity assay for newborn screening of the mucopolysaccharidoses and type 2 neuronal ceroid lipofuscinosis. Clin Chem. 2017;63(6):1118–1126. doi:10.1373/clinchem.2016.269167
20. Ohira M, Okuyama T, Mashima R. Quantification of 11 enzyme activities of lysosomal storage disorders using liquid chromatography-tandem mass spectrometry. Mol Genet Metab Rep. 2018;17:9–15. doi:10.1016/j.ymgmr.2018.08.005
21. Gort L, Granell MR, Fernández G, Carreto P, Sanchez A, Coll MJ. Fast protocol for the diagnosis of lysosomal diseases in nonimmune hydrops fetalis. Prenat Diagn. 2012;32(12):1139–1142. doi:10.1002/pd.3972
22. Verma J, Thomas DC, Sharma S, et al. Inherited metabolic disorders: prenatal diagnosis of lysosomal storage disorders. Prenat Diagn. 2015;35(11):1137–1147. doi:10.1002/pd.4663
23. Morrison A, Oussoren E, Friedel T, Cruz J, Yilmaz N. Pathway to diagnosis and burden of illness in mucopolysaccharidosis type VII- A European caregiver survey. Orphanet J Rare Dis. 2019;14(1):1–11. doi:10.1186/s13023-019-1233-z
24. Whitley CB, Spielmann RC, Herro G, Teragawa SS. Urinary glycosaminoglycan excretion quantified by an automated semimicro method in specimens conveniently transported from around the globe. Mol Genet Metab. 2002;75(1):56–64. doi:10.1006/mgme.2001.3271
25. Kubaski F, Osago H, Mason RW, et al. Glycosaminoglycans detection methods: applications of mass spectrometry. Mol Genet Metab. 2017;120(1–2):67–77. doi:10.1016/j.ymgme.2016.09.005
26. Osago H, Shibata T, Hara N, et al. Quantitative analysis of glycosaminoglycans, chondroitin/dermatan sulfate, hyaluronic acid, heparan sulfate, and keratan sulfate by LC-ESI-MS/MS. Anal Biochem. 2014;467:62–74. doi:10.1016/j.ab.2014.08.005
27. Oguma T, Toyoda H, Toida T, Imanari T. Analytical method for keratan sulfates by high-performance liquid chromatography/turbo-ionspray tandem mass spectrometry. Anal Biochem. 2001;290(1):68–73. doi:10.1006/abio.2000.4940
28. Oguma T, Toyoda H, Toida T, Imanari T. Analytical method of heparan sulfates using high-performance liquid chromatography turbo-ionspray ionization tandem mass spectrometry. J Chromatogr B Biomed Sci Appl. 2001;754(1):153–159. doi:10.1016/s0378-4347(00)00601-0
29. Fuller M, Rozaklis T, Ramsay SL, Hopwood JJ, Meikle PJ. Disease-specific markers for the mucopolysaccharidoses. Pediatr Res. 2004;56(5):733–738. doi:10.1203/01.PDR.0000141987.69757.DD
30. Saville JT, McDermott BK, Fletcher JM, Fuller M. Disease and subtype specific signatures enable precise diagnosis of the mucopolysaccharidoses. Genet Med. 2019;21(3):753–757. doi:10.1038/s41436-018-0136-z
31. Wang RY, da Silva Franco JF, López-Valdez J, et al. The long-term safety and efficacy of vestronidase alfa, rhGUS enzyme replacement therapy, in subjects with mucopolysaccharidosis VII. Mol Genet Metab. 2020;129(3):219–227. doi:10.1016/J.YMGME.2020.01.003
32. Kubaski F, Brusius-Facchin AC, Mason RW, et al. Elevation of glycosaminoglycans in the amniotic fluid of a fetus with mucopolysaccharidosis VII. Prenat Diagn. 2017;37(5):435–439. doi:10.1002/PD.5028
33. Chabas A, Giros ML, Guardiola A. Low beta-glucuronidase activity in a healthy member of a family with mucopolysaccharidosis VII. J Inherit Metab Dis. 1991;14(6):908–914. doi:10.1007/BF01800472
34. Tomatsu S, Montano AM, Dung VC, Grubb JH, Sly WS. Mutations and polymorphisms in GUSB gene in mucopolysaccharidosis VII (sly syndrome). Hum Mutat. 2009;30(4):511–519. doi:10.1002/humu.20828
35. Vervoort R, Gitzelmann R, Bosshard N, Maire I, Liebaers I, Lissens W. Low β-glucuronidase enzyme activity and mutations in the human β-glucuronidase gene in mild mucopolysaccharidosis type VII, pseudodeficiency and a heterozygote. Hum Genet. 1998;102(1):69–78. doi:10.1007/s004390050656
36. Shipley JM, Klinkenberg M, Wu BM, Bachinsky DR, Grubb JH, Sly WS. Mutational analysis of a patient with mucopolysaccharidosis type VII, and identification of pseudogenes. Am J Hum Genet. 1993;52(3):517–526. Available from: http://www.ncbi.nlm.nih.gov/pubmed/7680524.
37. Vervoort R, Islam MR, Sly W, et al. A pseudodeficiency allele (D152N) of the human beta-glucuronidase gene. Am J Hum Genet. 1995;57(4):798–804. Available from: http://www.ncbi.nlm.nih.gov/pubmed/7573038.
38. Gónzalez-Meneses A, Pineda M, Bandeira A, et al. Description of the molecular and clinical characteristics of the mucopolysaccharidosis type VII Iberian cohort. Orphanet J Rare Dis. 2021;16(1):445. doi:10.1186/s13023-021-02063-1
39. Giugliani R, Barth AL, Dumas MRC, et al. Mucopolysaccharidosis VII in Brazil: natural history and clinical findings. Orphanet J Rare Dis. 2021;16(1):1–9. doi:10.1186/s13023-021-01870-w
40. Guffon N, Froissart R, Fouilhoux A. A rare late progression form of Sly syndrome mucopolysaccharidosis. JIMD Rep. 2019;49(1):1–6. doi:10.1002/jmd2.12039
41. Parini R, Deodato F. Intravenous enzyme replacement therapy in mucopolysaccharidoses: clinical effectiveness and limitations. Int J Mol Sci. 2020;21(8):2975. doi:10.3390/ijms21082975
42. Fox JE, Volpe L, Bullaro J, Kakkis ED, Sly WS. First human treatment with investigational rhGUS enzyme replacement therapy in an advanced stage MPS VII patient. Mol Genet Metab. 2015;114(2):203–208. doi:10.1016/j.ymgme.2014.10.017
43. Cadaoas J, Boyle G, Jungles S, et al. Vestronidase alfa: recombinant human β-glucuronidase as an enzyme replacement therapy for MPS VII. Mol Genet Metab. 2020;130(1):65–76. doi:10.1016/j.ymgme.2020.02.009
44. McCafferty EH, Scott LJ. Vestronidase alfa: a review in mucopolysaccharidosis VII. BioDrugs. 2019;33(2):233–240. doi:10.1007/s40259-019-00344-7
45. Vogler C, Levy B, Galvin N, et al. A novel model of murine mucopolysaccharidosis type VII due to an intracisternal a particle element transposition into the beta-glucuronidase gene: clinical and pathologic findings. Pediatr Res. 2001;49(3):342–348. doi:10.1203/00006450-200103000-00007
46. Chen HH, Sawamoto K, Mason RW, et al. Enzyme replacement therapy for mucopolysaccharidoses; past, present, and future. J Hum Genet. 2019;64(11):1153–1171. doi:10.1038/S10038-019-0662-9
47. Montaño AM, Oikawa H, Tomatsu S, et al. Acidic amino acid tag enhances response to enzyme replacement in mucopolysaccharidosis type VII mice. Mol Genet Metab. 2008;94(2):178–189. doi:10.1016/J.YMGME.2008.01.007
48. Bigg PW, Baldo G, Sleeper MM, et al. Pathogenesis of mitral valve disease in mucopolysaccharidosis VII dogs. Mol Genet Metab. 2013;110(3):319–328. doi:10.1016/J.YMGME.2013.06.013
49. Harmatz P, Whitley CB, Wang RY, et al. A novel, randomized, placebo-controlled, blind-start, single-crossover Phase 3 study to assess the efficacy and safety of UX003 (rhGUS) enzyme replacement therapy in patients with MPS VII. Mol Genet Metab. 2017;120(1–2):S63. doi:10.1016/j.ymgme.2016.11.144
50. Qi Y, McKeever K, Taylor J, et al. Pharmacokinetic and pharmacodynamic modeling to optimize the dose of vestronidase alfa, an enzyme replacement therapy for treatment of patients with mucopolysaccharidosis type VII: results from three trials. Clin Pharmacokinet. 2019;58(5):673–683. doi:10.1007/s40262-018-0721-y
51. Ultragenyx Brasil Farmacêutica LTDA. MEPSEVIITM - Bula para Profissionais de Saúde; 2021. Available from: https://consultas.anvisa.gov.br/.
52. European Medicines Agency. Vestronidase alfa (Mepsevii): summary of product characteristics; 2018. Available from: http://www.ema.europa.eu/.
53. Ultragenyx Pharmaceutical Inc. MepseviiTM (vestronidase alfavjbk): US prescribing information; 2017. Available from: http://www.fda.gov/.
54. Nguyen QH, Witt RG, Wang B, et al. Tolerance induction and microglial engraftment after fetal therapy without conditioning in mice with Mucopolysaccharidosis type VII. Sci Transl Med. 2020;12(532). doi:10.1126/SCITRANSLMED.AAY8980/SUPPL_FILE/AAY8980_SM.PDF
55. Schwab ME, Brown JEH, Lianoglou B, et al. Fetal therapies and trials for lysosomal storage diseases: a survey of attitudes of parents and patients. Orphanet J Rare Dis. 2022;17(1):1–11. doi:10.1186/S13023-022-02178-Z/FIGURES/4
56. Giugliani R, Martins AM, Okuyama T, et al. Enzyme replacement therapy with pabinafusp alfa for neuronopathic mucopolysaccharidosis II: an integrated analysis of preclinical and clinical data. Int J Mol Sci. 2021;22(20):10938. doi:10.3390/IJMS222010938
57. Sisinni L, Pineda M, Coll MJ, et al. Haematopoietic stem cell transplantation for mucopolysaccharidosis type VII: a case report. Pediatr Transplant. 2018;22(7):e13278. doi:10.1111/petr.13278
58. Yamada Y, Kato K, Sukegawa K, et al. Treatment of MPS VII (Sly disease) by allogeneic BMT in a female with homozygous A619V mutation. Bone Marrow Transplant. 1998;21(6):629–634. doi:10.1038/sj.bmt.1701141
59. Furlan F, Rovelli A, Rigoldi M, et al. A new case report of severe mucopolysaccharidosis type VII: diagnosis, treatment with haematopoietic cell transplantation and prenatal diagnosis in a second pregnancy. Ital J Pediatr. 2018;44(Suppl 2). doi:10.1186/S13052-018-0566-X
60. Dubot P, Sabourdy F, Plat G, et al. First report of a patient with MPS type VII, due to novel mutations in GUSB, who underwent enzyme replacement and then hematopoietic stem cell transplantation. Int J Mol Sci. 2019;20(21):5345. doi:10.3390/ijms20215345
61. Gitzelmann R, Bosshard NU, Superti-Furga A, et al. Feline mucopolysaccharidosis VII due to β-glucuronidase deficiency. Vet Pathol. 1994;31(4):435–443. doi:10.1177/030098589403100405
62. Haskins ME, Desnick RJ, Diferrante N, Jezyk PF, Patterson DF. β-glucuronidase deficiency in a dog: a model of human mucopolysaccharidosis VII. Pediatr Res. 1984;18(10):980–984. doi:10.1203/00006450-198410000-00014
63. Birkenmeier EH, Davisson MT, Beamer WG, et al. Murine mucopolysaccharidosis type VII. Characterization of a mouse with beta-glucuronidase deficiency. J Clin Invest. 1989;83(4):1258–1266. doi:10.1172/JCI114010
64. Xing EM, Knox VW, O’Donnell PA, et al. The effect of neonatal gene therapy on skeletal manifestations in mucopolysaccharidosis VII dogs after a decade. Mol Genet Metab. 2013;109(2):183–193. doi:10.1016/j.ymgme.2013.03.013
65. Bigg PW, Sleeper MM, O’Donnell PA, et al. The effect of neonatal gene therapy with a gamma retroviral vector on cardiac valve disease in mucopolysaccharidosis VII dogs after a decade. Mol Genet Metab. 2013;110(3):311–318. doi:10.1016/j.ymgme.2013.06.015
66. Macsai CE, Derrick-Roberts ALK, Ding X, et al. Skeletal response to lentiviral mediated gene therapy in a mouse model of MPS VII. Mol Genet Metab. 2012;106(2):202–213. doi:10.1016/j.ymgme.2012.03.022
67. Bielicki J, McIntyre C, Anson DS. Comparison of ventricular and intravenous lentiviral-mediated gene therapy for murine MPS VII. Mol Genet Metab. 2010;101(4):370–382. doi:10.1016/j.ymgme.2010.08.013
68. Watson G, Sayles J, Chen C, et al. Treatment of lysosomal storage disease in MPS VII mice using a recombinant adeno-associated virus. Gene Ther. 1998;5(12):1642–1649. doi:10.1038/sj.gt.3300775
69. Xu L, Mango RL, Sands MS, Haskins ME, Ellinwood NM, Ponder KP. Evaluation of pathological manifestations of disease in mucopolysaccharidosis VII mice after neonatal hepatic gene therapy. Mol Ther. 2002;6(6):745–758. doi:10.1006/mthe.2002.0809
70. Baldo G, Wu S, Howe RA, et al. Pathogenesis of aortic dilatation in mucopolysaccharidosis VII mice may involve complement activation. Mol Genet Metab. 2011;104(4):608–619. doi:10.1016/j.ymgme.2011.08.018
71. Sleeper MM, Fornasari B, Ellinwood NM, et al. Gene therapy ameliorates cardiovascular disease in dogs with mucopolysaccharidosis VII. Circulation. 2004;110(7):815–820. doi:10.1161/01.CIR.0000138747.82487.4B
72. Derrick-Roberts ALK, Pyragius CE, Kaidonis XM, Jackson MR, Anson DS, Byers S. Lentiviral-mediated gene therapy results in sustained expression of β-glucuronidase for up to 12 months in the Gusmps/mpsand up to 18 months in the Gustm(L175F) Slymouse models of mucopolysaccharidosis type VII. Hum Gene Ther. 2014;25(9):798–810. doi:10.1089/hum.2013.141
73. Derrick-Roberts ALK, Panir K, Pyragius CE, Zarrinkalam KH, Atkins GJ, Byers S. Reversal of established bone pathology in MPS VII mice following lentiviral-mediated gene therapy. Mol Genet Metab. 2016;119(3):249–257. doi:10.1016/j.ymgme.2016.09.003
74. Xing EM, Wu S, Ponder KP. The effect of Tlr4 and/or C3 deficiency and of neonatal gene therapy on skeletal disease in mucopolysaccharidosis VII mice. Mol Genet Metab. 2015;114(2):209–216. doi:10.1016/j.ymgme.2014.12.305
75. Herati RS, Knox VW, O’Donnell P, D’Angelo M, Haskins ME, Ponder KP. Radiographic evaluation of bones and joints in mucopolysaccharidosis I and VII dogs after neonatal gene therapy. Mol Genet Metab. 2008;95(3):142–151. doi:10.1016/j.ymgme.2008.07.003
76. Mango R. Neonatal retroviral vector-mediated hepatic gene therapy reduces bone, joint, and cartilage disease in mucopolysaccharidosis VII mice and dogs. Mol Genet Metab. 2004;82(1):4–19. doi:10.1016/j.ymgme.2004.01.015
77. Smith LJ, Martin JT, O’Donnell P, et al. Effect of neonatal gene therapy on lumbar spine disease in mucopolysaccharidosis VII dogs. Mol Genet Metab. 2012;107(1–2):145–152. doi:10.1016/j.ymgme.2012.03.013
78. Richard M, Arfi A, Seguin J, Gandolphe C, Scherman D. Widespread biochemical correction of murine mucopolysaccharidosis type VII pathology by liver hydrodynamic plasmid delivery. Gene Ther. 2009;16(6):746–756. doi:10.1038/gt.2009.36
79. Hofling AA, Devine S, Vogler C, Sands MS. Human CD34+ hematopoietic progenitor cell-directed lentiviral-mediated gene therapy in a xenotransplantation model of lysosomal storage disease. Mol Ther. 2004;9(6):856–865. doi:10.1016/j.ymthe.2004.03.013
80. Donsante A, Miller DG, Li Y, et al. AAV vector integration sites in mouse hepatocellular carcinoma. Science. 2007;317(5837):477. doi:10.1126/science.1142658
81. Donsante A, Vogler C, Muzyczka N, et al. Observed incidence of tumorigenesis in long-term rodent studies of rAAV vectors. Gene Ther. 2001;8(17):1343–1346. doi:10.1038/sj.gt.3301541
82. Santos HS, Rodrigues L, Vera LNP, et al. In situ gene therapy. Curr Gene Ther. 2021;21(5):406–430. doi:10.2174/1566523221666210504103323
83. Bosch A, Perret E, Desmaris N, Heard JM. Long-term and significant correction of brain lesions in adult mucopolysaccharidosis type VII mice using recombinant AAV vectors. Mol Ther. 2000;1(1):63–70. doi:10.1006/mthe.1999.0005
84. Cearley CN, Wolfe JH, Single A. Injection of an adeno-associated virus vector into nuclei with divergent connections results in widespread vector distribution in the brain and global correction of a neurogenetic disease. J Neurosci. 2007;27(37):9928–9940. doi:10.1523/JNEUROSCI.2185-07.2007
85. Pagès G, Giménez-Llort L, García-Lareu B, et al. Intrathecal AAVrh10 corrects biochemical and histological hallmarks of mucopolysaccharidosis VII mice and improves behavior and survival. Hum Mol Genet. 2019;28(21):3610–3624. doi:10.1093/hmg/ddz220
86. Elliger SS, Elliger CA, Aguilar CP, Raju NR, Watson GL. Elimination of lysosomal storage in brains of MPS VII mice treated by intrathecal administration of an adeno-associated virus vector. Gene Ther. 1999;6(6):1175–1178. doi:10.1038/sj.gt.3300931
87. Liu G. Functional correction of CNS phenotypes in a lysosomal storage disease model using adeno-associated virus type 4 vectors. J Neurosci. 2005;25(41):9321–9327. doi:10.1523/JNEUROSCI.2936-05.2005
88. Liu G, Chen YH, He X, et al. Adeno-associated virus type 5 reduces learning deficits and restores glutamate receptor subunit levels in MPS VII mice CNS. Mol Ther. 2007;15(2):242–247. doi:10.1038/sj.mt.6300016
89. Hennig AK, Levy B, Ogilvie JM, et al. Intravitreal gene therapy reduces lysosomal storage in specific areas of the CNS in mucopolysaccharidosis VII mice. J Neurosci. 2003;23(8):3302–3307. doi:10.1523/JNEUROSCI.23-08-03302.2003
90. Gurda BL, de Guilhem De Lataillade A, Bell P, et al. Evaluation of AAV-mediated gene therapy for central nervous system disease in canine mucopolysaccharidosis VII. Mol Ther. 2016;24(2):206–216. doi:10.1038/mt.2015.189
91. Cubizolle A, Serratrice N, Skander N, et al. Corrective GUSB transfer to the canine mucopolysaccharidosis VII brain. Mol Ther. 2014;22(4):762–773. doi:10.1038/mt.2013.283
92. Poletto E, Baldo G, Gomez-Ospina N. Genome editing for mucopolysaccharidoses. Int J Mol Sci. 2020;21(2):500. doi:10.3390/ijms21020500
93. Poletto E, Colella P, Pimentel Vera LN, et al. Improved engraftment and therapeutic efficacy by human genome-edited hematopoietic stem cells with Busulfan-based myeloablation. Mol Ther Methods Clin Dev. 2022;25:392–409. doi:10.1016/j.omtm.2022.04.009
94. Schuh RS, Poletto É, Pasqualim G, et al. In vivo genome editing of mucopolysaccharidosis I mice using the CRISPR/Cas9 system. J Control Release. 2018;288:23–33. doi:10.1016/j.jconrel.2018.08.031
95. Schuh RS, Gonzalez EA, Tavares AMV, et al. Neonatal nonviral gene editing with the CRISPR/Cas9 system improves some cardiovascular, respiratory, and bone disease features of the mucopolysaccharidosis I phenotype in mice. Gene Ther. 2020;27(1–2):74–84. doi:10.1038/s41434-019-0113-4
96. Vera LNP, Schuh RS, Fachel FNS, et al. Brain and visceral gene editing of mucopolysaccharidosis I mice by nasal delivery of the CRISPR/Cas9 system. J Gene Med. 2022;24(4). doi:10.1002/jgm.3410
97. Kao WWY, Ferreira T, Dong F, et al. Somatic gene therapy of mucopolysaccharidosis with CRISPR/Cas9 genome editing. Invest Ophthalmol Vis Sci. 2016;57(12):115.
98. Parenti G, Giugliani R. The mucopolysaccharidoses. In: Physician’s Guide to the Diagnosis, Treatment, and Follow-Up of Inherited Metabolic Diseases. Springer International Publishing; 2022:1267–1286. doi:10.1007/978-3-030-67727-5_64
99. Marek J, Kuchynka P, Mikulenka V, et al. Combined valve replacement and aortocoronary bypass in an adult mucopolysaccharidosis type VII patient. Cardiovasc Pathol. 2021;50:107297. doi:10.1016/j.carpath.2020.107297
100. Toda Y, Takeuchi M, Morita K, et al. Complete heart block during anesthetic management in a patient with mucopolysaccharidosis type VII. Anesthesiology. 2001;95(4):1035–1037. doi:10.1097/00000542-200110000-00041
101. Young RD, Liskova P, Pinali C, et al. Large proteoglycan complexes and disturbed collagen architecture in the corneal extracellular matrix of mucopolysaccharidosis type VII (Sly Syndrome). Invest Ophthalmol Vis Sci. 2011;52(9):6720. doi:10.1167/iovs.11-7377
102. Hoyme HE, Jones KL, Higginbottom MC, O’Brien JS. Presentation of mucopolysaccharidosis VII (beta-glucuronidase deficiency) in infancy. J Med Genet. 1981;18(3):237–239. doi:10.1136/jmg.18.3.237
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.