Back to Journals » Clinical Ophthalmology » Volume 13
Dexamethasone implant in the management of diabetic macular edema from clinician’s perspective
Authors Urbančič M , Gardašević Topčić I
Received 26 February 2019
Accepted for publication 4 April 2019
Published 13 May 2019 Volume 2019:13 Pages 829—840
DOI https://doi.org/10.2147/OPTH.S206769
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Mojca Urbančič,1 Ivana Gardašević Topčić2
1Eye Hospital, University Medical Centre Ljubljana, Ljubljana, Slovenia; 2Department of Ophthalmology, General Hospital in Novo mesto, Ljubljana, Slovenia
Abstract: The aim of this article is to provide an overview of characteristics and principles of use of dexamethasone implant in patients with diabetic macular edema (DME). The condensed information about patient selection, dosing, and postinjection management is provided to make the clinician’s decisions easier in real-life practice. DME is a common complication of diabetes and the leading cause of visual loss in the working-age population. Inflammation plays an important role in the pathogenesis of DME. The breakdown of the blood–retinal barrier involves the expression of inflammatory cytokines and growth factors, including vascular endothelial growth factor (VEGF). Steroids have proved to be effective in the treatment of DME by blocking the production of VEGF and other inflammatory cytokines, by inhibiting leukostasis, and by enhancing the barrier function of vascular endothelial cell tight junctions. Dexamethasone intravitreal implant has demonstrated efficacy in the treatment of DME resistant to anti-VEGF therapy and in vitrectomized eyes. Data from clinical trials suggest that dexamethasone implant can be considered as first-line treatment in pseudophakic eyes. Dexamethasone implant is also the first-line therapy in patients not suited for anti-VEGF therapy, pregnant women, and patients unable to return for frequent monitoring. It has been shown that the maximum effect of dexamethasone implant on visual gain and retinal thickness occurs approximately 2 months after injection. Various treatment regimens are used in real-life situations, and reported reinjection intervals were usually <6 months. The number of retreatments needed decreased over time. Treatment algorithms should be personalized. Postinjection management and follow-up should consider potential adverse events such as intraocular pressure elevation and cataract.
Keywords: dexamethasone, diabetic macular edema, intravitreal implant, Ozurdex
Introduction
Diabetic macular edema (DME), defined as retinal thickening in the macula, is a common complication of diabetes. The global prevalence of diabetes is increasing, with diabetic retinopathy (DR) being the leading cause of visual loss in the working-age population in many countries. About one-third of patients with diabetes have signs of DR and about one-third of patients with DR have the vision-threatening disease. Whereas proliferative DR is the most common vision-threatening condition in patients with type 1 diabetes, DME is the most common cause of visual loss in patients with type 2 diabetes.1,2 DME may affect up to 7% of patients with diabetes. The risk factors of DME development are largely similar to those for DR.2,3
Inflammation plays an important role in the pathogenesis of DME. DR has features of chronic low-grade inflammation and is associated with significant increases in proinflammatory cytokines within the retina and functional changes to immune cells and tissue macrophages.4–6 Many studies have shown elevated levels of proinflammatory cytokines, chemokines, growth factors, and adhesion molecules in the vitreous or aqueous humor of patients with DR and DME.7–11 These elevations in ocular levels of inflammatory mediators do not correlate with plasma levels, indicating that the inflammation is locally driven.6,11 Activated microglia and leukocytes are sources of proinflammatory mediators. Increased levels of proinflammatory mediators, including vascular endothelial growth factor (VEGF), increased leukocyte adhesion, and leukostasis lead to capillary occlusions and blood–retinal barrier breakdown. Consequent molecular alterations, such as proteins of the tight junction complex, and cytokine-induced vascular cell death contribute to increased capillary permeability.6,12
In patients with DME, liquid accumulates in intracellular and extracellular spaces. Intracellular edema (cytotoxic edema) appears earlier, and extracellular edema (vasogenic edema) appears later as a result of blood–retinal barrier breakdown.13,14 Fluid accumulation together with inflammatory, microvascular, and neurodegenerative changes leads to disruption of normal retinal architecture and loss of visual function.4,15
Steroids were used in the treatment of various ocular disorders from many years. Although the exact mechanism of steroid action on ocular tissues is not fully understood, they have proved to be effective in the treatment of DME by blocking the production of VEGF and other inflammatory cytokines, by inhibiting leukostasis, and by enhancing the barrier function of vascular endothelial cell tight junctions.16 Long-term steroid use may also have a neuroprotective effect on the retina.17–19
Systemic steroids must be administered with caution to diabetic patients because they alter glycemic homeostasis and favor hypertension. However, this seems not to be a problem in the case of their intravitreal administration. From the other side, intravitreally administered corticosteroids are associated with increased risks of cataract development and elevation of intraocular pressure (IOP).18,20,21 Also, there is a higher risk of endophthalmitis and other procedure-related complications due to the intravitreal administration of the drug.
Nowadays, three fluorinated synthetic corticosteroids are used for the treatment of DME: triamcinolone acetonide (TA), dexamethasone phosphate (DEX), and fluocinolone acetonide (FA). They all lack mineralocorticoid activity, but they differ in glucocorticoid-receptor binding affinity and lipophilicity. Differences in binding affinities and lipophilicity may partly explain differences in their relative potencies.17,21 DEX is five times more potent than TA. It is also less lipophilic than TA and FA, causing less binding to the trabecular meshwork and the lens. Therefore, DEX has lower risks for IOP elevation and cataract formation.22
The aim of this article is to provide an overview about the characteristics and principles of use of dexamethasone implant in patients with diabetic macular edema. The condensed information about patient selection, dosing, and postinjection management should help the clinician deciding in real-life situations.
Dexamethasone implant
The short half-life of intravitreally injected DEX limits its clinical use. Extended-release systems have been developed to prolong drug retention in the vitreous. An extended-release biodegradable DEX implant (Ozurdex®; Allergan, Inc., Irvine, CA, USA) loaded with 700 μg of dexamethasone was the first intravitreally injectable drug implant approved for the treatment of DME.23 The rod-shaped DEX implant, approximately 0.46 mm in diameter and 6 mm in length, is injected into the eye with a special disposable injection device through pars plana (Figure 1). The biodegradable polymer matrix slowly degrades and releases DEX into the vitreous (Figure 2). It has been designed to release DEX into the vitreous for up to 6 months.
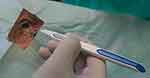 | Figure 1 Injection of dexamethasone implant. |
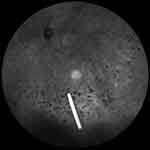 | Figure 2 Dexamethasone implant floating in the vitreous. |
Results of several trials demonstrated the efficacy of DEX implant in improving visual acuity (VA) and reducing central retinal thickness (CRT) in patients with DME. The first report showing the efficacy of DEX implant in treating DME comes from 2007.24 The study involved 315 patients with persistent macular edema resulting from various conditions, including DME. Authors compared two doses of DEX (350 μg and 700 μg) with no treatment. The analysis of 171 eyes with DME showed a statistically significant difference in the proportion of eyes achieving at least a 10-letter improvement in best-corrected visual acuity (BCVA) between the 700 μg DEX implant group and the observation group at days 60 (26% vs 9%; p=0.01) and 90 (33% vs 12%; p=0.007). The difference between the 700 μg DEX implant group and the observation group persisted through day 180 but was no longer statistically significant (30% vs 23%; p=0.4). A statistically significant difference in the proportion of eyes that achieved at least a 10-letter improvement in BCVA was also evident between the 350 μg DEX implant group and the observation group at day 60 (23% vs 9%; p=0.04), but not at day 90 or day 180 (p=0.31). There was a dose–response trend at all time points, but the differences between the 700 μg and 350 μg DEX implant groups were not statistically significant (p=0.14).25 The efficacy in different patterns of DME was similar.26
Macular edema: Assesment of Implantable Dexamethasone in Diabetes (MEAD) trials evaluated the efficacy and safety of DEX implant in treating DME. These were two randomized, multicenter, masked, sham-controlled, Phase III trials with identical protocols. These 3-year trials included 1,048 patients with DME, BCVA of 20/50 to 20/200 Snellen equivalent, and CRT of ≥300 μm by optical coherence tomography (OCT). Patients were randomized into three groups. The first group was treated with 700 μg DEX implant, the second with 350 μg DEX implant, and the third received a sham procedure. Retreatment according to predefined criteria was allowed after 6 months. After 3 years, 22.2% of patients treated with 700 μg DEX implant and 18.4% of patients treated with 350 μg DEX implant gained ≥15 letters from baseline BCVA, which was statistically significant in comparison with the sham group (12.0%; p<0.018). The mean number of treatments received over 3 years was 4.1, 4.4, and 3.3 with the 700 μg DEX implant group, 350 μg DEX implant group, and sham group, respectively. Rates of cataract-related adverse events (AEs) in phakic eyes were 67.9%, 64.1%, and 20.4% in the 700 μg DEX implant group, 350 μg DEX implant group, and sham group, respectively. An IOP of ≥35 mm Hg occurred in 6.6% in the 700 μg DEX implant group, in 5.2% in the 350 μg DEX implant group, but in only 0.9% in the sham group. Increases in IOP were controlled with medication or no therapy, and only two patients (0.6%) in the 700 μg DEX implant group and one (0.3%) in the 350 μg DEX implant group required trabeculectomy.27
The PLACID study was a controlled, multicenter study that randomized 253 patients with DME to 700 μg DEX implant followed by laser photocoagulation or to sham injection followed by laser photocoagulation. Reinjection was allowed after 6 months. Macular laser photocoagulation could be performed for both groups as needed every 3 months. Patients were followed for 12 months. The percentage of patients that gained 10 letters or more in BCVA at 12 months did not differ between treatment groups, but the percentage of patients was significantly higher in the combination group at month 1 (31.7% vs 11%; p<0.001) and month 9 (31.7% vs17.3%; p=0.007). Increased IOP was more common in the combination group than in the laser photocoagulation group. IOP elevations ≥35 mm Hg were observed in 4% of patients. However, surgery was not required to control IOP. Cataract-related AEs were more common among phakic patients in the combination group (22.2%) in comparison to the laser photocoagulation group (9.5%).28
The BEVORDEX study compared the efficacy of 700 μg DEX implant with bevacizumab. A total of 88 eyes with DME were divided into two groups, the first group receiving bevacizumab up to every 4 weeks and the second group receiving DEX implant up to every 16 weeks, both as needed (Pro Re Nata [PRN] regimen). At month 12, there was no difference between groups regarding a 10-letter visual gain (41% vs 40%; p=0.83). However, a higher number of eyes receiving DEX implant lost vision, mainly due to cataract formation.29 Patients in both groups maintained the visual improvement (43% vs 45%; p=0.99) at 24 months. The mean improvement in BCVA at 24 months was 6.9 letters in the DEX implant group and 9.6 letters in the bevacizumab group. At baseline, there were 26 pseudophakic eyes (29.5%): 16 in the DEX implant group and 10 in the bevacizumab group. In phakic eyes, the difference in mean BCVA change was more pronounced at 24 months, with the DEX implant group experiencing worse BCVA, most probably because of cataract formation. By 24 months, 11 of the 30 eyes treated with DEX implant (37%) and two of the 32 eyes treated with bevacizumab (6%) had cataract surgery. Patients treated with DEX implant had higher rates of increased IOP, but no glaucoma surgery was needed. Eyes treated with DEX implant received fewer injections than eyes treated with bevacizumab: in the first year 2.8 injections vs 9.1 injections and in the second year 2.2 injections vs 4.8 injections, respectively.30 A post hoc analysis of 68 study eyes (77%) that completed 2 years follow-up in the BEVORDEX study was carried out to determine whether early vision gains predict long-term visual outcomes. A short-term visual gain at 12 weeks strongly correlated with long-term visual gain, independent of treatment allocation or baseline lens status.31
A multicenter, open-label, randomized study compared the safety and efficacy of DEX implant versus ranibizumab in patients with DME. DEX implant was administered at baseline, at month 5, and at month 10. Ranibizumab 0.5 mg was given monthly, starting from baseline visit. Injections were repeated until maximum BCVA was achieved, meaning that the patient’s BCVA was stable for three consecutive monthly assessments. If BCVA was stable, treatment could be suspended, but monthly treatment was reinitiated if a decrease in BCVA occurred due to DME progression. The study included 363 eyes with DME. The mean BCVA change from baseline over 12 months was 4.34 letters with DEX implant and 7.6 letters with ranibizumab. DEX implant was noninferior to ranibizumab based on the prespecified noninferiority margin of five letters. This result was achieved with an average of 2.85 DEX implant and 8.7 ranibizumab injections. Ocular AEs were more frequent in the DEX implant group than in the ranibizumab group because of cataract formation and IOP increases. IOP increases were transient and managed with topical medication.32
A small retrospective study compared the effects of DEX implant and ranibizumab in patients with symmetrical bilateral DME recalcitrant to ranibizumab. Eleven patients received DEX implant in one eye, while the contralateral eye continued to receive ranibizumab monthly for 3 months. Mean BCVA improved in 3 months from 0.415 logMAR to 0.261 logMAR in eyes receiving DEX implant and from 0.394 logMAR to 0.269 logMAR in eyes receiving ranibizumab.33
The Diabetic Retinopathy Clinical Research (DRCR) network Phase II randomized clinical trial evaluated the potential benefit of adding intravitreous corticosteroid to anti-VEGF therapy. A total of 129 eyes with persistent DME, with BCVA of 20/32 to 20/320, and prior anti-VEGF therapy, were randomized to receive ranibizumab therapy alone or in combination with DEX implant. The addition of intravitreous DEX implant to continued ranibizumab therapy did not improve BCVA at 24 weeks more than continued ranibizumab therapy alone.34
The CHAMPLAIN study evaluated the safety and efficacy of DEX implant in vitrectomized eyes with DME. In this prospective multicenter study, 55 previously vitrectomized eyes with treatment-resistant DME received a single DEX implant injection. The mean BCVA gain from baseline was six letters after 8 weeks and three letters after 26 weeks. At 8 weeks, 30.4% of eyes gained 10 or more letters in BCVA. Cataract progressed in two of the 12 phakic eyes (17%) and IOP increased in 16% of eyes. Only one eye had an IOP increase >35 mm Hg, and it occurred after 8 weeks.35,36 Comparison of anatomic and functional improvement after DEX implant between nonvitrectomized (34) and vitrectomized (24) eyes with persistent DME showed no statistically significant differences in BCVA and CRT during 6 months of follow-up.37
Data from many real-life studies have shown that visual gains are higher in treatment-naive patients comparing to previously treated patients. This leads to the conclusion that early treatment is critical for favorable outcome.38–40
The official Ozurdex label in Europe23 recommends retreatment after approximately 6 months. However, the therapeutic effect in most eyes lasts around 4 months. Real-life studies have shown that the first reinjection interval was usually shorter than 6 months when using the PRN regimen.41–44
Based on available data, DEX implant is an important option for treating DME. Careful patient selection, optimal dosing, and postinjection management are crucial for obtaining optimal treatment results.
Patient selection
Anti-VEGF drugs are considered the first-line therapy for DME, but corticosteroids are also an important option for treating DME patients. Corticosteroids are mostly used as a second choice due to AEs, the most frequent being an increase in IOP and progression of cataract. In persistent DME not responding to anti-VEGF therapy, it is reasonable to switch to corticosteroids.45 There is no definite consensus regarding the definition of a nonresponder to anti-VEGF therapy. Consequently, the timing of the switch from anti-VEGF therapy to corticosteroid remains controversial. Findings from the DRCR network Protocol I and BEVORDEX clinical trials suggest that the long-term response to therapy may be predicted by the response after three anti-VEGF injections in 12 weeks.31,46 In the case of no visual and anatomical improvement after 3 to 6 anti-VEGF injections, a change of treatment should be considered,45 bearing in mind that long-standing DME, if left untreated, can lead to permanent damage to the retina.47 A recent retrospective real-life study showed that eyes with DME refractory to anti-VEGF therapy that were switched to DEX implant after three monthly anti-VEGF injections had better visual and anatomical outcomes at 12 months than those that continued with anti-VEGF therapy.48
The use of DEX implant as a first-line therapy could be warranted in patients in whom the use of anti-VEGF treatment is contraindicated or is expected to be suboptimal: patients with a history of a major cardiovascular event, pregnant patients, patients after vitrectomy, and patients unable or unwilling to return for frequent monitoring.
Anti-VEGF drugs detected in the systemic circulation after they had been injected intravitreally could be associated with an increased risk for systemic AEs in patients with a history of a major cardiovascular event.49,50 Although systemic concentrations were low, concerns have been raised regarding systemic anti-VEGF drug levels after intravitreous treatment, their potential impact on plasma free-VEGF levels, and possible association with an increased risk for systemic AEs. A meta-analysis assessing the risk in anti-VEGF-treated DME patients revealed that high-risk patients with DME that received monthly anti-VEGF treatment over the course of 2 years possibly have an increased risk of vascular-related death and cerebrovascular accidents.51 Although their findings were inconsistent with those from major clinical studies with lower drug exposure, the authors of two different meta-analyses suggested that cumulative exposure to anti-VEGF might be an independent risk factor for a major cardiovascular event.51,52
Progression of diabetic retinopathy during pregnancy can be dramatic. It occurs at approximately double the rate of nonpregnant women.53 Lack of documented experience and concerns about potential fetal side effects54 make the implementation of VEGF inhibition controversial. Consequently, anti-VEGF therapy is generally not offered to pregnant patients. Because of the destructive nature of laser photocoagulation treatment, DEX implant may be considered a better choice for pregnant women with DME. DEX implant seems to have minimal systemic exposure.55 In addition, glucocorticoid medication is accepted in pregnancy for a range of clinical indications.56 Data from retrospective observational case series on pregnant women with DME and treated with corticosteroids showed a prompt response to treatment without a clinically significant IOP increase. Given the durability of the DEX implant, on average one implant per patient was needed. That made cataract formation less likely.57
For patients after vitrectomy, removal of the vitreous humor leads to increased diffusion of molecules, such as oxygen and cytokines, including angiogenic cytokines (VEGF), to and from the retina.58 Even though a recent DRCR network trial showed that previous vitrectomy did not influence the outcomes of intravitreal anti-VEGF therapy,59 many studies reported accelerated diffusion and clearance of various drugs from the vitreous cavity in vitrectomized eyes. It made intravitreal pharmacotherapy less effective.60–64 To overcome the faster clearance of drugs in vitrectomized eyes, an implant with sustained drug release may be the best solution. In the CHAMPLAIN study, intravitreal treatment with DEX implant favorably improved VA and reduced DME in vitrectomized eyes with long-standing DME.35 A retrospective study compared improvements in BCVA and foveal thickness in vitrectomized and nonvitrectomized eyes with DME. During 6 months follow-up, there were no significant differences between the two groups.37
DEX implant can be offered as a first-line option for patients unable or unwilling to come for monthly anti-VEGF injections. However, these patients still require IOP monitoring.
The lens status assessment is a key element before starting treatment with DEX implant. Pseudophakic patients are good candidates for DEX implant. Phakic patients should be informed about the risk of cataract progression and a potential need for surgery. Although visually significant cataract formation is unlikely to develop early after initiating treatment with DEX implant, its incidence increases after the first year of therapy.27,29 In the MEAD study, over 75% of the cataract surgeries in the DEX implant groups were performed between 18 and 30 months. Cataract extraction significantly improved vision.27,29,30,65
With the aim of achieving optimal treatment results, personalized treatment with a thorough evaluation of patients’ general health and eye, in particular, is important. Predictive biomarkers help in assessing the most likely response to a particular treatment.66 Predictors of favorable treatment response with high vision gain were subretinal fluid, intraretinal cysts, and/or vitreomacular adhesion at baseline.67–69 On the other hand, disorganization of the inner retinal layers, disruption of the ellipsoid zone, and/or external limiting membrane, and a thin subfoveal choroid at baseline were predictors of poor VA after therapy.45,70–72 Hyperreflective spots (HRSs) were associated with poorer visual outcome in DME patients treated with DEX implant.73 The presence of a higher number of HRS and subretinal fluid may indicate a prevalent inflammatory component in DME.69,74 In a patient subgroup with a higher number of HRS, better morphological and functional outcomes were observed if treated with DEX implant73–76 compared to anti-VEGF treatment.74 A reduction of HRS and better morphological and functional outcomes after treatment with DEX implant in patients with DME73–76 were found in the patients’ subgroup with a higher number of HRS in comparison to anti-VEGF treatment.74
Not all patients are good candidates for DEX implant treatment. Absolute contraindications are advanced or uncontrolled glaucoma, communication with the anterior chamber, drug hypersensitivity, and local infection. Excluding a potential risk of active infection before considering therapy with DEX implant is necessary.
Dosing of DEX implant
The official Ozurdex label in Europe23 recommends retreatment after approximately 6 months. However, the therapeutic effect of DEX implant in most eyes is usually shorter than 6 months. To optimize treatment with DEX implant, the duration of the therapeutic effect must be taken into account.
Data from the PLACID study showed that the highest increase in BCVA from baseline was achieved 1 month after DEX implant injection. The maximum increase from baseline was 7.9 letters in the 7th month in patients retreated with DEX implant in the 6th month.28 Similarly, in the MEAD study, the highest increase in BCVA was noted 1.5 months after injection. Because the reinjection of DEX implant was not allowed before 6 months, marked fluctuations in BCVA and CRT were observed, particularly in the first year. Fluctuations in BCVA and CRT became smaller in the 2nd and 3rd years.27,77 The peak effectiveness of DEX implant in vitrectomized eyes with DME was seen from 2 to 3 months after injection.35 Other studies also reported the maximum increase in BCVA within 2 months after DEX implant injection.39,78–80
Several real-life studies have shown similar results regarding the number of retreatments during the first year and a mean duration between retreatment intervals. In a retrospective multicenter analysis of 79 eyes with DME treated with DEX implant and followed for at least 1 year after the first injection,38 72% of eyes did not require any additional treatment during the follow-up period. However, 21.5% of eyes required additional treatment before 6 months. The Chart Review of Ozurdex in Macular Edema study (CHROME) evaluated real-world use, efficacy, and safety of DEX implant in eyes with macular edema resulting from diabetes, retinal vein occlusion, and uveitis. The study included eyes receiving two or three DEX implant injections. The mean time to the first and second DEX implant reinjection in DME eyes was 5.8±0.5 months and 5.6±1.0 months, respectively.41 Aknin and Melki reported that 47% of patients needed one, two, or three additional DEX implant injections in the follow-up period of 18 months, with a mean reinjection interval of 5.6 months.81 Data from three studies in France showed consistent results regarding the use of DEX implant in DME patients with an average of 2.4 injections per year and 4.9 months interval between retreatments.82 Escobar-Barranco et al reported a median time of 4 months for the first and the second reinjection.39 Matonti et al observed that patients that received the second injection before the 5th month benefited from 9.9 letters more of visual gain at 12 months compared to patients retreated later than at the 5th month. At the baseline, there were only 0.6 letters of difference between these subgroups.83 Similarly, Sarao et al reported a difference of 0.11 logMAR in BCVA after 6 months in favor of the group of DME eyes treated with the PRN regimen in comparison to the group of DME eyes that received DEX implant only at baseline. The mean number of injections in the PRN group was 1.6 in 6 months.80 A recent systematic review of real-world studies done by Bucolo et al showed that the average mean retreatment time was 5.3±0.9 months. Retreatment was considered on a PRN basis at any time or starting from the 3rd or 4th month.44 The idea of a fixed regimen with a shorter interval of reinjections was evaluated in a prospective, multicenter, randomized clinical trial. The mean change in BCVA with 5-month fixed dosing of DEX implant was noninferior to the OCT-guided PRN regimen, which might be an option in simplifying treatment protocols for DME patients.84
The mean number of injections per year decreases with time and, conversely, mean time to retreatment increases. In the RELDEX study, the mean number of DEX implant injections over 3 years was 3.6, and the mean time to retreatment was 7.3 months over 3 years. In the first year, the mean number of injections was 1.5 and the mean time to retreatment was 5.7 months. In the second year, the mean number of injections was 1.4 and in the third year, it decreased to 1.1. Conversely, in the second year, the mean time to retreatment was 7.5 months and in the third year it lengthened to 10 months.65
Injection and reinjection criteria somewhat vary between different studies and real-life situations. According to recent guidelines for the management of DME,45,85 corticosteroids are mostly a second-line treatment in patients with DME. Patients should be switched from anti-VEGF therapy to corticosteroid treatment if there is less than a 50% reduction from baseline in the excess macular thickness after three- to four-monthly anti-VEGF injections, or if their BCVA has not improved to 20/40 after three- to six-monthly injections because of edema.86 In the MEAD study, patients were eligible for treatment if, among other criteria, BCVA was between 34 and 68 letters (20/200–20/50) and CRT was ≥300 μm. Retreatment eligibility criteria were ≥6 months from the most recent study treatment and residual edema with CRT >225 μm. A study protocol amendment in 2010 revised the anatomic criterion such that patients with CRT >175 μm or with evidence of intraretinal cysts or regions of retinal thickening within or outside the central retinal subfield seen on OCT were eligible for retreatment.27 In the PLACID study, eyes were required to have BCVA between 34 and 70 letters and CRT ≥275 μm. Retreatment was allowed after 6 months if CRT was ≥250 μm.28 Criteria in real-life situations can be more variable. Some real-life studies used a change in BCVA of five or more letters and/or a change in CRT of 50 or more μm42 or a change in BCVA of at least 10 letters and/or an increase in CRT of at least 150 μm.86 Regardless of the exact criteria for retreatment, it is essential to evaluate the functional and anatomical response to treatment when deciding on retreatment. If there is a functional and anatomical improvement after the treatment, it should continue until maximum possible improvement. If there is no functional and anatomical response, a tractional component may maintain edema, and vitrectomy should be considered. If there is a good anatomical response, but no or little functional improvement, this means that DEX implant is working, but structural changes in the retina, neuropathy, or cataract progression render functional improvement impossible. In such cases, the exact cause of the lack of functional improvement should be determined to make an optimal decision regarding subsequent treatment options.86
Based on current knowledge, it can be assumed that early retreatment may lead to better overall visual gain and fewer retreatments afterward. It seems that in most patients the first retreatment should be 4 to 5 months after the first DEX implant injection to achieve optimal results. Treatment algorithms should be personalized. From this perspective, the PRN regimen seems to be the most appropriate because it allows an individualized approach.
Postinjection management
Postinjection management and follow-up should consider potential AEs. In addition to IOP increase and cataract formation, other rare but potentially serious AEs such as retinal detachment, retinal tear, necrotizing retinitis, or endophthalmitis can develop. A recent meta-analysis reported the average time to development of viral retinitis to be 4 months, with cytomegalovirus as the most frequently observed agent.87
Despite the very low risk of endophthalmitis associated with DEX injection in DME patients,27,88 consequences may be devastating in terms of VA. The summary of product characteristics (SmPC) for Ozurdex (DEX implant)23 instructs administation of topical broad-spectrum antimicrobial drops following intravitreal DEX implant injection for 3 days after each injection. The use of antibiotic drops as a preventive method is mostly part of routine practice before and after different intraocular procedures.89–91 However, the application of povidone-iodine to the ocular surface before injection has been shown to be the only useful prophylactic measure supported by clinical trials.92 Furthermore, it has recently been shown that antibiotics do not offer protection against the risk of developing endophthalmitis after the intravitreal administration of anti-VEGF drugs and rates of endophthalmitis may be even higher in patients using topical antibiotics.93,94 An increasing proportion of resistant bacteria on the ocular surface may result in an increased risk of developing antibiotic resistant ocular infections.95 Due to repeated doses of topical antibiotics for long periods, patients receiving intravitreal therapy may be at higher risk. Two recent meta-analyses evaluated the incidence of endophthalmitis after treatment with various intravitreal injections associated with the use of topical antibiotics.96,97 The results supported the possibility that antibiotics are not required in the prophylaxis of endophthalmitis when administering intravitreal injections97 or may even be associated with a higher incidence of endophthalmitis.96 According to recent EURETINA expert consensus recommendations, perioperative antibiotics for intravitreal injections cannot be considered the standard of care because there is no evidence of endophthalmitis prevention.98 Instead, patients should be monitored regularly following the injection to allow early treatment if endophthalmitis occurs. The first biomicroscopy is advised between 2 and 7 days post application. Patients should be instructed to report any symptoms suggestive of ocular infection or other AEs.
An increase in IOP is mostly seen 45 to 60 days following intravitreal injection of DEX implant. However, a check for perfusion of the optic nerve head is advised immediately after the injection and tonometry should be performed within 30 min following the application. Regular monitoring of IOP is required afterward, and any elevation needs proper management. Increases in IOP are not typically observed at the safety visits within 3 weeks after DEX implant treatment. The MEAD study revealed that both mean IOP and the prevalence of IOP elevations were highest at 1.5 to 3 months after injection. These results suggested the need for a follow-up visit at 6 to 8 weeks postinjection, and IOP mostly returned to baseline levels by 6 months after injection.99 According to data from the SAFODEX study, which monitored IOP increase in eyes treated with DEX implant due to macular edema of various origin, there was a small percentage of patients in whom the IOP increase already occurred at 8 days and 1 month. A small percentage of late responders has also been reported in which the first increase in IOP was diagnosed at the third injection or later.100
There is no evidence that repeated DEX implant injections over time have any cumulative effect on IOP increase.99,100 The IOP increase observed in the first eye appears to be a good indicator of the expected IOP in the second eye in patients that receive bilateral injections of DEX implant. In patients with glaucoma or ocular hypertension at baseline, tolerance to IOP increase seems to be poor. According to the SAFODEX study results, there were 50% and 100% of high responders to DEX implant in those treated with dual or triple therapy at baseline, respectively.100 However, compared to uveitic patients or patients with retinal vein occlusion, DME patients seem to have a better pressure tolerance profile.100,101
A two-step algorithm was proposed by an expert panel of European ophthalmologists for monitoring and managing corticosteroid-induced IOP increase in patients with DME. The first step is risk stratification before treatment and the second step describes monitoring and treatment after steroid administration. During follow-up, patients developing an IOP increase should have baseline and periodical imaging and visual field testing. IOP-lowering medications are proposed only when IOP is >25 mm Hg or if diagnostic tests suggest glaucoma is developing. The panel agreed that there is no need for a specific management protocol for IOP increase induced by corticosteroid therapy compared to other causes of increased IOP.101
Recently, Spanish ophthalmologists proposed a protocol for managing and monitoring DME patients. This protocol is based on therapeutic response and potential IOP increase. According to this protocol, the first postinjection checkup should be performed 6 to 8 weeks after each injection and the second postinjection checkup on week 16 after the initial injection. If the therapeutic effect persists for 4 months, the patient may be monitored every 4 to 8 weeks to evaluate the further need for therapy. If there is a recurrence of edema in week 16, but BCVA is still stable, the patient can receive another injection or the injection is postponed for 4 weeks to reevaluate the situation. If there is a recurrence of edema and worse BCVA in week 16, the patient should receive another injection. If there is a considerable worsening of edema and BCVA, shortening the interval between postinjection checkups and a reinjection interval at 3 months should be considered. Based on the patient’s progress, reinjection intervals can be adjusted and gradually increased.86
Conclusion
Based on data from clinical trials and real-life clinical experience, DEX implant is a valuable option in treating DME patients. It provides similar rates of visual improvements as anti-VEGF therapy, but with fewer injections. AEs are manageable. Management and monitoring protocols serve as a guide to a personalized treatment with the aim of optimizing clinical results and reducing the burden of treatment.
Patient consent
Patient consent has been obtained for the publication of images.
Acknowledgments
Marcin Balcerzak of Farenta provided editorial support.
Disclosure
Allergan provided funding for the publication fees and editorial services. Both authors provided consultancy and received a speaker honoraria from Allergan, Novartis, and Bayer. The authors report no other conflicts of interest in this work.
References
1. Ding J, Wong TY. Current epidemiology of diabetic retinopathy and diabetic macular edema. Curr Diab Rep. 2012;12(4):346–354. doi:10.1007/s11892-012-0283-6
2. Yau JWY, Rogers SL, Kawasaki R, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35(3):556–564. doi:10.2337/dc11-1909
3. Lee R, Wong TY, Sabanayagam C, Epidemiology of diabetic retinopathy, diabetic macular edema and related vision loss. Eye Vis. 2015;2:17. doi:10.1186/s40662-015-0026-2
4. Antonetti DA, Barber AJ, Bronson SK, et al. Diabetic retinopathy: seeing beyond glucose-induced microvascular disease. Diabetes. 2006;55(9):2401–2411. doi:10.2337/db05-1635
5. Joussen AM, Poulaki V, Le ML, et al. A central role for inflammation in the pathogenesis of diabetic retinopathy. FASEB J. 2004;18(12):1450–1452. doi:10.1096/fj.03-1476fje
6. Tang J, Kern TS. Inflammation in diabetic retinopathy. Prog Retin Eye Res. 2011;30(5):343–358. doi:10.1016/j.preteyeres.2011.05.002
7. Dugel PU, Bandello F, Loewenstein A, Dexamethasone intravitreal implant in the treatment of diabetic macular edema. Clin Ophthalmol. 2015;9:1321–1335. doi:10.2147/OPTH.S79948
8. El-Asrar AM, Nawaz MI, Kangave D, et al. High-mobility group box-1 and biomarkers of inflammation in the vitreous from patients with proliferative diabetic retinopathy. Mol Vis. 2011;17:1829–1838.
9. Funatsu H, Noma H, Mimura T, Eguchi S, Hori S. Association of vitreous inflammatory factors with diabetic macular edema. Ophthalmology. 2009;116(1):73–79. doi:10.1016/j.ophtha.2008.09.037
10. Noma H, Mimura T, Yasuda K, Shimura M. Role of inflammation in diabetic macular edema. Ophthalmologica. 2014;232(3):127–135. doi:10.1159/000364955
11. Yoshimura T, Sonoda KH, Sugahara M, et al. Comprehensive analysis of inflammatory immune mediators in vitreoretinal diseases. PLoS One. 2009;4(12):e8158. doi:10.1371/journal.pone.0008158
12. Erickson KK, Sundstrom JM, Antonetti DA. Vascular permeability in ocular disease and the role of tight junctions. Angiogenesis. 2007;10(2):103–117.
13. Cunha-Vaz J. Diabetic macular edema. Eur J Ophthalmol. 1998;8(3):127–130.
14. Romero-Aroca P, Baget-Bernaldiz M, Pareja-Rios A, Lopez-Galvez M, Navarro-Gil R, Verges R. Diabetic macular edema pathophysiology: vasogenic versus inflammatory. J Diabetes Res. 2016;2016:2156273. doi:10.1155/2016/2156273
15. Daruich A, Matet A, Moulin A, et al. Mechanisms of macular edema: beyond the surface. Prog Retin Eye Res. 2018;63:20–68. doi:10.1016/j.preteyeres.2017.10.006
16. Daruich A, Matet A, Behar-Cohen F. Sustained-release steroids for the treatment of diabetic macular edema. Curr Diab Rep. 2015;15(11):99. doi:10.1007/s11892-015-0669-3
17. Edelman JL. Differentiating intraocular glucocorticoids. Ophthalmologica. 2010;224(Suppl 1):25–30. doi:10.1159/000315158
18. Lattanzio R, Cicinelli MV, Bandello F. Intravitreal steroids in diabetic macular edema. Dev Ophthalmol. 2017;60:78–90. doi:10.1159/000459691
19. Siqueira RC, Dos Santos WF, Scott IU, et al. Neuroprotective effects of intravitreal triamcinolone acetonide and dexamethasone implant in rabbit retinas after pars plana vitrectomy and silicone oil injection. Retina. 2015;35(2):364–370. doi:10.1097/IAE.0000000000000284
20. Moisseiev E, Loewenstein A. Diabetic macular edema: emerging strategies and treatment algorithms. Dev Ophthalmol. 2017;60:165–174. doi:10.1159/000459706
21. Schwartz SG, Scott IU, Stewart MW, Flynn HW
22. Cebeci Z, Kir N, Role of implants in the treatment of diabetic macular edema: focus on the dexamethasone intravitreal implant. Diabetes Metab Syndr Obes. 2015;8:555–566. doi:10.2147/DMSO.S73540
23.
24. Kuppermann BD, Blumenkranz MS, Haller JA, et al. Randomized controlled study of an intravitreous dexamethasone drug delivery system in patients with persistent macular edema. Arch Ophthalmol. 2007;125(3):309–317. doi:10.1001/archopht.125.3.309
25. Haller JA, Kuppermann BD, Blumenkranz MS, et al. Randomized controlled trial of an intravitreous dexamethasone drug delivery system in patients with diabetic macular edema. Arch Ophthalmol. 2010;128(3):289–296. doi:10.1001/archophthalmol.2010.21
26. Kuppermann BD, Chou C, Weinberg DV, Whitcup SM, Haller JA, Blumenkranz MS. Intravitreous dexamethasone effects on different patterns of diabetic macular edema. Arch Ophthalmol. 2010;128(5):642–643. doi:10.1001/archophthalmol.2010.44
27. Boyer DS, Yoon YH, Belfort R, et al. Three-year, randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with diabetic macular edema. Ophthalmology. 2014;121(10):1904–1914. doi:10.1016/j.ophtha.2014.04.024
28. Callanan DG, Gupta S, Boyer DS, et al. Dexamethasone intravitreal implant in combination with laser photocoagulation for the treatment of diffuse diabetic macular edema. Ophthalmology. 2013;120(9):1843–1851. doi:10.1016/j.ophtha.2013.02.018
29. Gillies MC, Lim LL, Campain A, et al. A randomized clinical trial of intravitreal bevacizumab versus intravitreal dexamethasone for diabetic macular edema: the BEVORDEX study. Ophthalmology. 2014;121(12):2473–2481. doi:10.1016/j.ophtha.2014.07.002
30. Fraser-Bell S, Lim LL, Campain A, et al. Bevacizumab or dexamethasone implants for DME: 2-year results (The BEVORDEX Study). Ophthalmology. 2016;123(6):1399–1401. doi:10.1016/j.ophtha.2015.12.012
31. Mehta H, Fraser-Bell S, Nguyen V, Lim LL, Gillies MC. Short-term vision gains at 12 weeks correlate with long-term vision gains at 2 years: results from the BEVORDEX randomised clinical trial of bevacizumab versus dexamethasone implants for diabetic macular oedema. Br J Ophthalmol. 2018;102(4):479–482. doi:10.1136/bjophthalmol-2017-310737
32. Callanan DG, Loewenstein A, Patel SS, et al. A multicenter, 12-month randomized study comparing dexamethasone intravitreal implant with ranibizumab in patients with diabetic macular edema. Graefes Arch Clin Exp Ophthalmol. 2017;55(3):463–473. doi:10.1007/s00417-016-3472-1
33. Thomas BJ, Yonekawa Y, Wolfe JD, Hassan TS. Contralateral eye-to-eye comparison of intravitreal ranibizumab and a sustained-release dexamethasone intravitreal implant in recalcitrant diabetic macular edema. Clin Ophthalmol. 2016;10:1679–1684.
34. Maturi RK, Glassman AR, Liu D, et al. Effect of adding dexamethasone to continued ranibizumab treatment in patients with persistent diabetic macular edema: a DRCR network phase 2 randomized clinical trial. JAMA Ophthalmol. 2018;136(1):29–38. doi:10.1001/jamaophthalmol.2017.4914
35. Boyer DS, Faber D, Gupta S, et al. Dexamethasone intravitreal implant for treatment of diabetic macular edema in vitrectomized patients. Retina. 2011;31(5):915–923. doi:10.1097/IAE.0b013e318206d18c
36. Nguyen QD, Brown DM, Marcus DM, et al. Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology. 2012;119(4):789–801. doi:10.1016/j.ophtha.2011.12.039
37. Medeiros MD, Alkabes M, Navarro R, Garcia-Arumí J, Mateo C, Corcóstegui B. Dexamethasone intravitreal implant in vitrectomized versus nonvitrectomized eyes for treatment of patients with persistent diabetic macular edema. J Ocul Pharmacol Ther. 2014;30(9):709–716. doi:10.1089/jop.2014.0010
38. Chhablani J, Bansal P, Veritti D, et al. Dexamethasone implant in diabetic macular edema in real-life situations. Eye. 2016;30(3):426–430. doi:10.1038/eye.2015.246
39. Escobar-Barranco JJ, Pina-Marín B, Fernández-Bonet M. Dexamethasone implants in patients with naïve or refractory diffuse diabetic macular edema. Ophthalmologica. 2015;233(3–4):176–185. doi:10.1159/000371770
40. Matonti F, Guigou S, Pommier S, et al. Dexamethasone implants in patients with naive diabetic macular edema. Ophthalmologica. 2016;235(4):244. doi:10.1159/000446296
41. Lam WC, Albiani DA, Yoganathan P, et al. Real-world assessment of intravitreal dexamethasone implant (0.7 mg) in patients with macular edema: the CHROME study. Clin Ophthalmol. 2015;9:1255–1268.
42. Panozzo G, Gusson E, Panozzo G, Dalla Mura G. Dexamethasone intravitreal implant for diabetic macular edema: indications for a PRN regimen of treatment. Eur J Ophthalmol. 2015;25(4):347–351. doi:10.5301/ejo.5000563
43. Scaramuzzi M, Querques G, Spina CL, Lattanzio R, Bandello F. Repeated intravitreal dexamethasone implant (Ozurdex) for diabetic macular edema. Retina. 2015;35(6):1216–1222. doi:10.1097/IAE.0000000000000443
44. Bucolo C, Gozzo L, Longo L, Mansueto S, Vitale DC, Drago F. Long-term efficacy and safety profile of multiple injections of intravitreal dexamethasone implant to manage diabetic macular edema: A systematic review of real-world studies. J Pharmacol Sci. 2018;138(4):219–232. doi:10.1016/j.jphs.2018.11.001
45. Schmidt-Erfurth U, Garcia-Arumi J, Bandello F, et al. Guidelines for the management of diabetic macular edema by the European Society of Retina Specialists (EURETINA). Ophthalmologica. 2017;237(4):185–222. doi:10.1159/000458539
46. Cicinelli MV, Cavalleri M, Querques L, Rabiolo A, Bandello F, Querques G. Early response to ranibizumab predictive of functional outcome after dexamethasone for unresponsive diabetic macular oedema. Br J Ophthalmol. 2017;101(12):1689–1693. doi:10.1136/bjophthalmol-2017-310242
47. Murakami T, Yoshimura N. Structural changes in individual retinal layers in diabetic macular edema. J Diabetes Res. 2013;2013:920713. doi:10.1155/2013/920713
48. Busch C, Zur D, Fraser-Bell S, et al. Shall we stay, or shall we switch? Continued anti-VEGF therapy versus early switch to dexamethasone implant in refractory diabetic macular edema. Acta Diabetol. 2018;55(8):789–796. doi:10.1007/s00592-018-1151-x
49. Avery RL, Castellarin AA, Steinle NC, et al. Systemic pharmacokinetics and pharmacodynamics of intravitreal aflibercept, bevacizumab, and ranibizumab. Retina. 2017;37(10):1847–1858. doi:10.1097/IAE.0000000000001493
50. Avery RL, Castellarin AA, Steinl NC, et al. Systemic pharmacokinetics following intravitreal injections of ranibizumab, bevacizumab or aflibercept in patients with neovascular AMD. Br J Ophthalmol. 2014;98(12):1636–1641. doi:10.1136/bjophthalmol-2013-304546
51. Avery RL, Gordon GM. Systemic safety of prolonged monthly anti-vascular endothelial growth factor therapy for diabetic macular edema: a systematic review and meta-analysis. JAMA Ophthalmol. 2016;134(1):21–29. doi:10.1001/jamaophthalmol.2015.4070
52. Ueta T, Noda Y, Toyama T, Yamaguchi T, Amano S. Systemic vascular safety of ranibizumab for age-related macular degeneration: systematic review and meta-analysis of randomized trials. Ophthalmology. 2015;121(11):
53. Kitzmiller JL, Block JM, Brown FM, et al. Managing preexisting diabetes for pregnancy: summary of evidence and consensus recommendations for care. Diabetes Care. 2008;31(5):1060–1079. doi:10.2337/dc07-1868
54. Polizzi S, Mahajan VB. Intravitreal anti-VEGF injections in pregnancy: case series and review of literature. J Ocul Pharmacol Ther. 2015;31(10):605–610. doi:10.1089/jop.2015.0056
55. Chang-Lin JE, Attar M, Acheampong AA, et al. Pharmacokinetics and pharmacodynamics of a sustained-release dexamethasone intravitreal implant. Invest Ophthalmol Vis Sci. 2011;52(1):80–86. doi:10.1167/iovs.10-5285
56. Götestam Skorpen C, Hoeltzenbein M, Tincani A, et al. The EULAR points to consider for use of antirheumatic drugs before pregnancy, and during pregnancy and lactation. Ann Rheum Dis. 2016;75(5):795–810. doi:10.1136/annrheumdis-2015-208840
57. Concillado M, Lund-Andersen H, Mathiesen ER, Larsen M. Dexamethasone intravitreal implant for diabetic macular edema during pregnancy. Am J Ophthalmol. 2016;165:7–15. doi:10.1016/j.ajo.2016.02.004
58. Stefánsson E. Physiology of vitreous surgery. Graefes Arch Clin Exp Ophthalmol. 2009;47(2):147–163. doi:10.1007/s00417-008-0980-7
59. Bressler SB, Melia M, Glassman AR, et al. Ranibizumab plus prompt or deferred laser for diabetic macular edema in eyes with vitrectomy before anti-vascular endothelial growth factor therapy. Retina. 2015;5(12):2516–2528. doi:10.1097/IAE.0000000000000617
60. Beer PM, Bakri SJ, Singh RJ, Liu W, Peters GB. 3rd, Miller M. Intraocular concentration and pharmacokinetics of triamcinolone acetonide after a single intravitreal injection. Ophthalmology. 2003;110(4):681–686. doi:10.1016/S0161-6420(02)01969-3
61. Chen YY, Chang PY, Wang JK. Intravitreal aflibercept for patients with diabetic macular edema refractory to bevacizumab or ranibizumab: analysis of response to aflibercept. Asia Pac J Ophthalmol. 2017;6(3):250–255. doi:10.22608/APO.2016186
62. Koyanagi Y, Yoshida S, Kobayashi Y, et al. Comparison of the effectiveness of intravitreal ranibizumab for diabetic macular edema in vitrectomized and nonvitrectomized eyes. Ophthalmologica. 2016;236(2):67–73. doi:10.1159/000446992
63. Laugesen CS, Ostri C, Brynskov T, et al. Intravitreal ranibizumab for diabetic macular oedema in previously vitrectomized eyes. Acta Ophthalmol. 2017;95(1):28–32. doi:10.1111/aos.13273
64. Yanyali A, Aytug B, Horozoglu F, Nohutcu AF. Bevacizumab (Avastin) for diabetic macular edema in previously vitrectomized eyes. Am J Ophthalmol. 2007;144(1):124–126. doi:10.1016/j.ajo.2007.02.048
65. Malclès A, Dot C, Voirin N, et al. Real-life study in diabetic macular edema treated with dexamethasona implant: the Reldex Study. Retina. 2017;37(4):753–760. doi:10.1097/IAE.0000000000001234
66. Cunha-Vaz J, Ribeiro L, Lobo C. Phenotypes and biomarkers of diabetic retinopathy. Prog Retin Eye Res. 2014;41:90–111. doi:10.1016/j.preteyeres.2014.03.003
67. Gerendas BS, Prager S, Deak G, et al. Predictive imaging biomarkers relevant for functional and anatomical outcomes during ranibizumab therapy of diabetic macular oedema. Br J Ophthalmol. 2018;102(2):195–203. doi:10.1136/bjophthalmol-2017-310483
68. Lee H, Kang KE, Chung H, Kim HC. Prognostic factors for functional and anatomic outcomes in patients with diabetic macular edema treated with dexamethasone implant. Korean J Ophthalmol. 2018;32(2):1–10. doi:10.3341/kjo.2017.0030
69. Zur D, Iglicki M, Busch C, et al. OCT biomarkers as functional outcome predictors in diabetic macular oedema treated with dexamethasone implant. Ophthalmology. 2018;125(2):288–294. doi:10.1016/j.ophtha.2017.08.031
70. Rayess N, Rahimy E, Ying GS, et al. Baseline choroidal thickness as a predictor for response to anti-vascular endothelial growth factor therapy in diabetic macular edema. Am J Ophthalmol. 2015;59(1):
71. Shin HJ, Lee SH, Chung H, Kim HC. Association between photoreceptor integrity and visual outcome in diabetic macular edema. Graefes Arch Clin Exp Ophthalmol. 2012;250(1):61–70. doi:10.1007/s00417-011-1774-x
72. Sun JK, Lin MM, Lammer J, et al. Disorganization of retinal inner layers as a predictor of visual acuity in eyes with center involved diabetic macular edema. JAMA Ophthalmol. 2014;132(11):1309–1316. doi:10.1001/jamaophthalmol.2014.2350
73. Chatziralli IP, Sergentanis TN, Sivaprasad S. Hyperreflective foci as an independent visual outcome predictor in macular edema due to retinal vascular diseases treated with intravitreal dexamethasone or ranibizumab. Retina. 2016;36(12):2319–2328. doi:10.1097/IAE.0000000000001070
74. Vujosevic S, Torresin T, Bini S, et al. Imaging retinal inflammatory biomarkers after intravitreal steroid and anti-VEGF treatment in diabetic macular oedema. Acta Ophthalmol. 2017;95(5):464–471. doi:10.1111/aos.13273
75. Chatziralli I, Theodossiadis P, Parikakis E, et al. Dexamethasone intravitreal implant in diabetic macular edema: real-life data from a prospective study and predictive factors for visual outcome. Diabetes Ther. 2017;8(6):1393–1404. doi:10.1007/s13300-017-0332-x
76. Hatz K, Ebneter A, Tuerksever C, Pruente C, Zinkernagel M. Repeated dexamethasone intravitreal implant for the treatment of diabetic macular oedema unresponsive to anti-VEGF therapy: outcome and predictive SD-OCT features. Ophthalmologica. 2018;239(4):205–214. doi:10.1159/000485852
77. Danis RP, Sadda S, Li XY, Cui H, Hashad Y, Whitcup SM. Anatomical effects of dexamethasone intravitreal implant in diabetic macular oedema: a pooled analysis of 3-year phase III trials. Br J Ophthalmol. 2016;100(6):796–801. doi:10.1136/bjophthalmol-2015-306823
78. Guigou S, Pommier S, Meyer F, et al. Efficacy and safety of intravitreal dexamethasone implant in patients with diabetic macular edema. Ophthalmologica. 2016;233(3–4):169–175. doi:10.1159/000381356
79. Lazic R, Lukic M, Boras I, et al. Treatment of anti-vascular endothelial growth factor-resistant diabetic macular edema with dexamethasone intravitreal implant. Retina. 2014;34(4):719–724. doi:10.1097/IAE.0b013e3182a48958
80. Sarao V, Veritti D, Furino C, et al. Dexamethasone implant with fixed or individualized regimen in the treatment of diabetic macular oedema: six-month outcomes of the UDBASA study. Acta Ophthalmol. 2017;95(4):e255–e260. doi:10.1111/aos.13273
81. Aknin I, Melki L. Longitudinal study of sustained-release dexamethasone intravitreal implant in patients with diabetic macular edema. Ophthalmologica. 2016;235(4):187–188. doi:10.1159/000446194
82. Querques G, Darvizeh F, Querques L, Capuano V, Bandello F, Souied EH. Assessment of the real-life usage of intravitreal dexamethasone implant in the treatment of chronic diabetic macular edema in France. J Ocul Pharmacol Ther. 2016;32(6):383–389. doi:10.1089/jop.2016.0010
83. Matonti F, Pommier S, Meyer F, et al. Long-term efficacy and safety of intravitreal dexamethasone implant for the treatment of diabetic macular edema. Eur J Ophthalmol. 2016;26(5):454–459. doi:10.5301/ejo.5000787
84. Ramu J, Yang Y, Menon G, et al. A randomized clinical trial comparing fixed vs pro-re-nata dosing of Ozurdex in refractory diabetic macular oedema (OZDRY study). Eye. 2015;29(12):1603–1612. doi:10.1038/eye.2015.214
85. Regillo CD, Callanan DG, Do DV, et al. Use of corticosteroids in the treatment of patients with diabetic macular edema who have a suboptimal response to anti-VEGF: recommendations of an expert panel. Ophthalmic Surg Lasers Imaging Retina. 2017;48(4):291–301. doi:10.3928/23258160-20170329-03
86. García-Layana A, Figueroa MS, Arias L, et al. Clinical decision-making when treating diabetic macular edema patients with dexamethasone intravitreal implants. Ophthalmologica. 2018;240(2):61–72. doi:10.1159/000486800
87. Takakura A, Tessler HH, Goldstein DA, et al. Viral retinitis following intraocular or periocular corticosteroid administration: A case series and comprehensive review of the literature. Ocul Immunol Inflamm. 2014;22(3):175–182. doi:10.3109/09273948.2013.866256
88. Schwartz SG, Flynn HW. Endophthalmitis associated with intravitreal anti-vascular endothelial growth factor injections. Curr Ophthalmol Rep. 2014;2(1):1–5. doi:10.1007/s40135-013-0033-1
89. Huang K, Sultan MB, Zhou D, Tressler CS, Mo J, Practice patterns of ophthalmologists administering intravitreal injections in Europe: a longitudinal survey. Clin Ophthalmol. 2016;10:2485–2488. doi:10.2147/OPTH.S117801
90. Samia-Aly E, Cassels-Brown A, Morris DS, Stancliffe R, Somner JE. A survey of UK practice patterns in the delivery of intravitreal injections. Ophthalmic Physiol Opt. 2015;35(4):450–454. doi:10.1111/opo.12217
91. Xing L, Dorrepaal SJ, Gale J. Survey of intravitreal injection techniques and treatment protocols among retina specialists in Canada. Can J Ophthalmol. 2014;49(3):261–266. doi:10.1016/j.jcjo.2014.03.009
92. Avery RL, Bakri SJ, Blumenkranz MS, et al. Intravitreal injection technique and monitoring: updated guidelines of an expert panel. Retina. 2014;34(Suppl 12):S1–S18. doi:10.1097/IAE.0000000000000399
93. Bhavsar AR, Stockdale CR, Ferris FL, et al. Update on risk of endophthalmitis after intravitreal drug injections and potential impact of elimination of topical antibiotics. Arch Ophthalmol. 2012;130(6):809–810. doi:10.1001/archophthalmol.2012.227
94. Storey P, Dollin M, Pitcher J, et al. The role of topical antibiotic prophylaxis to prevent endophthalmitis after intravitreal injection. Ophthalmology. 2014;121(1):283–289. doi:10.1016/j.ophtha.2013.08.037
95. Hsu J, Gerstenblith AT, Garg SJ, Vander JF. Conjunctival flora antibiotic resistance patterns after serial intravitreal injections without postinjection topical antibiotics. Am J Ophthalmol. 2014;157(3):514–518.e1. doi:10.1016/j.ajo.2013.10.003
96. Bande MF, Mansilla R, Pata MP, et al. Intravitreal injections of anti-VEGF agents and antibiotic prophylaxis for endophthalmitis: a systematic review and meta-analysis. Sci Rep. 2017;7(1):1–6. doi:10.1038/s41598-017-18412-9
97. Benoist d‘Azy C, Pereira B, Naughton G, Chiambaretta F, Dutheil F. Antibioprophylaxis in prevention of endophthalmitis in intravitreal injection: a systematic review and meta-analysis. PLoS One. 2016;11(6):e0156431. doi:10.1371/journal.pone.0156431
98. Grzybowski A, Told R, Sacu S, et al. 2018 Update on intravitreal injections: EURETINA expert consensus recommendations. Ophthalmologica. 2018;239(4):181–193. doi:10.1159/000486145
99. Maturi RK, Pollack A, Uy HS, et al. Intraocular pressure in patients with diabetic macular oedema treated with dexamethasone intravitreal implant in the 3-year MEAD study. Retina. 2016;36(6):1143–1152. doi:10.1097/IAE.0000000000001004
100. Malclès A, Dot C, Voirin N, et al. Safety of intravitreal dexamethasone implant (Ozurdex): the SAFODEX study. Incidence and risk factors of ocular hypertension. Retina. 2017;37(7):1352–1359. doi:10.1097/IAE.0000000000001369
101. Goñi FJ, Stalmans I, Denis P, et al. Elevated intraocular pressure after intravitreal steroid injection in diabetic macular edema: monitoring and management. Ophthalmol Ther. 2016;5(1):47–61. doi:10.1007/s40123-016-0052-8
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
