Back to Journals » Medical Devices: Evidence and Research » Volume 8
Device-length changes and implant function following surgical implantation of the KineSpring in cadaver knees
Authors McNicholas MJ, Gabriel S, Clifford AG, Hasler EM
Received 15 October 2014
Accepted for publication 22 November 2014
Published 6 January 2015 Volume 2015:8 Pages 47—56
DOI https://doi.org/10.2147/MDER.S75852
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Michael J McNicholas,1 Stefan M Gabriel,2 Anton G Clifford,2 Evelyne M Hasler2
1Aintree University Hospital, Teaching Hospital, Major Trauma Centre, NHS Foundation Trust, Liverpool, UK; 2Moximed, Hayward, CA, USA
Introduction: The KineSpring implant system has been shown to provide load reductions at the medial compartment of the knee, and has demonstrated clinical success in reducing pain and increasing function in patients with medial knee osteoarthritis. These results depend on the ability of the KineSpring to rotate, lengthen, and shorten to accommodate knee motions, and in response to knee position and loading.
Purpose: The present study was undertaken to determine length changes of the implanted KineSpring in response to a range of knee positions, external knee loads, and placements by different orthopedic surgeons.
Materials and methods: KineSpring system components were implanted in ten cadaver leg specimens by ten orthopedic surgeons, and absorber-length changes were measured under combined loading and in different positions of the knee.
Results and conclusion: Spring compression consistent with knee-load reduction, and device lengthening and shortening to accommodate knee loads and motions were seen. These confirm the functionality of the KineSpring when implanted medially to the knee.
Keywords: KineSpring, knee, function, preservation, offloading, osteoarthritis
Introduction
Knee osteoarthritis (OA) is a widespread disease that is affecting ever-younger patients with increasing frequency. This has spurred research efforts to reduce pain, preserve joints, delay replacement, and where possible restore joint function. High loads across the medial compartment of the OA knee in particular have been linked to both disease severity1,2 and progression,3 and so reduction of the loads at the knee is the basis for a number of potentially successful OA treatments, such as wedge insoles, braces,4–23 and high tibial osteotomy.24–27 A novel approach to reduce loads across the medial knee compartment is offered by the KineSpring system (Moximed, Hayward, CA, USA), which has been in clinical use for unicompartmental knee OA patients since mid-2008.
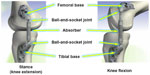
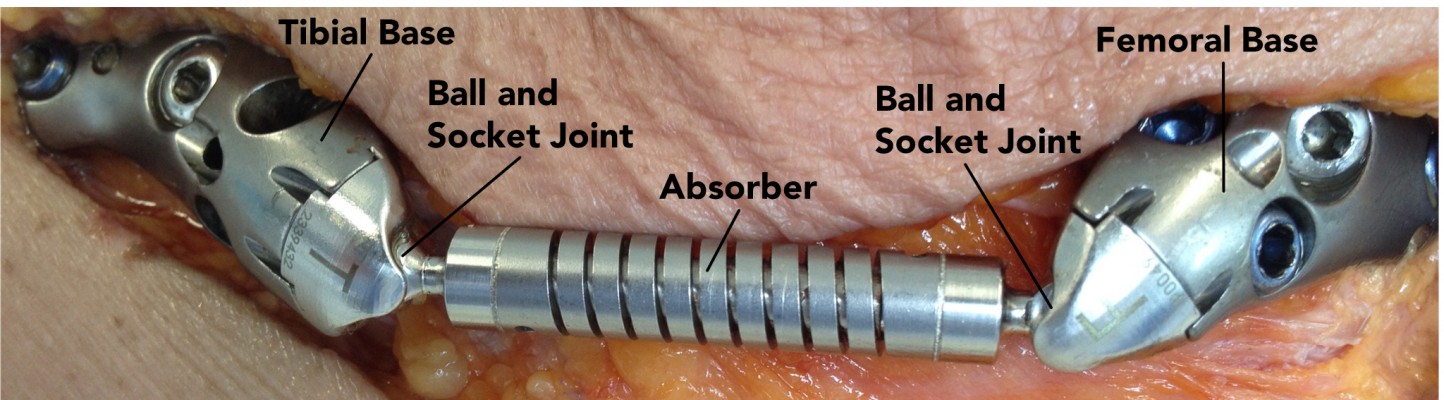
KineSpring treatment has resulted in pain relief and functional improvement in clinical studies with medium-term follow-up,28,29 and the active load reduction of the system has been demonstrated in biomechanical studies.30,31 The KineSpring reduces the load on the medial compartment through compression of its absorber at low knee-flexion angles, and accommodates knee motion via two ball-and-socket joints and a sliding piston (Figure 1). The ball-and-socket joints and sliding piston of the implant allow rotations and translations between the femoral and tibial bases in every direction, thereby accommodating knee motions.
Since the KineSpring is a novel prosthesis representing a different approach to OA treatment via joint-load reduction, questions, speculation, and misconceptions32 about its articulation and function exist. Additionally, the impact of surgical variability and joint stability on device function has not been reported.
The purpose of the present study was therefore to experimentally assess the compression–elongation function of the KineSpring after implantation on a number of cadaver specimens by a number of surgeons and subjected to quantified motions and external loads. Implantation of KineSpring components into a series of ten cadaver legs by ten different surgeons was followed by loading and manipulating each leg to place the knee in a variety of positions and under various external loads. Through measurement of the resulting device length, the accommodation of variations in surgical placement and anatomy, as well as different knee positions and loadings, by the KineSpring can be demonstrated.
Materials and methods
To determine the length changes of the absorber as a function of knee position and external loads, testing was carried out on cadaver knee specimens with the implant in place. Ten cadaver specimens, each consisting of a right or a left leg from the mid-shaft of the femur to the foot were used (Table 1). The specimens were utilized in a cadaveric laboratory surgical skills training course in which ten surgeons were carrying out their first KineSpring implantation after didactic lectures and under supervision.
During implantation, specimen numbers 3 and 4 (see Table 1) were found to have deficient medial knee structures (medial collateral ligament, medial joint capsule), allowing visualization directly into the knee joint in these two specimens. Even though KineSpring is not indicated in cases with soft-tissue deficit, such as this, the KineSpring device was implanted into these specimens to gain insight into surgical feasibility and the effect of joint instability on device function.
Each of ten orthopedic surgeons implanted the KineSpring device (Figure 2) into a different cadaver specimen. Implantations were performed according to the KineSpring surgical technique in a simulated surgical setting (Academy for Medical Training and Simulation, Muttenz, Switzerland) with standard orthopedic instruments and an instrument pack specifically designed for implantation of the KineSpring system (1-4022 KineSpring Procedure Pack). The surgical technique describes the proper positioning of the femoral base plate and correct alignment of the absorber under radiographic visualization, and fixation with bone screws of the femoral and tibial bases to the femur and tibia, respectively. The KineSpring is held at its nominally compressed length (equal to 4 mm of absorber compression) during implantation, and the bases are fixed to the bones while the knee is at or near 0° of flexion with the device held at this length. After base fixation, the absorber is released and allowed to lengthen and compress freely during use, as determined by the femoral pivot point location and the position of the knee.
Between implantation and testing, the implant components were temporarily removed and the legs were stored frozen. Prior to testing, the legs were fully thawed, and the proper fixation of the bases to the bones was checked. For the purposes of the test, the femoral and tibial access incisions along the medial side of the knee were extended to meet each other above the absorber to allow visual and instrument access to the absorber.
A 10–15 cm length of the proximal femoral shaft of each leg was exposed and held firmly within a stainless steel tube by radially opposing bolts (Figure 3). A sphere (~5 cm in diameter) welded to the end of the tube was clamped within a vise bolted to a post fixed to the test table. In this way, the femoral end of each leg was able to be fixed against translation and rotation during testing, and was able to be rotated as needed by temporarily loosening the vise around the sphere. The rest of the leg was free to rotate at the knee and ankle. An apparatus for load application was placed on each leg (Figure 3). The apparatus consisted of a partial boot (P.F.S.™ Plantar Fasciitis Night Splint; Bird and Cronin, Eagan, MN, USA), which cradled and held the foot firmly and immobilized the ankle, and a board bolted to the sole of the boot along with an arrangement of straps, which allowed for quantified application of loads and moments (Figure 3).
  | Figure 3 Apparatus used to hold the specimen for load application. |
A true lateral fluoroscopic image (true lateral is defined as when the posterior and distal aspects of the medial and lateral femoral condyles are superimposed in the view) was taken of each knee at 0° of knee flexion. The femoral pivot-point location is at the center of the femoral ball-and-socket joint, and this point is defined by the surgeon during the implantation procedure as being on the medial femoral epicondyle between 2 and 4 mm from the approximate center of Blumensaat’s line as it appears projected onto a sagittal plane in the lateral fluoroscopic image. The distances of the femoral pivot-point location to the distal and posterior condyle edges were measured in those fluoroscopic images, and the ratio of distal distance to posterior distance was calculated for each knee (Table 2). Comparison of the lateral-view fluoroscopic images between specimens allowed the variation in implantation position among specimens and surgeons to be characterized.
The loading conditions were as follows (Figure 4):
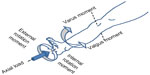  | Figure 4 Schematic depiction of load and moments applied to each leg specimen. |
- a 45 lbf (200 N) compressive axial load at the foot (along the femoral axis)
- a 26 Nm varus or valgus moment (about an anteroposterior axis at the knee)
- a 3 Nm internal or external rotation moment (about the tibial longitudinal axis).
The 45 lbf (200 N) axial load represents a load exceeding the nominal maximum 30 lbf (133 N) force carried by the absorber at its nominal minimum (4 mm compressed) length, which occurs around 0° of knee flexion. Zero degrees of knee flexion is the leg position corresponding to the heel-strike portion of the gait cycle. The 26 Nm of varus or valgus moment represents around 60% of the peak external knee-adduction moment reported during gait.1,33–35 The 3 Nm of internal or external rotation moment represents the value at which a plateau of the data for rotation versus moment was reported to have been reached during in vivo knee-rotation testing.36
The length of the absorber was measured with calibrated calipers for knee angles (as measured by a goniometer) of full hyperextension (terminal extension, if different from 0° flexion), 0° (straight leg), 15°, 30°, 45°, 60°, 90°, and full flexion of the unloaded leg. At 0° of knee flexion, axial load and moments were applied individually and in combination, and the absorber length corresponding to each of these loading conditions was also measured. Absorber-length change was calculated as the difference between the measured length in a given position and the measured free length of the absorber (all components in contact, but no compression of the absorber spring) (Figure 5).
  | Figure 5 Examples of absorber length measurement. |
Changes in absorber length versus knee-flexion angle and changes in absorber length versus applied load were compiled for the specimens to determine the compression–elongation functionality of the device.
Results
The effects of variability of the specimens in combination with variability of implantations by the surgeons can be seen in the variation of implant position. The position of the implant, as measured by the location of the femoral component on the femur, is shown in Table 2. With the exception of the low value (0.38), the remaining position-ratio values are relatively closely grouped (ranging from 0.6 to 0.84). This is reflected in the relatively small sample standard deviation of 0.142 from a sample mean of 0.71 (Table 2).
The absorber-length change as a function of knee-flexion angle is shown in Figure 6. A trend of increasing absorber length with increasing flexion was seen for all specimens. The absent medial structures in knees 3 and 4 resulted in those knees by default taking on a more valgus angulation during flexion than the other knees, which caused the increase in absorber length most evident around 45° of knee flexion (Figure 6). The amount of length change at any given flexion angle varied among the specimens, and the smallest variation occurred at 0°. The 95% confidence interval calculated from the data for the knees at 0° of flexion indicated uniform compression of the absorber (shortening of the absorber relative to its free length) at that knee position. This absorber compression remained for flexion of the knee less than around 15°. At around 15° of knee flexion, the implanted absorber was at its free length, and for knee flexion above 15°, the implanted absorber elongated from its free length (Figure 6).
Absorber-length changes corresponding to different external loading conditions at a given knee angle (0°) are shown in Figure 7. As can be seen, including the data from specimens 3 and 4 substantially increases the absorber-length changes when a valgus moment is applied. This is because of the increased valgus laxity in those two knees, due to their absent medial soft-tissue structures. A statistical comparison using a paired t-test analysis for the cases of no axial load and axial load of 45 lbf (200 N) for each specimen indicated no significant differences between the absorber length with or without the axial load, except for the case of internal rotation, when data from specimens 3 and 4 were included.
The loading conditions that resulted in the largest absorber-length change were the varus and valgus moments. For test runs both with and without axial load applied, the absorber-length changes due to the application of varus and valgus moments, both separately and in conjunction with internal or external rotation moments, resulted in significantly larger absorber-length changes than for no applied moments and for internal and external rotational moments applied separately. Application of a varus moment, with or without a concurrent internal or external rotation moment, resulted in an absorber-length decrease compared to the neutral condition (Figure 7). Application of a valgus moment, with or without a concurrent internal or external rotation moment, resulted in an absorber-length increase compared to the neutral condition (Figure 7). The observation that internal and external rotational moment has relatively little effect on absorber-length change was also quantified by a paired t-test analysis that showed no significant differences between combined loading cases for which presence or absence of internal or external rotational moments were the only difference (Figure 7).
Discussion
The goal of the KineSpring system is to apply accepted approaches of load reduction, joint conservation, and motion preservation to the clinical treatment of medial compartmental degenerative joint disease.37,38 Whenever the absorber-length is longer than or equal to its free length, the spring within the absorber is uncompressed, and the KineSpring is passive and carries no load. Whenever the absorber-length is shorter than its free length, the spring within the absorber is compressed, and the KineSpring is active, carrying a portion of the load passing through the knee joint. The present study demonstrates that the KineSpring functions when implanted at the knee by compressing (and thereby providing active load-carrying capacity) in a portion of the knee-flexion range corresponding to heel strike in gait (0°–15° of knee flexion), and by lengthening and remaining passive at higher knee-flexion angles (>15°). This is true in spite of the variation in implant position across a number of surgeons and specimens. The functionality of the KineSpring is also demonstrated in the present study, as it consistently lengthened to accommodate valgus angulation under external valgus moments, and shortened to accommodate varus angulation under external varus moments.
In general, the same loads and moments were applied to each specimen in the present study to ensure that load and moment magnitude did not become an additional variable possibly affecting the results. Although the magnitude of the axial load applied in the present study was relatively low in relation to body weight or loads occurring during common activities, such as gait, it was felt to be appropriate, since it is equal to 150% of the nominal maximum force carried by the implant.
It is expected that the effect of the axial load on the absorber-length may have been larger for a higher axial load. The length-change response to axial load application, however, seemed to have more to do with whether the application of the axial load resulted in a greater or lesser amount of varus or valgus angulation at the knee than with the proximal/distal effect of the load on the joint itself. This can be seen in the small but somewhat paradoxical lengthening trend of the absorber with the addition of axial load. We believe that this was due to an increase in the valgus moment on the knee that may occur naturally with axial load application (and other data from the present study strongly support lengthening with increased valgus moment).
The magnitude of 26 Nm for the applied varus and valgus moments was chosen with the goal of this being sufficient to bring each leg specimen to its limit of varus or valgus laxity without damaging bony or soft-tissue structures at the knee. Since 26 Nm is approximately 60% of the peak knee-adduction moment reported by other investigators,1,33–35 this seemed to be appropriate for those specimens with intact soft-tissue structures. In the structurally deficient specimens 3 and 4, approaching 26 Nm seemed to initiate tearing of the remaining tissues, especially when applying valgus moments. For this reason, the varus and valgus moments applied to specimens 3 and 4 were limited to 18 Nm and 13 Nm, respectively. Even at these lower varus and valgus moment levels, the absorber-length changes for specimens 3 and 4 exceeded those seen when the larger moments were applied to the other specimens.
The magnitude of 3 Nm for the applied internal and external rotational moments was also chosen with the goal of this being sufficient to bring each leg specimen to its limit of internal or external rotation laxity without damaging bony or soft-tissue structures at the knee. This magnitude of internal and external moment was shown by Coughlin et al to do just that,36 and this seems to be demonstrated also in the present study. The structurally deficient specimens 3 and 4 were subjected to the same magnitude of internal and external rotational moments as other specimens without apparent harm, but they did exhibit the largest excursions of all the specimens in response.
The effects of muscular stabilization of the knee to counteract the effects of external loads or to hold the knee in a given position were not included in this study. The likely effects of muscle loading would be 1) to decrease the angular deviation of the knee under external loads, tending to increase the varus, valgus, internal, or external rotations of the bones at the knee, which would have lessened the lengthening of the implant under those external loads; or 2) to fully close the femorotibial compartment, which around 0° of knee flexion would result in the implant being compressed to a length approaching or equaling its nominal implantation length (4 mm of compression).
The functionality of the KineSpring was shown across a number of specimens implanted by a number of different surgeons, and what may be especially noteworthy is that these were the first KineSpring implantations done by those surgeons. In spite of this, with the exception of a possible single outlier in position, the variation across the surgeons and specimens was relatively low. This suggests a robust surgical technique and implant design ensuring that its function of carrying load at low knee-flexion angles is maintained over a range of cases. The magnitude of this load-carrying capacity has been demonstrated by Gabriel et al in a previous study. That study also suggested that the amount of unloading provided by the KineSpring can be clinically significant and is comparable to the amount of load reduction achieved with other OA treatments, such as weight loss, braces, and high tibial osteotomy.31
The actual clinical significance of the KineSpring as a treatment for medial knee OA has also been demonstrated in clinical studies with short- and medium-term follow-up.28,29 Significant and substantial improvements in OA pain and knee function have been reported after 1 year and through follow-up periods up to 4 years.
We acknowledge several limitations in the present study. Among those is a relatively small sample size. Availability limited the number of specimens included, and during the study the sample size was further challenged by soft-tissue structure deficits in two of the specimens. In spite of this, the number of right- and left-leg specimens were equal, and they came from an equal number of male and female donors (five of each), spanning a range of ages (53–98 years old) and body mass index values (16–31).
Data collection and load and moment application were also subject to investigator interpretation and variations in setup between specimens of different length and girth. Although care was taken to double-check measurements and to apply loads and moments as consistently as possible, inconsistencies in angle and length measurements and in load and moment applications may have occurred. Some of the variation in the results may reflect this.
Additionally, the cadaver surgeries were done by orthopedic surgeons who were implanting the KineSpring for the first time. Although this presented a good opportunity to observe potential variation in implantation at the start of any learning curve that may exist for the KineSpring, variations in placement due to inexperience may also be reflected in the results. This may be both a benefit and a drawback of the present study. Having each of these surgeons perform the implantation on a different leg in the present study allowed demonstration of function over a range of conditions represented by the particular surgeon–leg combinations. This approach, however, did not allow the effects of particular aspects of surgical technique or anatomy on function to be determined.
In spite of these limitations, the results were relatively consistent across the tested specimens and between subsets of the data gathered under different test conditions, indicating consistent function of the KineSpring. Independently, this function has been shown to effectively reduce the load in the OA knee during simulated gait, and patient data suggest that it is a clinically relevant and effective treatment option for medial compartment OA. Even when loads and motions were applied in the present study to specimens with deficient medial structures, the implanted components remained intact and functional. Although surgical implantation would be contraindicated in patients with such medial structural deficiency and they would not be suitable candidates for the procedure, it is reassuring that the implant would seem to be capable of remaining safely intact and functional should such damage occur or be present in a KineSpring patient.
In summary, the implantable KineSpring load absorber has been shown to function by changing length in response to knee-flexion angle and loading across a number of different knees and after implantation by a number of different surgeons.
Disclosure
MJM received payment for surgeon training activities from Moximed, Hayward, CA, USA. SMG, AGC, and EMH are employees of Moximed.
References
Sharma L, Hurwitz DE, Thonar EJ, et al. Knee adduction moment, serum hyaluronan level, and disease severity in medial tibiofemoral osteoarthritis. Arthritis Rheum. 1998;41(7):1233–1240. | |
Thorp LE, Sumner DR, Wimmer MA, Block JA. Relationship between pain and medial knee joint loading in mild radiographic knee osteoarthritis. Arthritis Rheum. 2007;57(7):1254–1260. | |
Miyazaki T, Wada M, Kawahara H, Sato M, Baba H, Shimada S. Dynamic load at baseline can predict radiographic disease progression in medial compartment knee osteoarthritis. Ann Rheum Dis. 2002;61(7):617–622. | |
Brouwer RW, Jakma TS, Verhagen AP, Verhaar JA, Bierma-Zeinstra SM. Braces and orthoses for treating osteoarthritis of the knee. Cochrane Database Syst Rev. 2005;(1):CD004020. | |
Chew KT, Lew HL, Date E, Fredericson M. Current evidence and clinical applications of therapeutic knee braces. Am J Phys Med Rehabil. 2007;86(8):678–686. | |
Crenshaw SJ, Pollo FE, Calton EF. Effects of lateral-wedged insoles on kinetics at the knee. Clin Orthop Relat Res. 2000;(375):185–192. | |
Draganich L, Reider B, Rimington T, Piotrowski G, Mallik K, Nasson S. The effectiveness of self-adjustable custom and off-the-shelf bracing in the treatment of varus gonarthrosis. J Bone Joint Surg Am. 2006;88(12):2645–2652. | |
Draper ER, Cable JM, Sanchez-Ballester J, Hunt N, Robinson JR, Strachan RK. Improvement in function after valgus bracing of the knee. An analysis of gait symmetry. J Bone Joint Surg Br. 2000;82(7):1001–1005. | |
Finger S, Paulos LE. Clinical and biomechanical evaluation of the unloading brace. J Knee Surg. 2002;15(3):155–158; discussion 159. | |
Fisher DS, Dyrby CO, Mundermann A, Morag E, Andriacchi TP. In healthy subjects without knee osteoarthritis, the peak knee adduction moment influences the acute effect of shoe interventions designed to reduce medial compartment knee load. J Orthop Res. 2007;25(4):540–546. | |
Hewett TE, Noyes FR, Barber-Westin SD, Heckmann TP. Decrease in knee joint pain and increase in function in patients with medial compartment arthrosis: a prospective analysis of valgus bracing. Orthopedics. 1998;21(2):131–138. | |
Keating EM, Faris PM, Ritter MA, Kane J. Use of lateral heel and sole wedges in the treatment of medial osteoarthritis of the knee. Orthop Rev. 1993;22(8):921–924. | |
Kerrigan DC, Lelas JL, Goggins J, Merriman GJ, Kaplan RJ, Felson DT. Effectiveness of a lateral-wedge insole on knee varus torque in patients with knee osteoarthritis. Arch Phys Med Rehabil. 2002;83(7):889–893. | |
Kirkley A, Webster-Bogaert S, Litchfield R, et al. The effect of bracing on varus gonarthrosis. J Bone Joint Surg Am. 1999;81(4):539–548. | |
Lindenfeld TN, Hewett TE, Andriacchi TP. Joint loading with valgus bracing in patients with varus gonarthrosis. Clin Orthop Relat Res. 1997(344):290–297. | |
Lundin O, Styf JR. Intramuscular pressure in the leg and thigh related to tensile strap force during knee brace wear. An experimental study in man. Am J Sports Med. 1998;26(4):567–570. | |
Matsuno H, Kadowaki KM, Tsuji H. Generation II knee bracing for severe medial compartment osteoarthritis of the knee. Arch Phys Med Rehabil. 1997;78(7):745–749. | |
Pollo FE, Otis JC, Backus SI, Warren RF, Wickiewicz TL. Reduction of medial compartment loads with valgus bracing of the osteoarthritic knee. Am J Sports Med. 2002;30(3):414–421. | |
Sasaki T, Yasuda K. Clinical evaluation of the treatment of osteoarthritic knees using a newly designed wedged insole. Clin Orthop Relat Res. 1987(221):181–187. | |
Self BP, Greenwald RM, Pflaster DS. A biomechanical analysis of a medial unloading brace for osteoarthritis in the knee. Arthritis Care Res. 2000;13(4):191–197. | |
Tohyama H, Yasuda K, Kaneda K. Treatment of osteoarthritis of the knee with heel wedges. Int Orthop. 1991;15(1):31–33. | |
Wolfe SA, Brueckmann FR. Conservative treatment of genu valgus and varum with medial/lateral heel wedges. Indiana Med. 1991;84(9):614–615. | |
Yasuda K, Sasaki T. The mechanics of treatment of the osteoarthritic knee with a wedged insole. Clin Orthop Relat Res. 1987(215):162–172. | |
Aglietti P, Rinonapoli E, Stringa G, Taviani A. Tibial osteotomy for the varus osteoarthritic knee. Clin Orthop Relat Res. 1983(176):239–251. | |
Brouwer RW, van Raaij TM, Bierma-Zeinstra SM, Verhagen AP, Jakma TS, Verhaar JA. Osteotomy for treating knee osteoarthritis. Cochrane Database Syst Rev. 2007;(3):CD004019. | |
Coventry MB. Upper tibial osteotomy. Clin Orthop Relat Res. 1984(182):46–52. | |
Insall J, Shoji H, Mayer V. High tibial osteotomy. A five-year evaluation. J Bone Joint Surg Am. 1974;56(7):1397–1405. | |
London NJ, Smith J, Miller LE, Block JE. Midterm outcomes and predictors of clinical success with the KineSpring knee implant system. Clin Med Insights Arthritis Musculoskelet Disord. 2013;6:19–28. | |
London NJ, Smith J, Miller LE, Block JE. Bridging the osteoarthritis treatment gap with the KineSpring knee implant system: early evidence in 100 patients with 1-year minimum follow-up. Orthop Res Rev. 2013;5:65–73. | |
Clifford A, O’Connell M, Gabriel S, Miller LE, Block JE. The KineSpring load absorber implant: rationale, design and biomechanical characterization. J Med Eng Technol. 2011;35(1):65–71. | |
Gabriel SM, Clifford AG, Maloney WJ, O’Connell MK, Tornetta P 3rd. Unloading the osteoarthritic knee with a novel implant system. J Appl Biomech. 2013;29(6):647–654. | |
Citak M, Kendoff D, O’Loughlin PF, et al. Failed joint unloading implant system in the treatment of medial knee osteoarthritis. Arch Orthop Trauma Surg. 2013;133(11):1575–1578. | |
Amin S, Luepongsak N, McGibbon CA, LaValley MP, Krebs DE, Felson DT. Knee adduction moment and development of chronic knee pain in elders. Arthritis Rheum. 2004;51(3):371–376. | |
Hunt MA, Birmingham TB, Giffin JR, Jenkyn TR. Associations among knee adduction moment, frontal plane ground reaction force, and lever arm during walking in patients with knee osteoarthritis. J Biomech. 2006;39(12):2213–2220. | |
Zhao D, Banks SA, Mitchell KH, D’Lima DD, Colwell CW Jr, Fregly BJ. Correlation between the knee adduction torque and medial contact force for a variety of gait patterns. J Orthop Res. 2007;25(6):789–797. | |
Coughlin K, Dunleavy D, Tinsley A, Kaback L, Brattbakk B, Beynnon B. Internal-External Torque-Rotation Response of the Knee In Vivo. Washington: Orthopaedic Research Society; 2005. | |
Günther KP. Surgical approaches for osteoarthritis. Best Pract Res Clin Rheumatol. 2001;15(4):627–643. | |
Hanssen AD, Stuart MJ, Scott RD, Scuderi GR. Surgical options for the middle-aged patient with osteoarthritis of the knee joint. Instr Course Lect. 2001;50:499–511. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.