Back to Journals » Patient Preference and Adherence » Volume 18
Development, Reliability and Validity of the Medication Literacy Scale for Parents of Children with Epilepsy
Authors Wu X , Cai S, Zhou Y, Lan Y, Lin Y
Received 23 October 2023
Accepted for publication 21 December 2023
Published 17 January 2024 Volume 2024:18 Pages 165—176
DOI https://doi.org/10.2147/PPA.S446081
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Johnny Chen
Xiaokun Wu,1,* Shu Cai,1,* Ye Zhou,2 Yutao Lan,1 Yan Lin3
1School of Nursing, Guangdong Pharmaceutical University, Guangzhou, People’s Republic of China; 2Department of Nursing, Luzhou Traditional Chinese Medicine Hospital, Luzhou, People’s Republic of China; 3Department of Nursing, Guangzhou Women and Children’s Medical Center, Guangzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yutao Lan, Guangdong Pharmaceutical University, 283 Jianghai Avenue, Haizhu Distric, Guangzhou, People’s Republic of China, Tel +86 13710667351, Email [email protected]
Purpose: This study aimed to develop a medication literacy scale for parents of children with epilepsy (MLSPCE) and to test the reliability and validity of the scale.
Patients and Methods: The pilot scale was formulated based on the concept of medication literacy, the knowledge-attitude-practice model, and relevant literature reviews. It was formed through two rounds of expert consultations using the Delphi method. A survey of 657 parents of children with epilepsy, who were admitted to the neurology department or examined in the neuro-electrophysiological outpatient department of Guangzhou Women and Children Medical Center, using the pilot scale was conducted from October 2021 to January 2022 to test the reliability and validity of the scale questionnaire. The content validity of the scale questionnaire was assessed by consulting 20 neurology nursing, neurology clinician, and nursing education experts. Numbers, percentages, t-test, correlation analysis, Cronbach’s alpha reliability coefficient and factor analysis were used for data analysis.
Results: The MLSPCE included 34 items in four dimensions. Ten factors were drawn from the explorative factor analysis, with a cumulative variance contribution rate of 62.32%. The content validity index of each item on the 34-item scale was between 0.81 and 1.0, and the scale-content validity index/ average was 0.97. The correlation coefficient between each item and its dimension was between 0.399 and 0.760, the correlation coefficients between dimensions were between 0.150 and 0.382, and the correlation coefficients between each dimension and the total scale were between 0.390 and 0.845. Differences for all comparisons were statistically significant (P < 0.05). Cronbach’s alpha coefficient for the total scale was 0.864, and the split-half reliability of the total scale was 0.923.
Conclusion: All the statistical procedures performed in the validity and reliability stages of the study showed that MLSPCE is a valid and reliable tool for measuring medication literacy among Chinese parents of children with epilepsy.
Keywords: epilepsy in children, medication literacy, scale development, reliability test, validity test
Introduction
Epilepsy is the most common chronic neurologic condition among children.1 It does not affect all ages equally but rather has a bimodal distribution, with one peak appearing at the age of 5–9 years.2 Approximately one out of 150 children is diagnosed with epilepsy during the first 10 years of life.3 There are currently about 6 million children with epilepsy in China, with the number increasing annually by about 3,00,000.4 Until the 21st century, the main treatment for epilepsy in children has been anti-epileptic drugs. Taking anti-epileptic drugs regularly and as per the doctor’s advice is the key to ensuring their efficacy. However, children with epilepsy cannot manage their medication independently due to the lack of experience and knowledge regarding the composition, effects, and administration of medication.5 Many medication-related tasks, such as drug administration, monitoring of drug efficacy, and handling adverse drug reactions, need to be performed by the parents of children with epilepsy when the children are under their care.
Medication Literacy (ML) refers to an individual’s ability to obtain, understand, and process medication information to make informed decisions for safe and effective medication use.6 It is the manifestation of health literacy in the context of medication use, with inappropriate medication use being significantly associated with poor ML.7,8 Non-standard medication practices among parents of children with epilepsy are observed widely and mainly include low adherence to the recommended medication course.9 Such practices make children three times more likely to continue having epilepsy up to 4 years post-diagnosis.10 Therefore, reducing non-standard medication practices to ensure the safety and efficacy of medication in children with epilepsy is a clinical problem that needs to be solved urgently. To improve medication use among children with epilepsy to a certain extent, the ML of their parents should be improved. However, the current state of ML among parents is unknown, because, although tools to assess ML do exist, they have been created mainly for patients with hypertension and the general population and are not suitable for assessing parents of children with epilepsy. Therefore, this study aimed to develop a scale that can comprehensively assess the ML of parents of children with epilepsy in order to help healthcare workers quickly understand the reasons underlying problems in the medication management of these children.
Patients and Methods
Study Design
This was a cross-sectional, methodological, descriptive, and correlational study conducted to develop a medication literacy scale for parents of children with epilepsy (MLSPCE). The methods used for the study are summarized in Figure 1.
 |
Figure 1 Summary of the Study Methodology. |
Study Sample
From October 2021 to January 2022, convenience sampling was used to select parents of children with epilepsy, who were hospitalized in the neurology department or examined in the neuro-electrophysiological outpatient department of a tertiary general hospital in Guangzhou, China, for participating in a field survey. Survey data were obtained from a total of 657 parents. Inclusion and exclusion criteria were set for both children and parents. The inclusion criteria for children included in this study were: 1) age < 18 years, 2) met the 2017 International League Against Epilepsy criteria for clinical diagnosis of epilepsy,11 3) had no serious organic disease in the brain, such as encephalitis, brain tumor, or brain degeneration disease, and 4) was being treated with anti-epileptic drugs. The inclusion criteria for the parents included in this study were: 1) participated in the daily medication care of children with epilepsy, 2) had basic Chinese reading and writing ability, normal expression ability, and no communication barriers, and 3) provided consent for participation in the study by signing an informed consent form. The exclusion criterion for the children was: 1) had other systemic chronic diseases, malignant diseases, or organic lesions besides epilepsy. The exclusion criterion for the parents was: 1) had depression or mental illness. The culling criteria for this study were: 1) ≥ 10% missing items in a recovered MLSPCE questionnaire, 2) ≥ 10% items selected repeatedly, 3) one survey being filled out by two or more parents of a children with epilepsy.
Compliance with Ethical Standards
This investigation conformed to the principles outlined by the Declaration of Helsinki. It was also approved by the ethical committee of the Guangzhou Women and Children Medical Center (approval number: 2021–090A01; approval date: May 10, 2021).
Data Collection Tools
The Sociodemographic Questionnaire
The sociodemographic questionnaire is designed by the investigators included two parts: a sub-questionnaire for the children and a sub-questionnaire for the parents. The sub-questionnaire for the children components, such as age, gender, family history, duration of medication, and duration of epilepsy. The sub-questionnaire for the parents included components, such as the relationship between the parent and child with epilepsy, age, education level, occupation, and the time of handling the epilepsy medication of their child every day. Both sub-questionnaires were filled out by the parents.
The MLSPCE
Literature regarding children with epilepsy, the concept of ML, and the knowledge-attitude-practice (KAP) model was reviewed to form an item pool for the MLSPCE, which is in the Chinese language. During the literature review, some guidelines and the consensus of experts from institutions, such as the National Institute for Health and Clinical Excellence (NICE)12 and the International League Against Epilepsy (ILAE),11 were examined. Based on the concept of ML and the KAP model, the theoretical framework and components of the MLSPCE were determined (Figure 2). The MLSPCE questionnaire (scale questionnaire) contained a pool of 50 items related to disease and medication knowledge, medication attitude, medication ability, and medication behavior. Medication attitude and medication behavior were rated on a five-point Likert-type scale (1 = Never – 5 = Always or 1 = strongly agree – 5 = Strongly disagree). The other two dimensions were reported as single choices selected as a response to a multiple-choice item question. If the parents responded to an item question correctly, they would get 1 point, otherwise they would get 0 points.
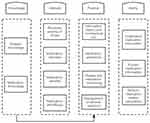 |
Figure 2 Theoretical framework for the Medication Literacy Scale for Parents of Children With Epilepsy. |
Two rounds of the Delphi method were used to finalize the pilot scale. We invited 20 clinical experts to participate in the Delphi method-based consultations. Their average age was 44.9 ± 6.43 years, their average work experience was 22.30 ± 7.84 years, and their highest educational qualification was at least a Bachelor’s degree. Out of the total 20, there were 14 experts with the title of deputy senior or above. Forty percent of the experts came from the field of neurology clinical medicine (n = 8), 50% came from neurology clinical nursing (n = 10), and the rest came from nursing education (n = 2). They have rich theoretical knowledge and clinical work experience related to epilepsy. We designed and used our own expert consultation form. Experts rated the importance of each item in this form using the five-point Likert-type scale (1 = Very unimportant – 5 = Very important).13 The mean importance score of an item was ≥ 4,14 and the coefficient of variation (CV) was < 0.3;15 these were the retention criteria for the items. The mean importance score of an item is the arithmetic average of the importance scores provided by experts for it. The CV is the ratio of the standard deviation of the importance score of an item and the mean. The smaller the CV value, the more concentrated is the expert’s opinion and the greater is the importance of the item.
The results of the first round of consultation revealed that the average importance score of 50 items in the item pool was between 2.15 and 4.95, and the CV value was between 0.00 and 0.49. Three of the items did not meet the retention criteria and were deleted (item 6: If I have questions about the medications my child is currently taking, I know who to consult; item 11: Sudden tapering or discontinuation of medication can trigger frequent seizures; item 26: Without taking anti-epileptic drugs, my child’s condition will worsen). We accepted the advice of the experts regarding accurate wording, avoidance of inducements, ease of understanding, etc. of the items, and revised the contents of 14 items accordingly. Some experts pointed out that there should be special emphasis on the administration method of Depakine used in Case 2 of the medication ability dimension, so we added one item accordingly (What is the correct way to take Depakine). We also accepted the input of two experts and merged two items with similar meanings into one item (Before taking the medicine, I will take the initiative to ask the medical staff about the drug used by the child, such as the name of the drug, its effect, dosage, adverse reactions, etc.). The results of the second Delphi method round showed that there were no items that needed to be deleted and no new changes required by the experts.
Pilot Testing
Some items for the scale questionnaire were removed or adjusted in accordance with expert opinions. Pilot testing was performed with a pilot scale including 47 items after their contents were validated. The 47-item pilot scale was administered to 49 parents. The readability, intelligibility, and response time of the pilot scale were then evaluated. The readability and intelligibility were explored through analysis of qualitative comments made by the patients; comments were made if the patients found any items confusing, upsetting, or difficult to respond to.16 The results showed that all parents found the number of items acceptable and the item contents understandable. The parents filled out the pilot scale in 6–20 min and did not give any negative feedback. These findings showed that the items of the pilot scale were clear, and its contents valid. After the pilot study, the pilot scale was administered to all parents. Results from these 49 parents included in the pilot testing were not included in the final, formal analysis.
Data Collection Process
A researcher handed out consent forms, sociodemographic questionnaires, and the scale questionnaire to 657 parents who agreed to participate in this study. The same researcher was responsible for the issue and recovery of the three types of forms. The study subjects were selected in strict accordance with the study inclusion and exclusion criteria. Informed consent was obtained from the parents before the questionnaire distribution process, and unified instructions were used to explain the purpose, content, and fill requirements of the survey, so as to ensure the consistency of the survey responses. To prevent parents from overlooking some items while filling out the forms, the researcher checked the forms on the spot after they were collected. If there were any missing items in a respondent’s form, the respondent was requested to immediately complete them to ensure an effective recovery rate. Besides, comprehensive assessment was carried out by reviewing the medical records of the children, so as to minimize the bias caused by human factors in the results of the investigation and improve the credibility of the data.
At the data collation stage, the recovered forms were numbered in the following order: 001,002. All data items were double-checked. If there was any inconsistency in the input results, it could be traced back to the corresponding original form for a check using the form number. Once all data were recorded, the forms that did not meet requirements were removed in strict accordance with the exclusion criteria.
Data Analyses
Percentages and means were used for descriptive statistics. For project analysis, this study adopted five common item screening methods using the IBM SPSS 23.0 software: discriminant analysis, correlation coefficient method, Cronbach’s alpha coefficient, tendency of dispersion, and principal component analysis. The results obtained using the above five item-screening methods were compiled, and the items selected for deletion more than two times were deleted from the scale questionnaire. For discriminant analysis, all scale questionnaires were first ranked according to the total score from high to low; the top 27% formed the high-score group, and the bottom 27% formed the low-score group. Then the average score of each item in the two groups was calculated, and the independent sample t-test was used to determine whether the difference in each item was statistically significant. The higher the t value, the higher was the discrimination degree of the item. When the t value was < 3.0 or the difference was not statistically significant (P > 0.05), the discrimination ability of the item was considered poor, and the item was considered for deletion from the scale questionnaire.17 The correlation coefficient method was used to calculate the correlation between each item and the total scale questionnaire. The greater the absolute value of the correlation coefficient (an r > 0.4 is generally considered to be very good, an r between 0.3 and 0.4 is considered to be good) and the more statistically significant (P < 0.05), the more representative are the items.18 Therefore, r < 0.3 was taken as the criterion for item deletion in this study. In the Cronbach coefficient analysis method, if the Cronbach coefficient increased after deleting an item, the item was considered for deletion from the scale questionnaire.19 For the discrete trend method, the CV was used as the index to evaluate the dispersion degree of items in the scale questionnaire, considering the need to eliminate the large difference in means caused by using two different methods of calculating an item score. If the CV was < 0.15, the item was considered for deletion.20 For the principal component analysis, we first performed Kaiser - Meyer - Olkin (KMO) and Bartlett tests to determine whether the data were suitable for factor analysis. If the KMO was > 0.8 and the Bartlett test revealed a significant difference (P < 0.05),21 principal component analysis could be performed. The criteria for considering item deletion were commonality < 0.2 and loading value < 0.4, or loading value > 0.4 in 2 or more factors.
For validity analysis, content validity index (CVI), exploratory factor analysis (EFA), and confirmatory factor analysis (CFA) were used. For structural validity, factor analysis was used to further screen the items retained after project analysis and to test the construct validity of the scale questionnaire. First, the IBM SPSS 23.0 software was used to conduct an exploratory factor analysis on a part of randomly selected questionnaire data (n = 300) to determine the potential structure of the scale questionnaire. Then, the AMOS 22.0 software was used to conduct a confirmatory factor analysis on the remaining questionnaire data (n = 320) to test the degree of fit between the scale questionnaire structure constructed by exploratory factor analysis and the initial theoretical hypothesis model and comprehensively evaluate the construct validity of the final scale questionnaire. Chi-square value (χ2), chi-square/ degree of freedom (χ2/df), root mean square residuals (RMR), root mean square error of approximation (RMSEA), incremental fit index (IFI), Tucker-Lewis (TLI), comparative fitness index (CFI), Parsimony goodness-of-fit index (PGFI), and Parsimony-adjusted normed fit index (PNFI) were used for confirmatory factor analysis in this study. When conducting the exploratory factor analysis, the criteria for deletion of items were: 1) the maximum loading value for he factor is < 0.4; 2) the loading value for ≥ 2 factors is > 0.4, and the difference is < 0.2; 3) the number of items in a factor is less than three (deletion is decided on a case-by-case basis); and 4) items are improperly categorized and unexplained.22 For the confirmatory factor analysis, the criteria for better model fit were: 1) χ2 significance probability value P < 0.05; 2) χ2/df < 2; 3) RMSEA < 0.05; 4) RMR < 0.05; 5) IFI, TLI, and CFI > 0.9; and 6) PGFI and PNFI > 0.5.23 For content validity analysis, we calculated the item-level CVI (I-CVI) using data previously provided by experts to score the importance of each item. The index of each item was calculated first, as follows: number of experts who provided an I-CVI = 4 or 5 /the total number of experts. Then, the CVI of the total scale questionnaire was calculated (S-CVI/Ave). When I-CVI was ≥ 0.78 and S-CVI/Ave was ≥ 0.90, the scale questionnaire was considered to have good content validity.
For the reliability analysis, the IBM SPSS 23.0 software was used to calculate Cronbach coefficient and split-half reliability of the total scale questionnaire and each dimension. For newly developed measuring tools, it is generally accepted that the Cronbach’s coefficient reaches 0.7.24
Results
Demographic Data
A total of 657 scale questionnaires were distributed, of which 620 valid questionnaires were recovered, giving an effective recovery rate of 94.36%. Of all children with epilepsy included in the study, 58.2% (n = 362) were male. The mean age of the children with epilepsy was 8.39 ± 4.47 years. The percentage of children aged > 3 years at epilepsy diagnosis was 51.5% (n = 319). The percentages of children who had taken anti-epileptic drugs for 1 year, 1 to 2 years, 2 to 3 years, and > 3 years were 12.9% (n = 80), 14.7% (n = 91), 19.8% (n = 123), and 52.6% (n = 326), respectively.
Among the parents of children with epilepsy, fathers accounted for 58.3% (n = 362) and mothers accounted for 41.6% (n = 258). The mean age of the parents was 36.38 ± 6.09 years. Among the parents, junior high school graduates (37.6%) made up the largest number, followed by senior high school or technical secondary school graduates (26.0%). The percentages of parents who were state officials, corporate employees, self-employed, healthcare workers, workers, farmers, and unemployed or otherwise were 3.5% (n = 22), 13.2% (n = 82), 17.7% (n = 110), 1.9% (n = 12), 18.5% (n = 115), 13.4% (n = 83), and 31.6% (n = 196), respectively.
Overall, most of the children with epilepsy included in this study were young and had been taking anti-epileptic drugs for a long time. Their parents had different ages, cultural backgrounds, and occupations.
Project Analysis
Although the difference in two items (item 21 and 36) between two groups was statistically significant (P < 0.05), the t value was found to be < 3.0 through discriminant analysis, and these items were, therefore, considered for deletion from the scale questionnaire. Correlation coefficient analysis revealed that the correlations between 14 items (item 8, 10, 12, 13, 21, 23, and 29–36) and the total scale questionnaire were < 0.3 and were statistically significant (P < 0.05). As eight items (items 29–36) out of these 14 items were from the dimension of medication ability, their deletion would affect the structure of the entire scale questionnaire. After consultation with experts, these eight items were retained for further testing, and the remaining six items were considered for deletion. Cronbach’s alpha coefficient was determined to be 0.870 using Cronbach’s coefficient method, and it was found to range between 0.860 and 0.875 after each item was deleted. After three items (items 18, 21, and 31) were deleted, Cronbach’s alpha coefficient was > 0.870. Therefore, these items were considered for deletion from the scale questionnaire. Using the tendency of dispersion method, the CV values of three items (items 18, 20, and 43) were found to be < 0.15, so they were considered for deletion. Using the KMO test and Bartlett’s test, the KMO was found to be 0.866, and χ2 was found to be 8132.897 (P < 0.05), indicating the suitability of the data for factor analysis. Then, twelve factors were found to explain 55.54% of the total variance and have eigenvalues higher than 1.00. Finally, only two items (item 18: Children diagnosed with epilepsy should be actively treated; item 21: I was worried that my child would have to take anti-epileptic drugs for a long time) were selected for deletion, and the MLSPCE was formed with 45 items in 4 dimensions.
Validity Analysis
After project analysis, exploratory factor analysis of the remaining 45 items was conducted. After performing exploratory factor analysis three times, we concluded that the MLSPCE structure was stable Eleven items were accordingly deleted from the scale questionnaire (Table 1). The KMO value obtained in the final factor analysis was 0.825, with χ2 =3288.695 (P < 0.05), indicating that the scale questionnaire was suitable for factor analysis. Ten factors that explained 62.32% of the total variance and had eigenvalues higher than 1.00 were found (Table 2). According to the contents of the items included in the scale questionnaire, the 10 extracted factors could be reasonably classified into four dimensions: disease and medication knowledge, medication attitude, medication ability, and medication behavior.
 |
Table 1 Process of Exploratory Factor Analysis of the Medication Literacy Scale for Parents of Children with Epilepsy (n = 320) |
 |
Table 2 Eigenvalue and Variance Contribution Rate of Each Factor of the Medication Literacy Scale for Parents of Children with Epilepsy |
After exploratory factor analysis, the MLSPCE, with four dimensions, 10 common factors, and 34 items, was formed. It was consistent with the original theoretical hypothesis. After confirmatory factor analysis, the χ2 value of the initial model was found to be significant (P < 0.05). The fit indices of the MLSPCE were: χ2/df = 1.462, RMR = 0.025, IFI = 0.917, TLI = 0.907, CFI = 0.916, RMSEA = 0.038, PGFI = 0.758, and PNFI = 0.706. All the fit indices were within the standard range, indicating that the actual item data fit the theoretical model well and the construct validity of the MLSPCE was ideal. These findings indicated that the scale can be used as a measurement tool for medication literacy among parents of children with epilepsy.
Twenty experts tested the scale questionnaire’s content validity. The CVI was used to assess the experts’ opinions. The content validity indices of the items were found to range between 0.81 and 1.00, while the S-CVI/Ave was found to be 0.97. These findings indicated that the MLSPCE has good content validity.
The correlations between the dimensions and total score and between dimensions are presented in Table 3. The correlation coefficients between the items contained in the four dimensions of the scale questionnaire and the dimensions they belong to ranged from 0.399 to 0.760. The correlation coefficients between each dimension and the total score ranged from 0.390 to 0.845.
 |
Table 3 Correlations Between Dimensions and the Total Score and Between Dimensions of the Medication Literacy Scale for Parents of Children with Epilepsy (r Values) |
Reliability Analysis
The reliability coefficient, α, of the MLSPCE (34 items) was found to be 0.864. Cronbach’s alpha coefficients for the dimensions ranged from 0.514 to 0.870. The split-half reliability coefficient of the MLSPCE (34 items) was 0.923. The split half reliability of each dimension ranged from 0.533 to 0.917. These findings indicated that the MLSPCE has good reliability (Table 4).
 |
Table 4 Reliability Analysis of the Medication Literacy Scale for Parents of Children with Epilepsy |
Discussion
This is the first study to develop a medication literacy scale specifically meant for parents of children with epilepsy and to explore the psychometric properties of this MLSPCE among Chinese parents. Although tools for assessing ML do exist at present, they are mainly intended for use in patients with hypertension25 and the general population26 and are unsuitable for assessing the ML of parents of children with epilepsy. Tools for assessing ML mostly evaluate an individual’s ability to understand and correctly interpret drug information, ignoring the individual’s attitudes towards the medication and their medication behavior. Thus, the results obtained using these tools cannot accurately and comprehensively reflect the level of individual ML.
The Process of Developing the MLSPCE Was Scientifically Rigorous
The operational definition of variables plays an important role in the preparation of an assessment scale. ML is a concept recently put forward in the field of drug use. In 2018, Pouliot et al6 proposed an international expert consensus on the concept of ML through the Delphi method in order to improve awareness regarding the importance of ML among global healthcare workers. Based on this concept, we defined the ML of parents of children with epilepsy as the ability to obtain, understand, process, and communicate information about anti-epileptic drugs, make wise medication decisions for the treatment of children with epilepsy, and administer anti-epileptic drugs safely and effectively. The KAP model implicates three continuous processes in human behavior development: knowledge, attitude, and practice.27 Knowledge is the basis of behavioral changes, and attitude is the motivation behind of behavioral changes. ML covers individual medication knowledge and attitude in consistency with the KAP model. The structure of the MLSPCE, developed based on the concept of ML and the KAP model, was found to be scientific and reasonable in the present study.
In the field of nursing, a scale is more than just a tool for collecting certain information about a patient. In order to make the scale reflect psychological, behavioral, and other conditions of parents of children with epilepsy in clinical practice, we used the Delphi method and invited a group of experts in the field of epilepsy to participate in the process of scale development. The Delphi method is a widely used and flexible method that is particularly useful in achieving consensus in a given area of uncertainty or in the absence of empirical evidence.28 The key to the implementing the Delphi method is the correct selection of experts, as this directly affects the reliability of the consultation results.24 To ensure that consultation results were representative and comprehensive, experts were selected from a wide variety of sources, ranging from hospitals to universities, and fields, including neurology clinical nursing, neurology clinical medicine, and nursing education. The number of consulted experts with an associate senior professor title or above was quite high. All of them had a bachelor’s degree or higher and had worked for more than 20 years in their field, indicating that they had rich theoretical knowledge and clinical work experience related to epilepsy.
The selection of scale items is key for the development of a scale. Choosing a scientific and reasonable method to select these items is particularly important to ensure the quality of the scale. In this study, five item-screening methods were used to screen items from the perspectives of discrimination, independence, internal consistency, sensitivity, and representativeness. When multiple consecutive items in the same dimension were to be deleted, which would affect the structure of the whole scale questionnaire, we sought the advice of experts to make a decision based on their own clinical experience. Data obtained through a variety of tests showed that the MLSPCE had a high level of reliability and could adequately measure what it was intended to measure.
The MLSPCE Has High Clinical Value
Since epilepsy is a chronic disease, children need to receive regular and appropriate anti-epileptic treatment for proper control of epilepsy. Long-term, regular medication is the key to ensuring the efficacy of anti-epileptic drugs. Children with epilepsy need to depend on their parents for medication management. However, many problems have been observed in how parents manage medication for their children, such as low medication adherence, medication errors, and failure to identify and deal with adverse drug effects.9,29 These problems can put patients at increased risk of seizures, increase the frequency of visits to the hospital, and increase healthcare costs.30 Therefore, reducing the occurrence of the above issues to ensure medication safety and efficacy in children with epilepsy is an urgent clinical problem to be solved. ML is an important factor affecting an individual’s medication behavior and plays a significant role in patient medication safety in clinical practice.7,8 Because there is no suitable tool to measure the ML of parents of children with epilepsy, it is necessary to develop a scientific and feasible tool for this purpose.
The MLSPCE finally formed in this study contains four dimensions and 34 items and has good reliability and validity. In the reliability analysis, the Cronbach’s alpha value was found to be 0.863 for the whole scale questionnaire. This was higher than the Cronbach’s alpha value reported for the ChMLM scale meant for the general population (0.72)26 and that reported for the C-MLSHP scale meant for hypertensive patients (0.849).25 Furthermore, the split-half reliability correlation coefficient of the MLSPCE (0.923) was higher than that of a 14-item English and Spanish MedLitRxSE scale meant for the general population31 (English: KR-20 = 0.81; Spanish: KR-20 = 0.77) and that of a 37-item Chinese C-MLSHP scale meant for hypertensive patients (0.893).25 Therefore, the newly developed scale was highly reliable for measuring what it was supposed to.
The better the validity of a measurement tool, the more the results obtained using it correspond to the reality.31 In the present study, the fit indices of the MLSPCE were: χ2/df = 1.462, RMR = 0.025, IFI = 0.917, TLI = 0.907, CFI = 0.916, RMSEA = 0.038, PGFI = 0.758, and PNFI = 0.706. All the parameters were within the standard range, indicating that the MLSPCE fit well with the theoretical model. The content validity indices of the items were found to range from 0.81 to 1.00, indicating that the MLSPCE has good content validity.
In general, the MLSPCE was developed to assess the ML of parents of children with epilepsy in four aspects in an up-to-date, detailed, user-friendly, and efficient manner. It can be used for parents of children who are aged 0–18 years and receiving anti-epileptic drugs. The scale can be used to quickly and comprehensively understand the ML of parents of children with epilepsy. Based on the scores in each dimension, problems in the medication management of parents can be identified in a timely manner and personalized medication education and intervention measures can be provided.
Limitations
This study is affected by several limitations. First, the participants were enrolled via convenience sampling from a tertiary general hospital in Guangzhou, China. The differences in demographic characteristics in other regions may affect the generalizability of the findings. Second, in the development and testing of the MLSPCE, the subjects were Chinese speaking parents in China. Therefore, the medication literacy in the contexts of other cultures, and countries are unknown. Finally, this study only completed the reliability and validity test of the scale, and did not carry out practical application evaluation in parents of children with epilepsy. In the subsequent study, the MLSPCE can be further modified and improved.
Conclusions
This study strictly complied with the development method of the medication literacy scales to create an MLSPCE for parents of children with epilepsy. The final MLSPCE contained 34 items in four dimensions. The disease and medication knowledge dimension contained 13 items, the medication attitude dimension contained seven items, the medication ability dimension contained four items, and the medication behavior dimension contained 10 items. The total scores of the scale range from 0 to 102, and the higher the score, the better is the ML of parents of children with epilepsy. The MLSPCE has good reliability and validity in evaluating the ML of parents of children with epilepsy. It is suitable for people with different education levels, ages, and careers, and can provide a basis for healthcare workers to provide out efficient and individualized interventions for children with epilepsy.
Acknowledgments
We would like to thank all the families, experts, and institutions who have participated in and contributed to this study as well as researchers who have contributed to the literature on which we have established our study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The author reports no conflicts of interest in this work.
References
1. Fowler SB, Hauck MJ, Allport S, et al. Knowledge and fears of parents of children diagnosed with epilepsy. J Pediatr Nurs. 2021;60:311–313. doi:10.1016/j.pedn.2021.08.008
2. Falco-Walter J. Epilepsy-definition, classification, pathophysiology, and epidemiology. Semin Neurol. 2020;40(6):617–623. doi:10.1055/s-0040-1718719
3. Aaberg KM, Gunnes N, Bakken IJ, et al. Incidence and prevalence of childhood epilepsy: a nationwide cohort study. Pediatrics. 2017;139:5. doi:10.1542/peds.2016-3908
4. Zhu F, Lang S, Wang X, et al. Long-term effectiveness of antiepileptic drug monotherapy in partial epileptic patients. Chinese Med J. 2015;128(22):3015–3022. doi:10.4103/0366-6999.168968
5. Berrier K. Medication errors in outpatient pediatrics. MCN Am J Matern Child Nurs. 2016;41(5):280–286. doi:10.1097/NMC.0000000000000261
6. Lee CH, Chang FC, Hsu SD, et al. Inappropriate self-medication among adolescents and its association with lower medication literacy and substance use. PLoS One. 2017;12(12):e189199. doi:10.1371/journal.pone.0189199
7. Shi S, Shen Z, Duan Y, et al. Association between medication literacy and medication adherence among patients with hypertension. Front Pharmacol. 2019;10:822. doi:10.3389/fphar.2019.00822
8. Modi AC, Wu YP, Rausch JR, et al. Antiepileptic drug nonadherence predicts pediatric epilepsy seizure outcomes. Neurology. 2014;83(22):2085–2090. doi:10.1212/WNL.0000000000001023
9. Modi AC, Rausch JR, Glauser TA. Early pediatric antiepileptic drug nonadherence is related to lower long-term seizure freedom. Neurology. 2014;82(8):671–673. doi:10.1212/WNL.0000000000000147
10. Fisher RS, Cross JH, D’Souza C, et al. Instruction manual for the ILAE 2017 operational classification of seizure types. Epilepsia. 2017;58(4):531–542. doi:10.1111/epi.13671
11. Nunes VD, Sawyer L, Neilson J, et al. Diagnosis and management of the epilepsies in adults and children: summary of updated NICE guidance. BMJ. 2012;344:e281. doi:10.1136/bmj.e281
12. Luo R, Xie W, Liu Y, et al. Development of evaluation index system for health education among adult acute leukemia patients with chemotherapy. Chin J Nurs. 2019;54(10):1482–1487.
13. Wang Y, Huang L, Feng Z, et al. Study on establishing urethral catheter maintenance strategies on the basis of evidence-based practice and Delphi method. Chin J Nurs. 2016;51(02):155–160.
14. Zhu K, Tao H. Construction of a comprehensive health assessment for community in-home elderly in Beijing based on modified delphi methods. Chinese General Practice. 2019;22(11):1341–1345.
15. Molassiotis A, Ellis J, Wagland R, et al. The Manchester cough in lung cancer scale: the development and preliminary validation of a new assessment tool. J Pain Symptom Manage. 2013;45(2):179–190. doi:10.1016/j.jpainsymman.2012.01.015
16. Liu S, Jin J, Zhang X, et al. The discrimination and reliability of functioning assessing tool based on ICF rehabilitation set in the assessment of aging disability. Chin J Rehabil Med. 2020;35(09):1044–1048.
17. Yang L, Yang Y, Zhang C, et al. Development and reliability and validity tests of elderly frailty assessment scale. Chin J Nurs. 2017;52(01):49–53.
18. Lv Y, Zheng Q. Mathematical models and evaluations in study of medical measuring scale. Chin J Clin Pharmacol Ther. 2007;2007(06):690–696.
19. He Q, Wang J, Zhang Y, et al. Item screening technique on clinical outcome rating scale which based on patient-reported outcomes. China J Tradition Chinese Med Pharm. 2011;26(01):112–114.
20. An GY, Pearce S. A Beginner’s guide to factor analysis: focusing on exploratory factor analysis. Tutorials Quant Methods Psychol. 2013;9(2):79–94. doi:10.20982/tqmp.09.2.p079
21. Barnes H, Faraz CA, Rubright JD. Development of the novice nurse practitioner role transition scale: an exploratory factor analysis. J Am Assoc Nurse Pract. 2021;34(1):79–88. doi:10.1097/JXX.0000000000000566
22. Xia Y, Yang Y. RMSEA, CFI, and TLI in structural equation modeling with ordered categorical data: the story they tell depends on the estimation methods. Behav Res Methods. 2019;51(1):409–428. doi:10.3758/s13428-018-1055-2
23. Hou S, Sun H, Fang X, et al. Quantitative index system for competency evaluation of nurses in post of integration of medical care and health service based on double helix model. J Nurs. 2021;28(22):25–29.
24. Zhong Z, Shi S, Duan Y, et al. The development and psychometric assessment of Chinese medication literacy scale for hypertensive patients (C-MLSHP). Front Pharmacol. 2020;11:490. doi:10.3389/fphar.2020.00490
25. Yeh YC, Lin HW, Chang EH, et al. Development and validation of a Chinese medication literacy measure. Health Expect. 2017;20(6):1296–1301. doi:10.1111/hex.12569
26. Macías YF, Glasauer P. Guidelines for Assessing Nutrition-Related Knowledge, Attitudes and Practices. Food and Agriculture Organization of the United Nations (FAO); 2014.
27. Powell C. The Delphi technique: myths and realities. J Adv Nurs. 2003;41(4):376–382. doi:10.1046/j.1365-2648.2003.02537.x
28. Yang CS, Song HY, Zhang LL, et al. Study on current status and influencing factors of medication compliance in children with epilepsy in China. Chinese Pharm Affairs. 2017;31(12):1513–1521.
29. Banks J, Varley J, Fitzsimons M, et al. Self-reported antiepilepsy medication adherence and its connection to perception of medication error. Epilepsy Behav. 2020;104(A):106896. doi:10.1016/j.yebeh.2019.106896
30. Sauceda JA, Loya AM, Sias JJ, et al. Medication literacy in Spanish and English: psychometric evaluation of a new assessment tool. J Am Pharm Assoc. 2012;52(6):e231–e240. doi:10.1331/JAPhA.2012.11264
31. Yazici E, Kose E, Turan C, et al. Outbreak anxiety scale: development, validity, and reliability. North Clin Istanb. 2021;8(5):443–453. doi:10.14744/nci.2021.69077
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
