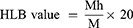Back to Journals » International Journal of Nanomedicine » Volume 17
Development of Squalene-Based Oil-in-Water Emulsion Adjuvants Using a Self-Emulsifying Drug Delivery System for Enhanced Antigen-Specific Antibody Titers
Authors Chae GE, Kim DW, Jin HE
Received 24 June 2022
Accepted for publication 15 November 2022
Published 9 December 2022 Volume 2022:17 Pages 6221—6231
DOI https://doi.org/10.2147/IJN.S379950
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yan Shen
Ga-Eul Chae,1 Dong Woo Kim,1 Hyo-Eon Jin1,2
1College of Pharmacy, Ajou University, Suwon, 16499, Republic of Korea; 2Research Institute of Pharmaceutical Science and Technology, Ajou University, Suwon, 16499, Republic of Korea
Correspondence: Hyo-Eon Jin, College of Pharmacy, Ajou University, Suwon, 16499, Republic of Korea, Tel +82-31-219-3466, Fax +82-504-152-4017, Email [email protected]
Introduction: A recombinant protein cannot induce sufficient immune response by itself. Various substances, including cytokine and mineral, have been used as adjuvants to enhance the immunogenicity and efficacy of vaccines; however, most of them induce excessive immune responses or exhibit cytotoxicity. In this study, a self-emulsifying drug delivery system (SEDDS), an isotropic mixture of oil, surfactant, and solvent, was designed for oil-in-water emulsions as a non-toxic adjuvant to increase immune response to antigens.
Methods: Squalene-based oil-in-water emulsions were prepared by SEDDS to assess its value as an adjuvant. Fifteen emulsions (F1–F15) were prepared by stirring two types of surfactants (Span® 85 and Kolliphor® RH40), and squalene and carboxymethyl cellulose (CMC) were added at different ratios. The physical properties and viscosity of the 15 emulsions were evaluated by measuring droplet size, zeta potential, and polydispersity index. The toxic effect of emulsions was assessed by acute toxicity test in mice. Mice were immunized twice with 1:1 mixtures of antigen and adjuvant (15 emulsions, phosphate-buffered saline, and commercial alum-based adjuvant). Antigen-specific antibody titers from immunized mice serum were measured by an indirect enzyme-linked immunosorbent assay.
Results: All emulsions exhibited droplet sizes ranging from 322 to 812 nm and maintained zeta potential values between − 30 mV to – 10 mV for 4 weeks, indicating good physical stability as a vaccine adjuvant. Additionally, all emulsions were non-toxic, and they induced humoral immunity at a similar level compared to commercial alum-based adjuvant in the first immunization. However, 12% squalene-based oil-in-water emulsion containing 0.5% of ultra-high viscosity CMC (F15) showed significantly higher immune response than a commercial adjuvant in the second immunization.
Conclusion: Squalene-based oil-in-water emulsions could be conveniently prepared using SEDDS technique and are non-toxic and stable at room temperature storage. Moreover, squalene-based oil-in-water emulsions show enhanced immune induction with antigen; hence, they can possibly be used as effective adjuvants.
Keywords: squalene, adjuvant, self-emulsifying drug delivery system, emulsion, vaccine
Introduction
Vaccines are drugs developed to protect the human body from infections by inducing immune responses to specific pathogenic substances before the actual infection. They are classified into live attenuated vaccine, mRNA vaccine, inactivated vaccine, and recombinant subunit vaccine based on the main ingredients of the antigen constituting the vaccine.1–4 Live attenuated vaccine, which removes the pathogenicity of the pathogen, and inactivated vaccine, which inactivates the antigen with heat or chemicals, induce an immune response similar to an actual infection.3,4 However, there is a limit to the risk of safety. The recombinant protein vaccine, which is purified proteins that have the antigenicity of the pathogen, produced through recombinant DNA technology, has no safety issues. However, these vaccines do not induce strong immunity by antigenic components themselves.3,4 Therefore, to induce a strong immune response to the antigen component, an adjuvant was mixed with the antigen for vaccine use.
Adjuvant is a substance that can increase immunity when inoculated in combination with antigens, it cannot induce an immune response by itself. Many cytokines and minerals have been developed for adjuvants, but most of them are highly cytotoxic and only a few adjuvants have been clinically approved by humans. There are four considerations when developing adjuvants: the primary being safety, adjuvants should be harmless as they are injected directly into the body with antigens.5–9 The second is storage stability, as vaccines contain a variety of ingredients, including antigens and adjuvants, they have to be stable for a long time at appropriate storage temperatures, and adjuvants should be able to contribute to stability. The third is the ease of manufacture, adjuvants should be able to be produced at a low cost through simple manufacturing methods. Finally, structural changes in the antigen could negatively affect the immune response. Therefore, the adjuvant should be able to stabilize the antigen.6,7 The most widely used adjuvant is alum. Alum induces a prolonged immune response at the injection site by electrostatically binding to the antigen and delaying the release of the antigen. The primary limitation of alum is that it can induce an effective and stable local immune response only, but not a T-cell-associated cellular immune response.10
Emulsions are mixtures of two immiscible liquids, such as water and oil.11,12 Emulsion, a form in which another liquid (dispersed phase) is dispersed into one liquid (continuous phase), could be divided into oil-in-water and water-in-oil emulsions depending on which material is continuous phase. The oil-in-water emulsion is an emulsion where water is the continuous phase and oil is the dispersed phase.13,14 This emulsion has been widely used in the pharmaceutical industry because it can load drugs that are soluble in the dispersed phase and deliver them to a specific site.15–18 Lipid emulsions have been developed to deliver nutrients (eg, Intralipid®, and Lipofundin S®) to patients who cannot eat through their mouths and are also used to treat toxicity caused by overdosed local anesthetics (eg, bupivacaine®) in veins.16–18 Lipid emulsions have recently been used as an adjuvant for the vaccine. MF59 used in the Influenza virus vaccine was a squalene-based oil-in-water emulsion developed by Novartis in 1990 and was used as a variety of vaccine ingredients, such as Aflunov®, Focetrea®, and Fluad®.19–21 MF59 is harmless to the body and can induce B cell-associated humoral immunity such as alum, as well as induce T cell-associated cellular immune responses, so its use as an adjuvant is of very high value.22 In general, high-pressure homogenization methods are used because high energy is required to form emulsions.23
In this study, we aim to formulate a squalene-based oil-in-water emulsion using a self-emulsifying drug delivery system (SEDDS) technique to use as an adjuvant. SEDDS is an isotropic mixture of oil, surfactants, and solvent that has been used for the design of formulations to improve the oral absorption of highly lipophilic drug compounds.24,25 SEDDS has the advantage of being able to form emulsions by easily mixing the two substances. In this study, 15 emulsions were formulated using SEDDS technique, and physicochemical properties (ie, size, zeta potential, polydisperse index, and viscosity), stability, and acute-toxicity were investigated. Then, adjuvant effect of the emulsions was assessed by immunization in Balb/c mice.
Materials and Methods
Chemical Reagents
Squalene, 99+%, Imject® alum, and Pierce™ TMB substrate kit were purchased from Thermo Fisher Scientific (Waltham, MA, USA). Span® 85 and Kolliphor® RH40 were purchased from TCI (Tokyo, Japan). Carboxymethylcellulose sodium salt (medium viscosity and ultra-high viscosity) was purchased from Sigma-Aldrich (St. Louis, MO, USA). Rabbit Anti-Mouse IgG H&L (HRP) was purchased from Abcam (Cambridge, UK). Isopropyl β-D-1-thiogalactopyranoside (IPTG) was purchased from Bioneer (Daejeon, Korea) and nickel-nitrilotriacetic acid (Ni-NTA) agarose was purchased from Qiagen (Hilden, Germany).
Animals
Six-week-old Balb/c mice were purchased from Orient Bio Inc. (Seongnam, Korea). All animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC) of Ajou University (IACUC 2021–0011).
Construction of Pseudo-Ternary Phase Diagram
The pseudo-ternary phase diagram that is used to identify the self-dispersion region of the emulsion was constructed with reference to the Ahmad’s method.14 Squalene was added in a ratio of 1:9 to 9:1 to an Smix that is a mixture of Span® 85 and Kolliphor® RH40 mixed in a ratio (w/w) of 1:1, 1:2, or 1:4. Then, a small amount of distilled water was added to the mixture and stirred at room temperature (RT) until the point of phase change was observed. The composition percentage of the calculated component based on the observed results was marked on a ternary plot using Chemix School software V9.00 (Arne Standnes, Bergen, Norway). In the diagram, the region of G and E shows where the gel is formed and self-disperses, respectively.
Calculation of HLB Value
The hydrophilic–lipophilic balance (HLB) value is an indicator of whether a surfactant is lipophilic or hydrophilic. Since all kinds of surfactants are amphiphilic, the surfactant suitable for use should be selected. It is calculated through the formula below the HLB value and ranges from 1 to 20. The closer the value is to 20, the more hydrophilic it is26,27
Mh denotes the molecular weight of the hydrophilic group, and M denotes the total molecular weight.
Preparation of Oil-in-Water Emulsion
Five milliliters of emulsion, containing 4%, 8%, and 12% squalene were prepared with Smix (a mixture of two surfactants, Span® 85 and Kolliphor® RH40), squalene, and carboxymethyl cellulose (CMC) solution in a 20 mL glass vial. The mixing ratio of Smix was selected through the pseudo-ternary phase diagram (see “Construction of pseudo-ternary phase diagram”). Next, 9 mL of Smix was prepared for 15 emulsions by mixing with two surfactants (at a ratio (w/w) of 1:1 with the widest region forming emulsions) by stirring at RT, 200 rpm for 20 min. Subsequently, 0.2, 0.4, and 0.6 mL of squalene oil was added to 0.3, 0.6, and 0.9 mL of Smix for 4%, 8%, and 12% squalene-containing emulsions, respectively. To form an oil-in-water emulsion, 4.5, 4, and 3.5 mL of deionized water and carboxymethyl cellulose (CMC) solutions (medium viscosity and ultra-high viscosity) were added in a dropwise manner to the mixture of squalene and Smix. All experiments were conducted with stirring at room temperature.
Measurement of Droplet Size, Zeta Potential, and Polydispersity Index (PDI)
We assess the stability of colloidal dispersion over time. The droplet size, zeta potential, and PDI were measured by dynamic light scattering (DLS) using ELSZ-1000 (Otsuka Electronics, Osaka, Japan). Emulsion, a mixture of two phases, is turbid and so each sample was diluted with deionized water containing Smix for light scattering measurement at RT.
Measurement of Viscosity
The viscosity of all the prepared squalene-based oil-in-water emulsions was evaluated using vibrational viscometers. The samples were sent to DAEHYUNSCIENCE (Seoul, Korea) for viscosity measurement, and the device used was the SV-10 model (A&D Company, Tokyo, Japan). All samples were measured in duplicate at 21–23°C.
Acute Toxicity Test
To assess the toxicity of all prepared squalene-based oil-in-water emulsions, a total of 60 Balb/c male mice were randomly divided into 4 mice per group. Each mouse was administered 200 µL of squalene-based o/w emulsions into the abdominal cavity and monitored for 7 days. The temperature was maintained at 21–23°C and humidity at 45–55%, water, feed, and bedding were available ad libitum.
Expression and Purification of rVP1
Recombinant Enterovirus 71 virus particle 1 (rVP1, EU703814.1) was expressed and purified from E. coli, as previously described.28 rVP1 was cloned in the pET28b vector and transformed into BL21-CodonPlus (DE3)-RIL competent cells (Agilent Technologies, USA) for protein expression. rVP1 was expressed in LB medium with 250 μM IPTG, purified using Ni-NTA agarose (Qiagen, Germany) according to the manufacturer’s instructions, and dialyzed with phosphate-buffered saline (PBS). The size of rVP1 was confirmed using SDS-polyacrylamide gel electrophoresis (PAGE).
Mouse Immunization and Measurement of rVP1-Specific Antibody Titers
To determine the value of squalene-based oil in water emulsion as an adjuvant, antigens, and emulsions were mixed in a 1:1 v/v ratio and then injected into the abdominal cavity of the mouse. Fifteen groups were injected with prepared F1-F15, and the other three groups were injected with PBS, only rVP1 (10 µg) and Imject® alum, respectively. The group injected with Imject® was used as positive control and the groups injected with PBS and antigen only were used for negative control. One week after the first immunization, blood was collected from retro-orbital plexus, and then leave the blood at RT for 20 min. Centrifugation was performed at 2000 x g, 10 min, and 4°C to obtain serum. Two weeks after primary immunization, the same amount of adjuvant and antigen were injected into the abdominal cavity of immunized mice for boosting. Serum was collected from the abdominal aorta a week after the booster shot.
rVP1-specific antibody titers were evaluated using mouse serum ELISA. A 96-well plate was coated with rVP1 at a concentration of 0.2 µg/well using a coating buffer (carbonate/bicarbonate buffer, pH 9.6) and incubated overnight at 4°C. Next day, the carbonate/bicarbonate coating buffer was discarded. The uncoated rVP1 protein was washed out twice with PBS. The plate was then blocked by adding 3% bovine serum albumin (BSA) solution (in PBS, pH 7.4) into the antigen-coated wells at RT for 1 h. Sera collected from the mice of each group was used as a primary antibody, and serial dilution was performed in a ratio of 1:500, 1:5000, and 1:10000 or 1:50000 in 0.1% BSA solution to calculate the antibody titer. After incubating at RT for 2 h, plates were washed three times with 0.1% PBS-T (PBS with 0.1% Tween® 20) for 5 min. HRP conjugated Rabbit Anti-Mouse IgG H&L (100 µL, diluted at a ratio of 1:10000 to 0.1% BSA solution) was added. After washing three times, 100 µL of 3,3’,5,5’-tetramethylbenzidine (TMB) substrate was added to each well. Once the color changed sufficiently to indicate the occurrence of the reaction, 100 µL of 2 M H2SO4 was added to stop the reaction substrate and enzyme. The intensity of the color that appears in all the wells was measured at 450 nm using a microplate reader (BioTek, VT, USA).
Statistical Analysis
Data were analyzed using Student’s t-test and one-way analysis of variance (ANOVA) along with Dunnett’s test conducted with GraphPad Prism 7 (GraphPad Software, San Diego, CA, USA). Data are presented as mean ± standard error (SEM). A p-value less than 0.05 was considered to be statistically significant.
Results
Determination of Appropriate Mixing Ratio of Two Surfactants
Smix mixed in a ratio of 1:1, 1:2, and 1:4 of Span® 85 and Kolliphor® RH40 was prepared to identify a region in which emulsions were self-dispersed. Then, a pseudo-ternary phase diagram was constructed for each ratio (Figure 1). The E region was the largest in emulsions with a 1:1 mixture of Span® 85 and Kolliphor® RH40 (Figure 1A). The 1:2 and 1:4 ratio mixtures showed similar size of E region, whereas the 1:2 ratio mixture showed the largest of G region (Figure 1B and C).
 |
Figure 1 Pseudo-ternary phase diagrams for appropriate surfactants mixing ratio of Span® 85 and Kolliphor® RH40. Surfactants mixing ratios of (A) 1:1, (B) 1:2, and (C) 1:4. |
The HLB values of the 1:1, 1:2, and 1:4 ratio mixture of Span® 85 and Kolliphor® RH40 were 8.4, 10.5, and 12.36, respectively.
Preparation of Squalene-Based Oil-in-Water Emulsion
Squalene-based oil-in-water emulsions were prepared by SEEDS. A mixing ratio of 1:1 between the two surfactants (Span® 85 and Kolliphor® RH40) was selected for Smix through the pseudo-ternary phase diagram. Subsequently, squalene was added to the Smix to obtain final concentrations of 4%, 8%, and 12% and stirred sufficiently. Two types of 1% CMC solutions were prepared by dissolving medium and ultra-high viscosities of CMC powder in deionized water. Each CMC solution and deionized water were added slowly in a dropwise manner to form an emulsion with uniform droplet size. The compositions of squalene-based oil-in-water emulsions are presented in Table 1 and images of all formulated emulsions are shown in Figure 2.
 |
Table 1 Compositions of Squalene-Based Oil-in-Water Emulsions |
Assessment of Stability of Colloidal Dispersion Over Time
The droplet size, zeta potential, and PDI of all prepared squalene-based oil-in-water emulsions were measured by the DLS technique (Table 2). To assess the stability of the oil-in-water emulsion based on long-term storage, the droplet size, zeta potential, and PDI were measured again 4 weeks after the emulsions were prepared (Table 2). As a result, the droplet size range at week 0 was 358.57 ± 13.92–811.60 ± 23.45 nm, and that of the zeta potential was –27.19 ± 0.61 mV to –12.76 ± 0.56 mV. PDI values at week 0 ranged from 0.19 ± 0.02–0.38 ± 0.00, which is acceptable for pharmaceutical PDI as monodispersity.29 After 4 weeks, droplet size range was 322.1 ± 14.61–701.17 ± 20.15 nm, that of zeta potentials were –30.74 ± 0.41 mV to –10.56 ± 0.35 mV, and PDI values ranged from 0.29 ± 0.00–0.36 ± 0.01. Emulsions were stable for 4 weeks at RT storage.
 |
Table 2 Droplet Size, Zeta Potential, and Polydispersity Index (PDI) of Oil-in-Water Emulsions at 0 and 4 Weeks |
Viscosity Study
Viscosity measurements over time were conducted in duplicates except for two samples with extremely low viscosity (F1, F6). The viscosity of all emulsion samples is presented in Table 3.
 |
Table 3 Viscosity of Squalene-Based Oil-in-Water Emulsions |
Emulsions with high viscosity CMC were found to have higher viscosity than that of samples with medium viscosity CMC or without CMC. F15 emulsion with the highest percentages of squalene and ultra-high viscosity CMC showed the highest viscosity (24.63 mPa·s; Table 3).
Assessment of the Toxic Effects of Squalene-Based Oil-in-Water Emulsions
Approximately 200 µL of emulsions were injected into the mouse to evaluate the toxicity of the prepared squalene-based oil-in-water emulsions. All 60 Balb/c mice injected with emulsions of different compositions survived the experimental period (7 days). This is represented by Kaplan-Meier survival curve shown in Figure 3.
 |
Figure 3 Kaplan–Meier survival curve. For acute toxicity test, mice were intraperitoneally injected with squalene-based oil-in-water emulsions (F1–F15). All mice survived for 7 days. |
Mice Immunization and Assessment of Immune Response to rVP1
Sera collected from mice injected with antigen (rVP1) and prepared with squalene-based oil-in-water emulsions were used to assess antigen-specific antibody titers through indirect ELISA (Figures 4 and 5). Mice injected with PBS and only antigen were used as negative control, and the commercially available aluminum-based Imject® adjuvant was used as positive control. In case of primary mice immunization with all formulation groups, rVP1-specific antibody titers slightly increased at dilution ratios of 1:500, 1:5000, and 1:10000 compared to the PBS-injected group (Figure 4). However, there was no significant difference in the groups (F1–F15) at dilution ratios of 1:500, 1:5000, and 1:10000 compared to the Imject®-injected mice group (Figure 4). In case of the second mice immunization with all formulation groups, the absorbance of rVP1-specific antibody titers slightly increased at dilution ratios of 1:500, 1:5000, and 1:50000 compared to the group injected with PBS or antigen (Figure 5). However, there was no significant difference in F1–F14 groups at dilution ratios of 1:500, 1:5000, and 1:50000 compared to the Imject®-injected mice group (Figure 5A and B). rVP1-specific antibody titers of F15 (12% squalene-based oil-in-water emulsion with 0.5% ultra-high viscosity CMC) at dilution ratios of 1:5000 and 1:50000 were significantly different from those of the Imject®-injected mice group (Figure 5C).
Discussion
SEDDS formulations can be composed of simple binary systems: drug and lipophilic phase, or drug, lipophilic phase, and surfactant, which are isotropic mixtures of oils, surfactants, solvents, and cosolvents, which emulsify spontaneously to manufacture fine oil-in-water emulsions when mixed into aqueous phase under agitation.30–32 SEDDS has been used for designing formulations to improve the oral absorption of highly hydrophobic drugs.33,34 Here, we used SEDDS technique to formulate squalene-based oil-in-water emulsions as the adjuvant.
MF59®, a famous squalene-based adjuvant, is an oil-in-water emulsion containing squalene (4.3%) in citric acid buffer with stabilizing nonionic surfactants, Tween 80 (0.5%) and Span® 85 (0.5%). It has been approved for use in human influenza vaccines.35 Squalene-based oil-in-water emulsion has been extensively studied, as squalene induces immune response through CD4 memory T cell production.36 Therefore, we designed squalene-based oil-in-water emulsions containing increasing amounts of squalene (4% for F1–F5, 8% for F6–F10, and 12% for F11–F15) in deionized water with stabilizing non-ionic solubilizers and emulsifying agents, Span® 85 (3% for F1–F5, 6% for F6–F10, and 9% for F11–F15) and Kolliphor® RH40 (3% for F1–F5, 6% for F6–F10, and 9% for F11–F15). Further, 0.25% and 0.5% of medium or ultra-high viscosity of carboxy methyl cellulose (CMC) were added to increase the viscosities of oil-in-water emulsions (Table 1).
The phase changes observed according to the contents of squalene, Smix, and water are shown in the pseudo-ternary phase diagram (Figure 1). Smix with a mixing ratio of 1:1 (mixture of Span® 85 and Kolliphor® RH40) showed the widest emulsion region (E region), indicating this mixing ratio to be appropriate in preparing the squalene-based oil-in-water emulsion. An HLB value of 8.4 shows that a 1:1 ratio mixture was suitable as an oil-in-water emulsifier.37,38 Compositions of squalene-based oil-in-water emulsions are shown in Table 1. An adjuvant electrostatically binds to the antigen and slowly releases the antigen from the injection site. As a result, the antigen is continuously exposed to antigen presenting cells (APCs) at the injection site, inducing long-term immune responses. A sustained exposure of lymphoid tissues to vaccine antigens promotes humoral immunity.39 Therefore, we considered that increasing the viscosity of the adjuvant would slowly release antigens, facilitating continuous stimulation of APCs. In this study, we used CMC to increase the adjuvant viscosity. CMC is widely used as a viscosity enhancer in various industries, owing to its safety in humans.40 Moreover, it is known to stabilize emulsions. We used two types of CMC, with medium and ultra-high viscosity, to assess the effect of different adjuvant viscosities on immune responses (Tables 1 and 3). We added 0.25% and 0.5% of medium or ultra-high viscosity CMC for enhanced viscosity in squalene-based oil-in-water emulsions (Table 1). The high percentages of squalene and ultra-high viscosity CMC showed the highest viscosity (F15 = 24.63 mPa·s, Table 3).
The measured droplet size shows that the emulsion contained droplets (358.57–811.60 nm) of similar or slightly smaller size compared to other squalene-based oil-in-water emulsions41 (Table 2). In general, the droplet size of emulsion used in the pharmaceutical products ranges from 0.1 to 10 µm,15,42 suggesting that prepared emulsions (F1–F15) were of suitable sizes for use as an adjuvant. The zeta potential values are indicators of the stability of emulsions; here, the zeta potential values of F1, F6, and F11 (4, 8, 12% of squalene without CMC, respectively) tended to increase from –25.54 ± 0.12 to –13.00 ± 0.31, as the water content decreased and oil content increased (Table 2). Zeta potential changes for 4 weeks tended to decrease as the viscosity increased due to the contribution of CMC to the stability of emulsion (Table 3). The viscosity measured as part of the physical assessment was found to be higher in emulsions with ultra-high viscosity than in samples with medium viscosity CMC or without CMC (Table 3). A previous study reported the use of two types of non-ionic surfactants, Span® 80 and Cremophor® ELP, for 3%, 5%, and 10% of squalene-based oil-in-water emulsions as adjuvants for the delivery of a combination vaccine containing a porcine circovirus type 2 (PCV2) antigen and inactivated Mycoplasma hyopneumoniae (J101 strain) antigen.41 As a result, a particle size of up to 2.5 µm was obtained, and the zeta potential exhibited values ranging from –43 mV to +11 mV.41 Our emulsions (Span® 85, Kolliphor® RH40, and CMC) showed droplet sizes in the nano range and maintained zeta potential values between −30 mV to –10 mV for 4 weeks, indicating good physical stability as a vaccine adjuvant.
The toxic effect of squalene-based oil-in-water was evaluated via acute toxicity test. All mice groups injected with 200 µL of all 15 emulsions survived for 1 week without abnormality, suggesting non-toxicity of the formulations (Figure 3). Results of ELISA of serum collected from the first immunized mice, which were injected with antigen and formulated emulsions, revealed that emulsions of a particular composition induced a level of immune response similar to that of commercially available alum-based adjuvant, imject® (Figure 4A–C). There was no significant difference between the groups (F1–F15) injected with emulsions at all dilution ratios compared with the Imject®-injected mice group (Figure 4). Results of ELISA of serum collected from the second immunization at 2 weeks after the first immunization showed that the rVP1-specific immune responses significantly increased in F15 at a serum dilution ratio of 1:5000 and 1:50000 compared to the Imject®-injected mice group (Figure 5C). F15 is composed of 12% squalene-based oil-in-water emulsion with 0.5% ultra-high viscous CMC. The high percentage of squalene (12%) and high viscosity (24.63 mPa·s) may affect the effective immune induction to antigens as an adjuvant. Previous studies have reported that sustained exposure of lymphoid tissues to vaccine antigens is vital to promote humoral immunity.39 Therefore, several approaches using microneedle, osmotic pump, hydrogel depot, and nanocarrier have been used to achieve sustained release of antigens.39,43,44 Proof-of-concept studies evaluating sustained vaccine delivery using osmotic pumps have clearly demonstrated the benefits of prolonged exposure compared to bolus administration.45 Sustained release leads to more robust germinal center responses, higher antibody titers, and targeting of a more diverse set of epitopes than does bolus administration of the same vaccine.45,46 In this study, we designed a squalene-based oil-in-water emulsion containing CMC—for increasing the viscosity of emulsions—which enabled sustained antigen retention.
Conclusion
In this study, through the pseudo-ternary phase diagram, it was possible to obtain the appropriate ratio of mixture to prepare squalene-based emulsions. Squalene-based oil-in-water emulsion could be easily prepared using SEDDS. The evaluation of physical properties such as droplet size, zeta potential, and PDI demonstrated that the prepared emulsions were stable at room temperature for long periods and harmless to the body, based on the acute toxicity test. Oil-in-water emulsion containing of squalene could be used as adjuvants to induce immune responses.
Ethics Approval and Informed Consent
All animal experiments were conducted according to the Guide for the Care and Use of Laboratory Animals issued by the National Institute of Health and approved by the Animal Care and Use Committee of Ajou University (IACUC 2021-0011).
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. 2022R1A2C1004714). This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI17C0587, HV22C0222).
Disclosure
The authors declare no conflicts of interest.
References
1. Plotkin SA. Vaccines: past, present and future. Nat Med. 2005;11(4 Suppl):S5–S11. doi:10.1038/nm1209
2. Di Pasquale A, Preiss S, Tavares Da Silva F, Garcon N. Vaccine adjuvants: from 1920 to 2015 and beyond. Vaccines. 2015;3(2):320–343. doi:10.3390/vaccines3020320
3. Zepp F. Principles of vaccine design-Lessons from nature. Vaccine. 2010;28(Suppl 3):C14–C24. doi:10.1016/j.vaccine.2010.07.020
4. Dumpa N, Goel K, Guo Y, et al. Stability of vaccines. AAPS Pharm Sci Tech. 2019;20(2):42. doi:10.1208/s12249-018-1254-2
5. Brito LA, Malyala P, O’Hagan DT. Vaccine adjuvant formulations: a pharmaceutical perspective. Semin Immunol. 2013;25(2):130–145. doi:10.1016/j.smim.2013.05.007
6. Petrovsky N, Aguilar JC. Vaccine adjuvants: current state and future trends. Immunol Cell Biol. 2004;82(5):488–496. doi:10.1111/j.0818-9641.2004.01272.x
7. Shah RR, Hassett KJ, Brito LA. Overview of vaccine adjuvants: introduction, history, and current status. Methods Mol Biol. 2017;1494:1–13.
8. Perrie Y, Mohammed AR, Kirby DJ, McNeil SE, Bramwell VW. Vaccine adjuvant systems: enhancing the efficacy of sub-unit protein antigens. Int J Pharm. 2008;364(2):272–280. doi:10.1016/j.ijpharm.2008.04.036
9. O’Hagan DT, De Gregorio E. The path to a successful vaccine adjuvant–’the long and winding road’. Drug Discov Today. 2009;14(11–12):541–551. doi:10.1016/j.drudis.2009.02.009
10. Kool M, Fierens K, Lambrecht BN. Alum adjuvant: some of the tricks of the oldest adjuvant. J Med Microbiol. 2012;61(Pt 7):927–934. doi:10.1099/jmm.0.038943-0
11. Kale SN, Deore SL. Emulsion micro emulsion and nano emulsion: a review. Syst Rev Pharm. 2016;8(1):39–47. doi:10.5530/srp.2017.1.8
12. Wong SF, Lim JS, Dol SS. Crude oil emulsion: a review on formation, classification and stability of water-in-oil emulsions. J Petrol Sci Eng. 2015;135:498–504. doi:10.1016/j.petrol.2015.10.006
13. McClements DJ, Jafari SM. Improving emulsion formation, stability and performance using mixed emulsifiers: a review. Adv Colloid Interface Sci. 2018;251:55–79. doi:10.1016/j.cis.2017.12.001
14. Azeem A, Rizwan M, Ahmad FJ, et al. Nanoemulsion components screening and selection: a technical note. AAPS Pharm Sci Tech. 2009;10(1):69–76. doi:10.1208/s12249-008-9178-x
15. Barkat AK, Naveed A, Haji MSK, et al. Basics of pharmaceutical emulsions: a review. Afr J Pharm Pharmacol. 2011;5:25. doi:10.5897/AJPP11.698
16. Gutiérrez JM, González C, Maestro A, Solè I, Pey C, Nolla J. Nano-emulsions: New applications and optimization of their preparation. Curr Opin Colloid Interface Sci. 2008;13(4):245–251. doi:10.1016/j.cocis.2008.01.005
17. Chahar P, Cummings III KC . Liposomal bupivacaine: a review of a new bupivacaine formulation. J Pain Res. 2012;5:257–264. doi:10.2147/JPR.S27894
18. Eren Cevik S, Tasyurek T, Guneysel O. Intralipid emulsion treatment as an antidote in lipophilic drug intoxications. Am J Emerg Med. 2014;32(9):1103–1108. doi:10.1016/j.ajem.2014.05.019
19. O’Hagan DT. MF59 is a safe and potent vaccine adjuvant that enhances protection against influenza virus infection. Expert Rev Vaccines. 2007;6(5):699–710. doi:10.1586/14760584.6.5.699
20. El Sahly H. MF59™ as a vaccine adjuvant: a review of safety and immunogenicity. Expert Rev Vaccines. 2010;9(10):1135–1141. doi:10.1586/erv.10.111
21. Schultze V, D’Agosto V, Wack A, Novicki D, Zorn J, Hennig R. Safety of MF59 adjuvant. Vaccine. 2008;26(26):3209–3222. doi:10.1016/j.vaccine.2008.03.093
22. Wack A, Baudner BC, Hilbert AK, et al. Combination adjuvants for the induction of potent, long-lasting antibody and T-cell responses to influenza vaccine in mice. Vaccine. 2008;26(4):552–561. doi:10.1016/j.vaccine.2007.11.054
23. Schultz S, Wagner G, Urban K, Ulrich J. High‐pressure homogenization as a process for emulsion formation. Chem Eng Technol. 2004;27(4):361–368. doi:10.1002/ceat.200406111
24. Pouton CW. Formulation of self-emulsifying drug delivery systems. Adv Drug Deliv Rev. 1997;25(1):47–58. doi:10.1016/S0169-409X(96)00490-5
25. Gursoy RN, Benita S. Self-emulsifying drug delivery systems (SEDDS) for improved oral delivery of lipophilic drugs. Biomed Pharmacother. 2004;58(3):173–182. doi:10.1016/j.biopha.2004.02.001
26. Boyd J, Parkinson C, Sherman P. Factors affecting emulsion stability, and the HLB concept. J Colloid Interface Sci. 1972;41(2):359–370. doi:10.1016/0021-9797(72)90122-1
27. Pasquali RC, Taurozzi MP, Bregni C. Some considerations about the hydrophilic-lipophilic balance system. Int J Pharm. 2008;356(1–2):44–51. doi:10.1016/j.ijpharm.2007.12.034
28. Kim YG, Lee Y, Kim JH, et al. Self-assembled multi-epitope peptide amphiphiles enhance the immune response against enterovirus 71. Nanomaterials. 2020;10(12):2342. doi:10.3390/nano10122342
29. Danaei M, Dehghankhold M, Ataei S, et al. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics. 2018;10(2):57. doi:10.3390/pharmaceutics10020057
30. Gao P, Jiang Z, Luo Q, Mu C, Cui M, Preparation YX. Evaluation of Self-emulsifying Drug Delivery System (SEDDS) of cepharanthine. AAPS Pharm Sci Tech. 2021;22(7):245. doi:10.1208/s12249-021-02085-9
31. Nigade PM, Patil SL, Tiwari SS. Self emulsifying drug delivery system (SEDDS): a review. Int J Pharm Biol Sci. 2012;2(2):42–52.
32. Shah NH, Carvajal MT, Patel CI, Infeld MH, Malick AW. Self-emulsifying drug delivery systems (SEDDS) with polyglycolyzed glycerides for improving in vitro dissolution and oral absorption of lipophilic drugs. Int J Pharm. 1994;106(1):15–23. doi:10.1016/0378-5173(94)90271-2
33. Salawi A. Self-emulsifying drug delivery systems: a novel approach to deliver drugs. Drug Deliv. 2022;29(1):1811–1823. doi:10.1080/10717544.2022.2083724
34. Gao P, Rush BD, Pfund WP, et al. Development of a supersaturable SEDDS (S-SEDDS) formulation of paclitaxel with improved oral bioavailability. J Pharm Sci. 2003;92(12):2386–2398. doi:10.1002/jps.10511
35. Ko EJ, Kang SM. Immunology and efficacy of MF59-adjuvanted vaccines. Hum Vaccin Immunother. 2018;14(12):3041–3045. doi:10.1080/21645515.2018.1495301
36. Lou-Bonafonte JM, Martinez-Beamonte R, Sanclemente T, et al. Current insights into the biological action of squalene. Mol Nutr Food Res. 2018;62(15):e1800136. doi:10.1002/mnfr.201800136
37. Yoshioka T, Sternberg B, Florence AT. Preparation and properties of vesicles (niosomes) of sorbitan monoesters (Span 20, 40, 60 and 80) and a sorbitan triester (Span 85). Int J Pharm. 1994;105(1):1–6. doi:10.1016/0378-5173(94)90228-3
38. Tran T, Rades T, Mullertz A. Formulation of self-nanoemulsifying drug delivery systems containing monoacyl phosphatidylcholine and Kolliphor((R)) RH40 using experimental design. Asian J Pharm Sci. 2018;13(6):536–545. doi:10.1016/j.ajps.2017.09.006
39. Boopathy AV, Mandal A, Kulp DW, et al. Enhancing humoral immunity via sustained-release implantable microneedle patch vaccination. Proc Natl Acad Sci U S A. 2019;116(33):16473–16478. doi:10.1073/pnas.1902179116
40. Feddersen RL, Thorp SN. Chapter 20 - Sodium carboxymethylcellulose. In: Whistler RL, Bemiller JN, editors. Industrial Gums.
41. Bastola R, Seo JE, Keum T, et al. Preparation of squalene oil-based emulsion adjuvants employing a self-emulsifying drug delivery system and assessment of mycoplasma hyopneumoniae-specific antibody titers in BALB/c mice. Pharmaceutics. 2019;11(12):667. doi:10.3390/pharmaceutics11120667
42. Liu B, Hu X. Hollow Micro- and Nanomaterials: Synthesis and Applications. Advanced Nanomaterials for Pollutant Sensing and Environmental Catalysis. Elsevier; 2020:1–38. doi:10.1016/B978-0-12-814796-2.00001-0
43. An W, Defaus S, Andreu D, In Vivo R-GP. Sustained release of peptide vaccine mediated by dendritic mesoporous silica nanocarriers. Front Immunol. 2021;12:684612. doi:10.3389/fimmu.2021.684612
44. Luzuriaga MA, Shahrivarkevishahi A, Herbert FC, Wijesundara YH, Gassensmith JJ. Biomaterials and nanomaterials for sustained release vaccine delivery. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2021;13(6):e1735. doi:10.1002/wnan.1735
45. Cirelli KM, Carnathan DG, Nogal B, et al. Slow delivery immunization enhances HIV neutralizing antibody and germinal center responses via modulation of immunodominance. Cell. 2019;177(5):1153–1171 e1128. doi:10.1016/j.cell.2019.04.012
46. Roth GA, Picece V, Ou BS, Luo W, Pulendran B, Appel EA. Designing spatial and temporal control of vaccine responses. Nat Rev Mater. 2022;7(3):174–195. doi:10.1038/s41578-021-00372-2
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.