Back to Journals » Infection and Drug Resistance » Volume 15
Development of Highly Sensitive Sandwich ELISA for the Early-Phase Diagnosis of Chikungunya Virus Utilizing rE2-E1 Protein
Authors Islamuddin M, Ali A, Khan WH , Ali A, Hasan SK, Abdullah M, Kato K, Abdin MZ, Parveen S
Received 3 November 2021
Accepted for publication 3 June 2022
Published 28 July 2022 Volume 2022:15 Pages 4065—4078
DOI https://doi.org/10.2147/IDR.S347545
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Mohammad Islamuddin,1,2 Abuzer Ali,3 Wajihul Hasan Khan,4 Amena Ali,5 Syed Kazim Hasan,1 Mohd Abdullah,6 Kentaro Kato,2 Malik Zainul Abdin,7 Shama Parveen1
1Molecular Virology Laboratory, Centre for Interdisciplinary Research in Basic Sciences, Jamia Millia Islamia, New Delhi, 110025, India; 2Laboratory of Sustainable Animal Environment, Graduate School of Agricultural Science, Tohoku University, Miyagi, Japan; 3Department of Pharmacognosy, College of Pharmacy, Taif University, Taif, 21944, Saudi Arabia; 4Molecular Virology Lab, Department of Microbiology, All India Institute of Medical Sciences (AIIMS), New Delhi 110029, India; 5Department of Pharmaceutical Chemistry, College of Pharmacy, Taif University, Taif, 21944, Saudi Arabia; 6Microbiology Laboratory, Ansari Health Center, Jamia Millia Islamia, New Delhi 110025, India; 7Department of Biotechnology, School of Chemical
and Life Sciences, Hamdard University, New Delhi 110026, India
Correspondence: Mohammad Islamuddin; Shama Parveen, Email [email protected]; [email protected]
Introduction: Chikungunya is caused by an alpha virus transmitted to humans by an infected mosquito. Infection is generally considered to be self-limiting and non-critical. Chikungunya infection may be diagnosed by severe joint pain with fever, but it is difficult to diagnose because the symptoms of chikungunya are common to many pathogens, including dengue fever. Diagnosis mainly depends on viral culture, reverse transcriptase polymerase chain reaction (RT-PCR), and IgM ELISA. Early and accurate diagnosis of the virus can be achieved by the application of PCR methods, but the high cost and the need for a thermal cycler restrict the use of such methods. On the other hand, antibody-based IgM ELISA is considered to be inexpensive, but antibodies against chikungunya virus (CHIKV) only develop after 4 days of infection, so it has limited application in the earlier diagnosis of viral infection and the management of patients. Because of these challenges, a simple antigen-based sensitive, specific, and rapid detection method is required for the early and accurate clinical diagnosis of chikungunya.
Methods: The amino acid sequence of CHIKV ectodomain E1 and E2 proteins was analyzed using bioinformatics tools to determine the antigenic residues, particularly the B-cell epitopes and their characteristics. Recombinant E2-E1 CHIKV antigen was used for the development of polyclonal antibodies in hamsters and IgG was purified. Serological tests of 96 CHIKV patients were conducted by antigen-capture ELISA using primary antibodies raised against rCHIKV E2-E1 in hamsters and human anti-CHIKV antibodies.
Results: We observed high specificity and sensitivity, of 100% and 95.8%, respectively, and these values demonstrate the efficiency of the test as a clinical diagnostic tool. There was no cross-reactivity with samples taken from dengue patients.
Discussion: Our simple and sensitive sandwich ELISA for the early-phase detection of CHIKV infection may be used to improve the diagnosis of chikungunya.
Keywords: chikungunya, sandwich ELISA, recombinant E2-E1, B-cell epitopes, IgG, hamster antibodies
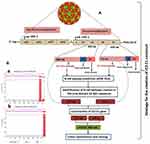
Graphical Abstract:
Introduction
Chikungunya fever is a mosquito-transmitted viral illness of humans that has been identified in various parts of Africa, Americas, the western Pacific, Southeast Asia, and India.1 It is an enveloped, plus-sense, single-stranded RNA virus, generally measuring 11.7 kb in length.2 CHIKV is a vector-borne virus belonging to the genus Alphavirus and family Togaviridae, consisting of 30 species which can be antigenically categorized into seven complexes based on the E1 and E2 enveloped glycoproteins. Of the two enveloped glycoproteins of alphavirus, E1 and E2, E1 protein accommodates highly conserved cross-reactive epitopes and E2 structural protein is the point of neutralizing epitopes. Genetic studies on the gene sequence of E1 have revealed that it has emerged as three different genotypes: Asian, East/Central/South African (ECSA), and West African strains.3–5 Chikungunya fever is mainly treated as self-limiting and non-lethal, but in recent outbreaks hemorrhagic and neurological complications have been observed. The signs and symptoms of CHIKV overlap with dengue virus infection, suggesting the need for a differential laboratory diagnosis in India, where both viruses are endemic;6 it is difficult to diagnose CHIKV infection in the initial stages because of the resemblance of chikungunya fever to dengue fever. Despite knowing that the early diagnosis of chikungunya and dengue infections is important, in resource-limited set-ups, the sophisticated laboratory diagnostic assays needed to differentiate between CHIKV and dengue virus infections may be absent and/or costly, necessitating epidemiological, sign of illness, and symptom-based methods for diagnosis. Various approaches are used to test CHIKV infection, including reverse transcriptase polymerase chain reaction (RT-PCR),7–11 real-time RT-PCR,12–15 reverse transcriptase loop-mediated isothermal amplification (RT-LAMP),16 and enzyme-linked immunosorbent assay (ELISA) (IgM/IgG antibodies).17,18 RT-PCR is often used to detect the viral genome, which is a gold standard for confirmed CHIKV infection;19 however, in resource-limited settings, PCR is often not easily available. One serologic testing method is the indirect fluorescent antibody (IFA) technique. Although IFA tests have good sensitivity and specificity for CHIKV, this method requires specific material that may not be available in diagnostic laboratories worldwide. Virus culture and isolation can be carried out for the actual diagnosis of CHIKV infection and/or to explain the immune response; however, these techniques are not broadly performed in medical clinics and hospitals since they require specific equipment and trained, skilled laboratory workers. The anti-CHIKV immunoglobulin M (IgM)-capture ELISA kit has been widely utilized to help in the clinical evaluation of patients with uncertain CHIKV infection.20 The majority of patient antibodies are detectable 3–5 days after the onset of illness, toward the end of the intense period of infection, which limits the sensitivity of IgM detection kits.21 Serology based on diagnosis is used to confirm the infection, but paired sera are required to prove the increase in the specific antibody titer in convalescent serum. Recombinant E1 and E2 protein-based ELISAs and immune chromatography assays (ICAs) exhibited sensitivities of 77.5% and 100% and specificities of 95% and 100%, respectively,22,23 but were not very successful. A monoclonal antibody identified in the serum of patients against the capsid protein of CHIKV was found to be IgM based.24 The detection of antibodies or viral antigen depends on various techniques, including ELISA, immunofluorescence assay (IFA), and lateral flow immunochromatographic assay (LFCA), and an important factor in these diagnostic approaches is a robust and accurate reaction between the viral antigen and the antibody. Although various protein-based diagnostic tools are ready for use to detect CHIKV infection, recent CHIKV diagnostic techniques are limited by low sensitivity.25,26 As a result, we need more sensitive methods to improve the diagnosis of chikungunya. Consequently, the advancement of rapid and reliable unique antigen-based diagnostic research is of essential significance for clinical care (ie early diagnosis of severe infection, case validation, and differential diagnosis from other viral infections). As of 2016, in India (Delhi), early diagnosis has been prolonged, mainly because of the unavailability of correct diagnostics. Therefore, in this study we attempted to detect immunogenic features of the recombinant E2-E1 chimeric antigen and to establish a sandwich ELISA using anti-rE2-E1 hamster polyclonal antibody for the early detection of CHIKV infection.
A comprehensive immuno-informatics analysis of the B-cell epitopes of CHIKV E2 and E1 proteins was carried out to identify the high-scoring epitopes on the ectodomain region of E2 and E1 proteins. Ectodomain recombinant chimeric E2-E1 protein was generated and hamster antibodies were developed and purified against recombinant E2-E1 chimeric antigen. Antigen-capture ELISA was performed in the serum samples of chikunguya patients using anti-rE2-E1 chimeric antigen anti-CHIKV hamster antibodies. The detection of CHIKV viral infection can be very efficient in the early as well as subclinical stages of the disease.
Methods
Study Samples
In total, 96 CHIKV-positive, 36 CHIKV-negative, and 22 dengue-positive serum samples were collected from the outpatient department of Dr. M.A. Ansari Health Centre, Jamia Millia Islamia, New Delhi, India, in 2016. Written informed consent was obtained from the enrolled patients before the sample collection. CHIKV positivity was confirmed by the CHIKV IgM rapid test and PCR for the E1 gene, and negativity for dengue by NS1 and PCR for the CprM region. Dengue-positive serum samples were confirmed by NS1 and PCR for the CprM region, and negativity for CHIKV was confirmed by the IgM rapid test and PCR for the E1 gene. The circulating strain of CHIKV in Delhi during 2016 was the ECSA genotype.
Chikungunya Viral Strain and Selection of Ectodomain Region of E2 and E1 Viral Protein
The currently circulating chikungunya E2 and E1 full-length gene sequences from India were obtained in FASTA format from the NCBI database. The accession number for the full genome sequence is KX619424. The sequence of E2 and E1 was identified by an online tool of TMHMM server v.2.0 (http://www.cbs.dtu.dk/services/TMHMM/). The ectodomain region of the E2 and E1 proteins of CHIKV was identified and chosen for antigenic study. The number of epitopes and their potential score in the ectodomain sequence of E2 and E1 proteins were also utilized for B-cell prediction, using an online prediction server. For sequence optimization and construct generation, the region of the ectodomain sequence with the greatest number of epitopes with a potential score was selected.
B-Cell Epitope Prediction of rE2-E1 Protein of Chikungunya Virus
B-cell epitope prediction methods from the Immune Epitope Database (IEDB) were applied to identify the location of linear B-cell epitopes in the rE2-E1 sequence. The essential criteria for predicting B-cell epitopes are antigenicity, hydrophilicity, flexibility, surface accessibility, and linear epitope predictions.27 To examine the antigenicity, surface accessibility, flexibility, hydrophilicity, and linear epitope predictions of the highest antigenic part of the sequence, we used the Kolaskar and Tongaonkar antigenicity scale,28 Karplus and Schulz flexibility prediction,29 Parker hydrophilicity predictor,30 and Emini surface accessibility prediction.31 Because many laboratory experiments have found that a protein’s antigenic component is located in the β-turn region, Chou and Fasman’s β-turn prediction technique32 was utilized to locate these regions in the sequence.
Optimization and Custom Synthesis of rE2-E1 Genes
The rE2-E1 genes were commercially synthesized following a codon optimization process based on the prokaryotic host. The optimized genes were synthesized commercially by GenScript, USA, with BamHI and XhoI restriction sites to clone into the pET28a vector. The synthetic constructs were further confirmed by double digestion and colony PCR.33
Cloning of Designed Construct
The pET28a vector was used to clone the synthesized nucleic acid construct of rE2-E1. The E. coli DH5 strain was utilized for transformation and clone replication. The transformed colonies were identified by screening the colonies on Luria Bertani (LB) agar medium with a kanamycin concentration of 30 µg/mL.33
Expression and Purification of rE2-E1 Protein of Chikungunya Virus
For recombinant protein synthesis, the expression clone of rE2-E1 was transformed into E. coli BL21 (DE3) bacterial expression host cells. IPTG (isopropyl-D-thiogalactopyranoside) was added at a concentration of 0.5 mM and incubated for 4 hours at 37°C with shaking at 250 rpm to induce the expression of rE2-E1. The harvested induced bacterial culture was resuspended in a lysis buffer. His-tagged rE2-E1 was allowed to bind with Ni-NTA (Qiagen, Hilden, Germany) for 1.5 hours at room temperature under slow shaking. The lysate–resin mixture was put onto a 20 mL purification column, which was then washed with a pH 6.5 urea buffer. Finally, urea buffer pH 4 was used to elute the protein content. Coomassie Blue-stained 12% SDS-PAGE was used to assess the quality and amount of isolated recombinant E2-E1 protein.34,35
SDS–PAGE and Western Blotting
SDS–PAGE was carried out in denaturing conditions with 12% acrylamide gels. Protein samples were combined with 4× SDS loading buffer (1 M Tris–HCl, pH 6.8, 8% SDS, 0.4 M dithiothreitol, 40% glycerol, 0.8% bromophenol blue) and boiled for 10 minutes at 98°C before separating on 12% SDS–PAGE for Western blots. Nitrocellulose membranes were used to transfer the proteins (Whatman, Kent, UK). The membranes were incubated with an anti-6xHis tag mouse monoclonal antibody (Invitrogen, Carlsbad, CA, USA), followed by horseradish peroxidase (HRP)-conjugated AffiniPure goat anti-mouse IgG, after which they were blocked with 5% non-fat milk at room temperature for 30 minutes (Beijing Dingguo, Beijing, China). The immunological response was then identified using luminol (5-amino-2,3-dihydrophthalazine-1,4-dione).36
Immunization and Production of Anti-rE2-E1 Antibody
Seven-to-eight-week-old golden Syrian hamsters were immunized subcutaneously with 100 g of pure rE2-E1 immunogen (antigen) in 100 mL of PBS mixed with an equivalent amount of Freund’s adjuvant (Beijing Dingguo). Three booster doses with rE2-E1 antigen and Freund’s incomplete adjuvant were administered at 10-day intervals. An ELISA was used to assess the antibody titer 10 days following each booster dose. When the titer value reached an adequate level, the hamsters were killed by administration of an analgesic and the total blood was collected. Ammonium sulfate precipitation and protein A affinity column chromatography were used to purify the anti-rE2-E1 antibody.
Western Blot Analysis
The rE2-E1 protein was electrophoretically transferred onto a nitrocellulose membrane after being resolved by SDS-PAGE.10 The membrane was blocked overnight with 5% BSA before being incubated overnight at 4°C with a 1:2000 dilution of rE2-E1 hamster polyclonal antibody. The membrane was thoroughly washed in TBS containing 0.05% Tween-20 before being incubated for 1 hour at room temperature with HRP-conjugated goat anti-mouse IgG antibodies (Genei, Bangalore, India) at a 1:10,000 dilution. After that, the blot was stained for 2–5 minutes with a precipitated 3,3′,5,5′-tetramethylbenzidine solution containing H2O2 (Genei, Bangalore). The membrane was thoroughly rinsed with distilled water after immunostaining, and the response pattern was promptly recorded.37
Purification of Anti-CHIKV Human IgG and Anti-rE2-E1 Hamster IgG Antibody
Anti-CHIKV IgG and anti-rE2-E1 hamster IgG polyclonal antibody was collected from the serum of chikungunya patients and from immunized hamsters, respectively, with a high antibody titer as measured by an indirect ELISA. After being precipitated with 40% ammonium sulfate, serum was dialyzed against 0.01 M sodium phosphate pH 8.0. DEAE cellulose column chromatography was used to fractionate the dialyzed sera, and the protein was eluted with 0.01 M sodium phosphate with increasing concentrations of NaCl (0.2–0.6 M NaCl).38
ELISA for Optimization of Anti-rE2-E1 Hamster IgG Antibody
The purified rE2-E1 protein was coated (0.25 μg/well) on a 96-microtiter ELISA plate (Nunc, Thermo Fisher Scientific) overnight at 4°C. The unbound protein was removed by washing the microtiter plate three times with PBS containing 0.05% Tween-20 (PBS-T), blocked with PBS (pH 7.4) containing 5% BSA overnight at 4°C, and washed once more with PBS-T. Purified anti-rE2-E1 hamster IgG antibody was diluted (1:10 to 100, 500, 1000, 2000, 5000, 10,000, 20,000, 50,000, 100,000, and 200,000-fold) and 100 μL of the diluted antibodies was allowed to interact with the coated rE2-E1 protein in the ELISA wells at 37°C for 3 hours. The wells were washed with PBS-T buffer three times. The microtiter plate was incubated for 30 minutes at 37°C with 100 µL of 1:10,000 diluted anti-hamster HRP (Genei, Bangalore), followed by 2 minutes at room temperature with 100 µL of 3,3′,5,5′-tetramethylbenzidine (TMB). The reaction was terminated by adding 100 µL of 1 N H2SO4 to the solution. The optical density (OD) of the mixture was read at 450 nm.39
Development of Antigen-Capture ELISA
A 96-well microtiter plate was covered with a purified human anti-CHIKV IgG antibody in PBS buffer (1:2000-fold dilution) and incubated at 4°C overnight. The microtiter plate was washed three times with PBS-T, blocked with PBS (pH 7.4) containing 5% BSA overnight at 4°C, and washed once more with PBS-T. After that, 100 µL of the CHIKV-positive, CHIKV-negative, and dengue-positive serum sample was added and incubated for 2 hours at 37°C. The samples were washed and treated with 100 µL of a 1:2000 dilution of purified rE2-E1 hamster IgG antibody for 3 hours at 37°C. The microtiter plate was washed and incubated for 30 minutes at 37°C with 100 µL of 1:10,000 diluted anti-hamster HRP (Genei, Bangalore), followed by 2 minutes at room temperature with 100 µL of TMB. The reaction was terminated by adding 100 µL of 1 N H2SO4 to the solution. The OD of the mixture was read at 450 nm.39
Statistical Analysis
Antigen-capture ELISA was used to test 96 CHIKV-positive, 36 CHIKV-negative, and 22 dengue-positive serum samples for CHIKV positivity, and the data were evaluated statistically using the t-test in GraphPad Prism software. The level of significance was determined at a 1% level.
Results
Evaluation of Ectodomain Amino Acid Sequence of E2 and E1
TMHMM server v.2.0 software was used to identify the E2 and E1 ectodomain sequences. Out of 423 amino acids, we found 350 amino acid sequences constituting the ectodomain part of CHIKV E2 protein (Figure 1A), whereas CHIKV E1 ectodomain comprised 401 amino acids (Figure 1).
rE2-E1 Gene Sequence Alignment for Non-Optimized Genes (NOG) and Codon-Optimized Genes (COG) of CHIKV
In the bacterial host system, expressing viral membrane proteins is challenging. Most viral membrane proteins have been shown to be toxic to the bacterial expression system.40,41 The challenges associated with expression in frequently used bacterial expression strains under conventional culture conditions are exacerbated by leaky expression of toxic genes.40 In addition, the unstable nature of inserts may have an impact on the expression levels of proteins. Limited expression in E. coli is possibly due to the distinction in heterologous gene expression. This is mainly due to their codon usage for bacterial tRNA bias, high AT content, or RNA secondary structure. With these considerations, we attempted rE2-E1 ectodomain gene optimization based on the bacterial cell strain (Figure 2). The gene was chemically produced after codon optimization, abiding by the codon preferences of E. coli as a primary host. Thus, the sequences for the ectodomain part of the rE2-E1 gene were optimized according to the prokaryotic expression host system, following a sequence optimization strategy to change substandard codon usage for bacterial tRNA preference, to enhance the secondary mRNA structure, and to delete the AT-rich region for the maximal expression of the proteins. Thus, the gene frame was changed under the same protein sequence for optimal expression. The optimized gene for ectodomain rE2-E1 of the CHIKV sequence was synthesized by commercial means (GenScript, USA) and inserted in the pET28a vector. Clones were confirmed by the DNA sequencing method.
B-Cell Epitope Prediction
The optimized amino acid sequence of rE2-E1 was assessed for recognition of features such as antigenicity, accessibility, hydrophilicity, polarity, and flexibility. B-cell epitopes have various features that are essential for successful identification through B cells. These characteristics include antigenicity, surface accessibility, hydrophilicity, and β-turn prediction. The proteins of interest were scanned to recognize B-cell epitopes through several web-based designed tools accessible in the IEDB. After cross processing all the data derived from the previous B cell epitope prediction tools, the amino acids sequence of rE2-E1 was found to be the most suitable domain for inducing B cell response (Figure 3). The physiochemical features and structural properties of the rE2-E1 protein assist the appearance of B-cell epitope in the recombinant E2-E1 antigen.
Cloning, Expression, and Purification of rE2-E1
Cloning was successfully performed into the pET28a vector with codon-optimized genes. For the transformation and propagation, E. coli strain DH5α was used, and the recombinant pET-28a vector harboring the E2-E1 protein gene was verified by restriction digestion methods. The transformation was done into E. coli strain BL21 (DE3) and the clone was confirmed through isolation of the plasmid and restriction digestion (Figure 4A). The E. coli strain BL21 (DE3) was developed in LB medium with different concentrations of IPTG to obtain the optimum expression level of rE2-E1 protein at 37°C. A significant level of protein expression was achieved at 0.5 mM concentration with a molecular weight of approximately 51 kDa (rE2-E1) (Figure 4B, lane 3). Purification of rE2-E1 was carried out using an Ni-NTA affinity chromatograph, under denaturing conditions with increasing concentrations of imidazole, such as 50, 100, 200, and 300 mM. The results of SDS-PAGE demonstrate that the protein of interest, rE2-E1, was eluted in significant quantities at an imidazole concentration of 200 mM (Figure 4D, lane 4).
Western Blotting for Confirmation of Expression and Purification
The 51 kDa recombinant rE2-E1 protein was induced during the exponential phase growth by utilizing 0.5 mM IPTG for 5 hours at 37°C. A sharp condensed and clear band appeared after induction, which corresponded to 51 kDa on SDS-PAGE, as shown in lane 3 of Figure 4B. After IPTG induction, the purified rE2-E1 protein showed a single clear band corresponding to 51 kDa without any impurities, as observed in lane 4 of Figure 4D. In order to validate that the 51 kDa band appearing on the SDS-PAGE gel was recombinant rE2-E1 protein, a Western blot was carried out utilizing the anti-His tag antibody. A 51 kDa band was detected that corresponded to the His tagged-rE2-E1 fusion protein (Figure 4E), signifying the successful cloning, expression, and purification of recombinant rE2-E1 protein (Figure 4C and E).
Anti-rE2-E1 Hamster IgG Antibody Optimization
Antibodies produced against rE2-E1 antigen from the sera of hamster blood were purified using affinity column chromatography. SDS-PAGE was performed for the analysis of the purified IgG, and a prominent band appeared on the gel corresponding to the expected protein. The optimum concentration of purified hamster IgG antibody produced against rE2-E1 was determined by indirect ELISA utilizing recombinant rE2-E1 antigen (0.25 μg/well) and serially diluted purified IgG antibody (1:100 to 1:20,000) (Figure 5A). The findings of the assay showed that the ideal concentration of the IgG antibody is 1:2000 dilution. So, 1:2000-fold IgG antibody dilution was utilized for further studies.
Determination of Antigen-Capture Sandwich ELISA in CHIKV-Infected Patient Serum Samples
Antigen (rE2-E1)-capture or sandwich ELISA was developed utilizing anti-rE2-E1 hamster antibody for the early diagnosis of chikungunya. For the evaluation in this study, 96 CHIKV-positive serum samples were used, 36 serum samples were collected from healthy donors as negative controls, and 22 dengue-positive serum samples were used to check the cross-reactivity. Assays were performed in triplicate on each individual sample, and the average of the three assays was calculated. The mean absorbance values, in terms of the OD of the CHIKV-positive and CHIKV-negative serum samples, were found to be 0.713 and 0.128, respectively (Figure 5B). On the basis of these OD values and by the application of ROC curve analysis, the cut-off value was calculated and was found to be 0.275. A total of 92 out of 96 serum samples of CHIKV patients were found to be positive when incubated with anti-rE2-E1 hamster antibody. All of the control serum samples from the healthy donors were found to be negative with respect to the OD cut-off value. Among negative and positive serum samples, the OD value was found to be significant at the 1% level. The sensitivity of anti-rE2-E1 hamster antibody for capturing CHIKV and/or antigen was 95.8%, and the specificity was found to be 100% when utilized with normal control samples from the healthy donors.
Discussion
At present, clinical diagnosis is difficult during the early phase of CHIKV infection, as the clinical symptoms are similar among the mosquito-transmitted viruses.42,43 Thus, molecular diagnostic methods are important to accurately diagnose the infection, as CHIKV belongs to the genus Alphavirus of the Togaviridae family, while dengue virus, along with Japanese encephalitis virus and zika virus, belongs to the genus Flavivirus of the Flaviviridae family.44–46 Clinically, CHIKV and dengue virus both induce febrile illness, but dengue virus infection has a worse prognosis. Infection outbreaks caused by chikungunya and dengue viruses often take place in the same geographical region and with the same vectors, the majority of which are Aedes aegypti. As a result, laboratory confirmation of CHIKV and dengue virus infection is required to initiate control measures in the event of an epidemic or endemic outbreak. In India, the CHIKV epidemic affected approximately 1.3 million individuals in 13 states,47 prompting public health and administrative officials to be concerned about how to handle the issue.48 Owing to the lack of antiviral treatments and vaccines for CHIKV, a specific detection method is urgently required. Several methods for detecting chikungunya and dengue viruses have been described, including immunofluorescence technology, plaque assay, viral isolation, and multiplex RT-PCR;49 however, the difficulty of carrying out these procedures has led to the use of more practical methods, such as ELISA and RT-PCR.50 For CHIKV detection, the RT-PCR test is extremely specific and sensitive, but the chemicals and equipment are too expensive for general use.51 The use of antigen ELISA and lateral flow tests has been reported for the detection of CHIKV E1 and E2 antigens,52 but these methods have several limitations. The detection of serum IgM in CHIKV-infected patients is a valuable diagnostic tool since it may be identified as soon as 5–7 days after onset of fever and lasts for many weeks.53 Previously, the entire virus was employed as an antigen to detect IgM in the patient’s serum; however, it can present a biohazard danger. Live virus culture requires a BSL3 laboratory and high levels of competence. As a result, rather than using the entire live virus for CHIKV diagnostics, recombinant DNA technology is now utilized to generate a particular protein. Given the lack of an antigen detection method for CHIKV and the impediments of commercially accessible antibody detection techniques, the antibody sandwich ELISA test described in this research offers a wide range of applications in clinical CHIKV diagnosis. The CHIKV spike is mainly made up of triplets of enveloped glycoproteins E2 and E1, which wrap the viral surface as membrane-embedded types. The spike proteins of the virus help the virus adhere to the surface of host cells and enter the cytoplasm of the host cells. During viral infection, E1 glycoprotein is a type II fusion protein, which helps in membrane fusion, and E2 is a type I enveloped glycoprotein, which has been linked to receptor binding throughout the CHIKV life cycle.54,55
B-cell epitopes are protein antigenic areas identified by immunoglobulin molecule binding sites. The B-cell epitopes discovered in CHIKV E1 and E2 antigens may offer clues to the immunological response in CHIKV infection, since epitopes are the target of pathogenesis, and the goals of diagnostics and vaccine development. Although epitopes may be classified as conformational or linear, B-cell epitopes are mostly linear, which could be useful for the creation of vaccines and the diagnosis of CHIKV infection.56 The assessment data show that the prominent B-cell epitopes found in the rE1 and rE2 ectodomain coat proteins cover all important characteristics, such as antigenicity, hydrophilicity, polarity, flexibility, surface accessibility, and the presence of fewer α-helices and maximum β-turn areas. The anti-chimeric E2-E1 antibody offers adequate capacity to detect a particular antigen on the basis of similarity seen among the locations of antigenic regions and the peptide sequence of epitopes of a recombinant antigen. The current findings show that the recombinant antigen produced from the B-cell epitope ectodomain E1 and E2 (chimeric E2-E1) gene of CHIKV is useful in a bacterial system. The E1 and E2 coat proteins of CHIKVs may be used to detect antigen and antibodies, and the current findings highlight the specificity of the epitope ectodomain coat protein produced via recombinant technology. The immunogenic ability of anti-chimeric rE2-E1 hamster antibody against recombinant antigen has clearly shown its selectivity for trapping viral antigen. However, the usefulness of recombinant structural proteins (capsid, E1 and E2) as antigens for CHIKV serum-based diagnosis has been explored and several disadvantages have been found.22,57
CHIKV chimeric rE2-E1 envelope proteins were produced in an E. coli bacterial cell as CHIKV-specific testing agents in this study. CHIKV-positive and CHIKV-negative plasma samples were utilized to test the seroreactivity of CHIKV rE2-E1 envelope proteins, while anti-dengue virus plasma samples were utilized to test the cross-reactivity of rE2-E1 protein with dengue infection. When utilized with normal control samples from healthy people, the sensitivity of rE2-E1 antibodies for collecting antigen was 95.8%, while specificity was found to be 100%. Others have shown that the sensitivity of CHIKV rE2-E1 antibody for collecting antigen was greater than that of CHIKV E2 and E1.22,35 This disparity can be elucidated through the fact that the CHIKV E2-E1 recombinant antigen has more B-cell epitopes than the E2 and E1 proteins. Protein-based CHIKV diagnostic kits have been used and are based on several methods, including ELISA, IFA, and ICA.25,58–61 Thus, the rE2-E1 IgG antibody generated by immunization with rE2-E1 antigen can be used in the direct IFA to detect viral antigen in the early phase of CHIKV infection.
In conclusion, the data from serological testing from the rE2-E1 antigen utilizing anti-rE2-E1 antibody reported in the research offer a wide range of applications in clinical diagnosis and treatment planning for CHIKV. The method will become an attractive, promising and inexpensive test for the earlier diagnosis of CHIKV infection owing to the high rates of sensitivity and specificity found throughout the assessment. The findings of the ELISA employing polyclonal antibodies to the rCHIKV E2-E1 chimeric protein may be used to identify CHIKV infection in patients with chikungunya fever in the early stages of illness. Antigen-capture ELISA was shown to be negative for all of the healthy subjects, demonstrating its outstanding specificity. As our method offers several advantages over existing diagnostics used in resource-limited settings, it has the potential to transform disease management through enabling accessible evidence-based laboratory diagnosis.
Ethics Statement
The immunization study was performed on female Syrian golden hamsters. All animal studies were approved (ethics approval no. 1360) by the Hamdard University Animal Ethics Committee (JHAEC) and Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Government of India. The animal experiments were performed according to the Guidelines for Animal Experiments of the CPCSEA, Government of India. The use of the patients’ sera (96 CHIKV-positive, 36 CHIKV-negative, and 22 dengue-positive serum samples) in this study was approved (no. 16/9/150/JMI/IEC/2017) by the Jamia Millia Islamia at New Delhi Institutional Ethics Review Board. All of the CHIKV and dengue virus patients consented to participate in the study and provided written informed consent before the sample collection, and the study was conducted in accordance with the Declaration of Helsinki.
Acknowledgments
MI thanks DST-SERB, Government of India, for providing financial assistance (DST no. PDF/2016/003753), and JSPS, Tokyo, Japan, for providing a fellowship during the writing of the manuscript (JSPS/OF322, ID no. P19108). Dr. Abuzer Ali is thankful to Taif University Researchers Supporting Project (no. TURSP‐2020/124), Taif University, Taif, Saudi Arabia. We are grateful to Dr. Mohd Aslam (Advisor, Department of Biotechnology, Government of India) for logical instruction in this study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflict of interest in this work.
References
1. Powers AM, Logue CH. Changing patterns of chikungunya virus: re-emergence of a zoonotic arbovirus. J Gen Virol. 2007;88:2363–2377. doi:10.1099/vir.0.82858-0
2. Khan AH, Morita K, Parquet MDC, Hasebe F, Mathenge EGM, Igarashi A. Complete nucleotide sequence of chikungunya virus and evidence for an internal polyadenylation site. J Gen Virol. 2002;83:3075–3084. doi:10.1099/0022-1317-83-12-3075
3. Powers AM, Brault AC, Tesh RB, Weaver SC. Re-emergence of Chikungunya and O’nyong-nyong viruses: evidence for distinct geographical lineages and distant evolutionary relationships. J Gen Virol. 2002;81:471–479.
4. Schuffenecker I, Iteman I, Michault A, et al. Genome microevolution of chikungunya viruses causing the Indian Ocean outbreak. PLoS Med. 2006;3:e263. doi:10.1371/journal.pmed.0030263
5. Strauss JH, Strauss EG, Kuhn RJ. Budding of alphaviruses. Trends Microbiol. 1995;3:346–350. doi:10.1016/S0966-842X(00)88973-8
6. Lumsden WH. An epidemic of virus disease in Southern Province, Tanganyika Territory, in 1952–53. II. General description and epidemiology. Trans R Soc Trop Med Hyg. 1955;49:33–57. doi:10.1016/0035-9203(55)90081-X
7. Hasebe F, Parquet MC, Pandey BD, et al. Combined detection and genotyping of Chikungunya virus by a specific reverse transcription-polymerase chain reaction. J Med Virol. 2002;67(3):370–374. doi:10.1002/jmv.10085
8. Pfeffer M, Linssen B, Parke MD, Kinney RM. Specific detection of chikungunya virus using a RT-PCR/nested PCR combination. J Vet Med B Infect Dis Vet Public Health. 2002;49(1):49–54. doi:10.1046/j.1439-0450.2002.00535.x
9. Pastorino B, Bessaud M, Grandadam M, Murri S, Tolou HJ, Peyrefitte CN. Development of a TaqMan RT-PCR assay without RNA extraction step for the detection and quantification of African Chikungunya viruses. J Virol Methods. 2005;124(1–2):65–71. doi:10.1016/j.jviromet.2004.11.002
10. Joseph AY, Babu VS, Dev SS, et al. Rapid detection and characterization of Chikungunya virus by RT-PCR in febrile patients from Kerala, India. Indian J Exp Biol. 2008;46(8):573–578.
11. Panning M, Hess M, Fischer W, Grywna K, Pfeffer M, Drosten C. Performance of the RealStar Chikungunya virus real-time reverse transcription-PCR kit. J Clin Microbiol. 2009;47(9):3014–3016. doi:10.1128/JCM.01024-09
12. Carletti F, Bordi L, Chiappini R, et al. Rapid detection and quantification of Chikungunya virus by a one-step reverse transcription polymerase chain reaction real-time assay. Am J Trop Med Hyg. 2007;77(3):521–524. doi:10.4269/ajtmh.2007.77.521
13. Grivard P, Le Roux K, Laurent P, et al. Molecular and serological diagnosis of Chikungunya virus infection. Pathol Biol. 2007;55(10):490–494. doi:10.1016/j.patbio.2007.07.002
14. Laurent P, Le Roux K, Grivard P, et al. Development of a sensitive real-time reverse transcriptase PCR assay with an internal control to detect and quantify chikungunya virus. Clin Chem. 2007;53(8):1408–1414. doi:10.1373/clinchem.2007.086595
15. Santhosh SR, Parida MM, Dash PK, et al. Development and evaluation of SYBR Green I-based one-step real-time RT-PCR assay for detection and quantification of Chikungunya virus. J Clin Virol. 2007;39(3):188–193. doi:10.1016/j.jcv.2007.04.015
16. Parida MM, Santhosh SR, Dash PK, et al. Rapid and real-time detection of Chikungunya virus by reverse transcription loop-mediated isothermal amplification assay. J Clin Microbiol. 2007;45(2):351–357. doi:10.1128/JCM.01734-06
17. Wang R, Ongagna-Yhombi SY, Lu Z, et al. Rapid diagnostic platform for colorimetric differential detection of dengue and chikungunya viral infections. Anal Chem. 2019;91:5415–5423. doi:10.1021/acs.analchem.9b00704
18. Bagno FF, Godói LC, Figueiredo MM, et al. Chikungunya E2 protein produced in E. coli and HEK293-T cells-comparison of their performances in ELISA. Viruses. 2020;12(9):939. doi:10.3390/v12090939
19. Pfeffer M, Proebster B, Kinney RM, Kaaden OR. Genus-specific detection of alphavirus by a semi-nested reverse transcription polymerase chain reaction. Am J Trop Med Hyg. 1997;57(6):709–718. doi:10.4269/ajtmh.1997.57.709
20. Mourya DT, Mishra AC. Chikungunya fever. Lancet. 2006;368:186–187. doi:10.1016/S0140-6736(06)69017-X
21. Blacksell SD, Mammen MP, Thongpaseuth S, et al. Evaluation of the Panbio dengue virus nonstructural 1 antigen detection and immunoglobulin M antibody enzyme-linked immunosorbent assays for the diagnosis of acute dengue infections in Laos. Diagn Microbiol Infect Dis. 2008;60:43–49. doi:10.1016/j.diagmicrobio.2007.07.011
22. Cho B, Jeon BY, Kim J, et al. Expression and evaluation of Chikungunya virus E1 and E2 envelope proteins for serodiagnosis of Chikungunya virus infection. Yonsei Med J. 2008;49:828–835. doi:10.3349/ymj.2008.49.5.828
23. Priya R, Khan M, Rao MK, Parida M. Cloning, expression and evaluation of diagnostic potential of recombinant capsid protein based IgM ELISA for chikungunya virus. J Virol Methods. 2014;203:15–22. doi:10.1016/j.jviromet.2014.03.005
24. Damle RG, Jayaram N, Kulkarni SM, et al. Diagnostic potential of monoclonal antibodies against the capsid protein of chikungunya virus for detection of recent infection. Arch Virol. 2016;161:1611–1622. doi:10.1007/s00705-016-2829-4
25. Mardekian SK, Roberts AL. Diagnostic options and challenges for dengue and chikungunya viruses. Biomed Res Int. 2015;2015:834371. doi:10.1155/2015/834371
26. Burdino E, Calleri G, Caramello P, Ghisetti V. Unmet needs for a rapid diagnosis of chikungunya virus infection. Emerg Infect Dis. 2016;22:1837–1839. doi:10.3201/eid2210.151784
27. Fieser TM, Tainer JA, Geysen HM, Houghten RA, Lerner RA. Influence of protein flexibility and peptide conformation on reactivity of monoclonal anti-peptide antibodies with a protein alpha-helix. Proc Natl Acad Sci U S A. 1987;84:8568–8572. doi:10.1073/pnas.84.23.8568
28. Kolaskar AS, Tongaonkar PC. A semi-empirical method for prediction of antigenic determinants on protein antigens. FEBS Lett. 1990;276:172–174. doi:10.1016/0014-5793(90)80535-Q
29. Vihinen M, Torkkila E, Riikonen P. Accuracy of protein flexibility predictions. Proteins. 1994;19:141–149. doi:10.1002/prot.340190207
30. Parker JC, Rippe B, Taylor AE. Fluid filtration and protein clearances through large and small pore populations in dog lung capillaries. Microvasc Res. 1986;31:1–17. doi:10.1016/0026-2862(86)90002-6
31. Emini EA, Hughes JV, Perlow DS, Boger J. Induction of hepatitis A virus-neutralizing antibody by a virus-specific synthetic peptide. J Virol. 1985;55:836–839. doi:10.1128/jvi.55.3.836-839.1985
32. Chou PY, Fasman GD. Empirical predictions of protein conformation. Annu Rev Biochem. 1978;47:251–276. doi:10.1146/annurev.bi.47.070178.001343
33. Kumar M, Sudeep AB, Arankalle VA. Evaluation of recombinant E2 protein-based and whole-virus inactivated candidate vaccines against chikungunya virus. Vaccine. 2012;30:6142–6149. doi:10.1016/j.vaccine.2012.07.072
34. Afshari E, Amini-Bayat Z, Hosseinkhani S, Bakhtiari N. Cloning, expression and purification of Pseudomonas putida ATCC12633 Creatinase. Avicenna J Med Biotechnol. 2017;9:169–175.
35. Yathi KK, Bhasker S, Chinnamma M. Determination of B cell epitopes and evaluation of antigen capture ELISA for the earlier diagnosis of CHIK virus using anti-rCHIK E1 rabbit antibodies. J Immunol Methods. 2013;393:45–52. doi:10.1016/j.jim.2013.04.005
36. Zhao SH, Yang H, Hou W, et al. Rapid neutralization testing system for zika virus based on an enzyme-linked immunospot assay. ACS Infect Dis. 2020;6:811–819. doi:10.1021/acsinfecdis.9b00333
37. Desrochers GF, Cornacchia C, McKay CS, Pezacki JP. Activity-based phosphatidylinositol kinase probes detect changes to protein-protein interactions during Hepatitis C virus replication. ACS Infect Dis. 2018;4:752–757. doi:10.1021/acsinfecdis.8b00047
38. Buerano CC, Natividad FF, Contreras RC, et al. Antigen sandwich ELISA predicts RT-PCR detection of dengue virus genome in infected culture fluids of Aedes albopictus C6/36 cells. Southeast Asian J Trop Med Public Health. 2008;39:817–821.
39. Kashyap RS, Morey SH, Chandak NH, Purohit HJ, Taori GM, Daginawala HF. Detection of viral antigen, IgM and IgG antibodies in cerebrospinal fluid of Chikungunya patients with neurological complications. Cerebrospinal Fluid Res. 2010;13(7):12. doi:10.1186/1743-8454-7-12
40. Arechaga I, Miroux B, Karrasch S, et al. Characterisation of new intracellular membranes in Escherichia coli accompanying large scale over-production of the b subunit of F(1)F(o) ATP synthase. FEBS Lett. 2000;482:215–219. doi:10.1016/S0014-5793(00)02054-8
41. Saïda F, Uzan M, Odaert B, Bontems F. Expression of highly toxic genes in E. coli: special strategies and genetic tools. Curr Protein Pept Sci. 2006;7:47–56. doi:10.2174/138920306775474095
42. Chen LH, Wilson ME. Dengue and chikungunya infections in travelers. Curr Opin Infect Dis. 2010;23:438–444. doi:10.1097/QCO.0b013e32833c1d16
43. Hoarau JJ, Jaffar Bandjee MC, Krejbich Trotot P, et al. Persistent chronic inflammation and infection by Chikungunya arthritogenic alphavirus in spite of a robust host immune response. J Immunol. 2010;184:5914–5927. doi:10.4049/jimmunol.0900255
44. Garoff H, Sjoberg M, Cheng RH. Budding of alphaviruses. Virus Res. 2004;106:103–116. doi:10.1016/j.virusres.2004.08.008
45. Bhatt S, Gething PW, Brady OJ, et al. The global distribution and burden of dengue. Nature. 2013;496:504–507. doi:10.1038/nature12060
46. Fauci AS, Morens DM. Zika virus in the Americas—Yet another arbovirus threat. N Engl J Med. 2016;374:601–604. doi:10.1056/NEJMp1600297
47. Arankalle VA, Shrivastava S, Cherian S, et al. Genetic divergence of Chikungunya viruses in India (1963–2006) with special reference to the 2005–2006 explosive epidemic. J Gen Virol. 2007;88:1967–1976. doi:10.1099/vir.0.82714-0
48. Dash PK, Parida MM, Santhosh SR, et al. East Central South African genotype as the causative agent in reemergence of Chikungunya outbreak in India. Vector Borne Zoonotic Dis. 2007;7:519–527. doi:10.1089/vbz.2006.0648
49. Islam MA, El Zowalaty ME, Islam S, et al. RT-PCR assay for simultaneous detection of dengue and chikungunya viruses. Int J Mol Sci. 2020;21(21):8281. doi:10.3390/ijms21218281
50. Kim YC, López-Camacho C, Garcia-Larragoiti N, et al. Development of an E2 ELISA methodology to assess chikungunya seroprevalence in patients from an Endemic Region of Mexico. Viruses. 2019;11(5):407. doi:10.3390/v11050407
51. Edwards CJ, Welch SR, Chamberlain J, et al. Molecular diagnosis and analysis of Chikungunya virus. J Clin Virol. 2007;39:271–275. doi:10.1016/j.jcv.2007.05.008
52. Reddy A, Bosch I, Salcedo N, et al. Development and validation of a rapid lateral flow E1/E2-antigen test and ELISA in patients infected with emerging Asian Strain of chikungunya virus in the Americas. Viruses. 2020;12(9):971. doi:10.3390/v12090971
53. Staples E, Powers A, Tomashek K, Lanciotti R, Hunsperger E, Munoz J. Preparedness and response for Chikungunya Virus Introduction in the Americas. Washington, DC: Pan American Health Organization; 2011.
54. Kielian M. Class II virus membrane fusion proteins. Virology. 2005;344:38–47. doi:10.1016/j.virol.2005.09.036
55. Bréhin AC, Rubrecht L, Navarro-Sanchez ME, et al. Production and characterization of mouse monoclonal antibodies reactive to Chikungunya envelope E2 glycoprotein. Virology. 2008;371:185–195. doi:10.1016/j.virol.2007.09.028
56. Jiang L, Zhou JM, Yin Y, Fang DY, Tang YX, Jiang LF. Selection and identification of B-cell epitope on NS1 protein of dengue virus type 2. Virus Res. 2010;150:49–55. doi:10.1016/j.virusres.2010.02.012
57. Cho B, Kim J, Cho JE, Jeon BY, Park S. Expression of the capsid protein of Chikungunya virus in a baculovirus for serodiagnosis of Chikungunya disease. J Virol Methods. 2008;154:154–159. doi:10.1016/j.jviromet.2008.07.031
58. Shukla J, Khan M, Tiwari M, et al. Development and evaluation of antigen capture ELISA for early clinical diagnosis of chikungunya. Diagn Microbiol Infect Dis. 2009;65:142–149. doi:10.1016/j.diagmicrobio.2009.06.017
59. Okabayashi T, Sasaki T, Masrinoul P, et al. Detection of chikungunya virus antigen by a novel rapid immunochromatographic test. J Clin Microbiol. 2015;53:382–388. doi:10.1128/JCM.02033-14
60. Fumagalli MJ, de Souza WM, Esposito DLA, et al. Enzyme-linked immunosorbent assay using recombinant envelope protein 2 antigen for diagnosis of chikungunya virus. Virol J. 2018;15:112. doi:10.1186/s12985-018-1028-1
61. Jain J, Okabayashi T, Kaur N, et al. Evaluation of an immunochromatography rapid diagnosis kit for detection of chikungunya virus antigen in India, a dengue-endemic country. Virol J. 2018;15:84. doi:10.1186/s12985-018-1000-0
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.