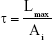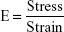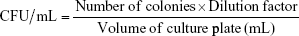Back to Journals » Drug Design, Development and Therapy » Volume 12
Development of controlled release silicone adhesive–based mupirocin patch demonstrates antibacterial activity on live rat skin against Staphylococcus aureus
Authors David SR, Malek N, Mahadi AH, Chakravarthi S, Rajabalaya R
Received 16 July 2017
Accepted for publication 21 November 2017
Published 8 March 2018 Volume 2018:12 Pages 481—494
DOI https://doi.org/10.2147/DDDT.S146549
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sukesh Voruganti
Sheba R David,1 Nurafiqah Malek,1 Abdul Hanif Mahadi,2 Srikumar Chakravarthi,3 Rajan Rajabalaya1
1PAPRSB Institute of Health Sciences, Universiti Brunei Darussalam, Bandar Seri Begawan, Brunei Darussalam; 2Centre for Advanced Material and Energy Sciences (CAMES), Universiti Brunei Darussalam, Bandar Seri Begawan, Brunei Darussalam; 3School of Medicine, Perdana University, Jalan MAEPS Perdana, Serdang, Selangor, Malaysia
Background: Peritonitis is the most serious complication of peritoneal dialysis. Staphylococcus aureus infections could lead to peritonitis which causes reversal of peritoneal dialysis treatment back to hemodialysis. The aim of this study was to develop a controlled release silicone adhesive-based mupirocin patch for prophylactic effect and analyze its antibacterial effectiveness against S. aureus.
Methods: The matrix patches were prepared by using different polymers, with and without silicone adhesive, dibutyl sebacate and mupirocin. The patches were characterized for mechanical properties, drug content, moisture content, water absorption capacity and Fourier transform infrared spectrum. In vitro release studies were performed by using Franz diffusion cell. In vitro disk diffusion assay was performed on the Mueller–Hinton Agar plate to measure the zone of inhibition of the patches. The in vivo study was performed on four groups of rats with bacterial counts at three different time intervals, along with skin irritancy and histopathologic studies.
Results: The patches showed appropriate average thickness (0.63–1.12 mm), tensile strength (5.08–10.08 MPa) and modulus of elasticity (21.53–42.19 MPa). The drug content ranged from 94.5% to 97.4%, while the moisture content and water absorption capacities at two relative humidities (75% and 93%) were in the range of 1.082–3.139 and 1.287–4.148 wt%, respectively. Fourier transform infrared spectra showed that there were no significant interactions between the polymer and the drug. The highest percentage of drug release at 8 hours was 47.94%. The highest zone of inhibition obtained was 28.3 mm against S. aureus. The in vivo studies showed that the bacterial colonies were fewer at 1 cm (7×101 CFU/mL) than at 2 cm (1.3×102 CFU/mL) over a 24-hour period. The patches were nonirritant to the skin, and histopathologic results also showed no toxic or damaging effects to the skin.
Conclusion: The in vitro and in vivo studies indicated that controlled release patches reduced the migration of S. aureus on the live rat skin effectively, however, a longer duration of study is required to determine the effectiveness of the patch on a suitable peritonitis-induced animal model.
Keywords: transdermal delivery, matrix patch, peritonitis, antibacterial, dialysis infection, histopathology
Introduction
Peritonitis is the most serious complication caused by Staphylococcus aureus during peritoneal dialysis (PD). A common complication that is encountered by the PD patient is peritonitis, which causes the patient to revert back from PD into hemodialysis treatment. Peritonitis can lead to loss of capability of renal function in dialysis, such as decreased ultrafiltration, peritoneal membrane failure and leads to sclerosis.1,2 There is also a higher incidence of morbidity with mortality caused by peritonitis. Peritonitis is usually caused by infection from Gram-positive and Gram-negative bacteria and sometimes by fungi. Gram-positive bacteria that usually can lead to peritonitis are from Staphylococcus family, such as S. aureus. This study, therefore, focuses on S. aureus, which is one of the major causes of peritonitis among PD patients. S. aureus bacteria are present in the nose and on the skin as normal skin flora, which can increase the risk of causing exit-site infection and peritonitis from 2- to 6-fold.1,2 A nasal cream is used to reduce the incidence of infection.
The currently recommended treatment for peritonitis is the administration of antibiotics via intraperitoneal (IP) route. It is preferred to administer antibiotics through the IP route than via intravenous and oral routes for peritonitis, as IP route results in high local dosage level of antibiotics in dialysate.3 Moreover, bioavailability of antibiotics in the peritoneal cavity can be limited by their high molecular weight, protein-binding capacity and lipid solubility that will cause the antibiotics become ineffective when administered via the intravenous or oral route.4
Although there are many antibiotics in use for the prevention of peritonitis, mupirocin (MP) has been recommended widely, since it has prophylactic effects toward S. aureus as it has shown effectiveness in terms of killing S. aureus in several studies on application as ointment at the catheter exit site, while changing the dressing. Application of antibiotic ointment at the exit site helps in reducing the infection by the causative microorganism. However, alcohol present as an adjuvant in ointment causes holes in the catheter from the formulation. The holes in the catheter formed by the cream lead to leakage of the contents of the catheter and necessitate replacement of the catheter, which further increases the number of hospital visits. This causes inconvenience to the patient and decrease in quality of life as well as increase in health care cost.
MP has a property of inhibiting bacterial protein synthesis by binding to bacterial isoleucyl t-RNA synthetase. It acts by interrupting the peptide chain elongation, in turn blocking the A site of ribosomes that leads to misreading of the genetic code, thus preventing the attachment of oligosaccharide side chain to the glycoprotein. MP is effective in eradicating methicillin-susceptible and methicillin-resistant strains of streptococci and staphylococci. Although the application of MP topically at the exit site of PD patients showed a significant decrease in the incidence of ESI, peritonitis was still prevalent.1,5–7
There are several routes of drug administration for antibacterial agents, such as topical, parenteral and enteral routes. Transdermal route of antibiotic administration is the focus of interest in this study. Recently, transdermal drug delivery approaches are being used widely as they proved to be advantageous in delivering selected drugs and showed uniformity in transferring the drug over time in the plasma. They show improved therapeutic efficacy and drug safety, as they can be controlled and monitored over time.8–10 This study aims to develop and test the patches in terms of antibacterial effectiveness to prevent the migration of bacteria. Therefore, it could be used at the catheter site for PD patients who encountered peritonitis, as a way to reduce microbial contamination.
Materials and methods
Materials
MP was purchased from Pi Chemicals Ltd. (Shanghai, China). Eudragit RS 100 (ethyl acrylate, methyl methacrylate copolymers with trimethylammonium ethyl methacrylate) was gifted by Evonik Röhm GmbH (Arnsberg, Germany). Ethyl cellulose (EC; ethoxy content 48.0%–49.5%, viscosity 18–22 MPa) was received from Dow Chemicals (Midland, MI, USA). Dow Corning® BIO-PSAAC7-4201 silicone adhesive was obtained as a gift from Dow Corning Corporation (Midland, MI, USA). Polyvinyl pyrrolidone (PVP; Kollidon® 30) was obtained as a gift sample from BASF Chemical Company (Ludwigshafen, Germany). Dibutyl sebacate (DBS) was purchased from Sigma-Aldrich Chemie GmbH (Riedstr, Germany). All other chemicals used in the study were of analytical grade and were used as received.
Formulation of transdermal matrix patch
The polymeric matrix patches were prepared by solvent casting methods by dissolving 400 mg of Eudragit RS 100 and 300 mg of EC in 5 mL of chloroform with the help of slow magnetic stirrer in a beaker.11–13 Also, 100 mg of copolymer (PVP) and 3 mL of silicone adhesive were added with uniform stirring. Then, to the above solution, DBS plasticizer at 20% of the total polymer weight and 20–40 mg of MP were added and stirred for 30 min. Then, the total mass was slowly poured into the center of stainless steel rings having a backing layer of aluminum foil and left to be dried at room temperature for 5 days. The dried patches were then stored in a desiccator or in an airtight container until further use (Table 1). Similar patches were prepared without silicone adhesive.
Characterization of matrix patch
Matrix patch thickness and tensile strength
Digital micrometer (Mitutoyo, Tokyo, Japan) was used for determining patch thickness (Table 1). The tensile strength was measured with a load of 50 kN at 5 mm/min extension speed using an Instron 4204 (Instron, Buckinghamshire, UK) tensilometer. The test was performed according to the method D 882 – 75D of the American Society for Testing Materials for six samples of each formulation patch.11,12,14 A temperature of 25°C±2°C and humidity of 56%±2% were maintained for the test (Table 1). The below equation was used for the calculation of tensile strength:
|
where τ is the tensile strength, Lmax is the maximum load and Ai is the initial cross-sectional area of the sample.
Determination of modulus of elasticity
The modulus of elasticity reflects the stiffness or elasticity of the transdermal patches. This indicates resistance to distortion of the films, which was calculated by plotting the stress–strain curve by using an Instron 4204 tensilometer.15 The modulus of elasticity is represented as the ratio of applied stress over strain in the region of elastic deformation, which was determined using the following formula (Table 1):
|
where E is the modulus of elasticity.
Drug content
An accurate weighed portion of the patch was dissolved in a suitable solvent in which the drug is soluble and then the solution was shaken continuously for 24 hours in a shaker incubator; later, the whole solution was sonicated.16 After sonication and subsequent filtration, the drug in solution was analyzed by high-performance liquid chromatography. The separations were carried out with C18 column (250×4.6 mm, 5 μm) at 25°C and the mobile phase combination of ammonium acetate (0.05 M adjusted to pH 5.0 with acetic acid) and acetonitrile 60:40 (v/v). The flow rate was 0.5 mL/min, the ultraviolet detection was set at 230 nm and the injected volume was 10 μL (Table 1).
Moisture content
The matrix patches were independently weighed and preserved in a desiccator for a day at 40°C. They were consecutively reweighed until a constant weight was established. The initial and constant final patch weight difference was noted to calculate the moisture content percentage. Six readings were recorded and the average was calculated as reported by Rajabalaya et al.17 The values are presented in Table 1.
|
Water absorption capacities
Water absorption capacity was determined by weighing the dry patches initially. Then, they were placed in two different relative humidities (RH) for 24 hours at room temperature. Saturated sodium chloride solution and saturated ammonium hydrogen phosphate solution yield 75% and 93% humidities, respectively. The weight of the patch was recorded periodically till the weight was constant. The average of six readings was recorded (Table 1).17
|
Fourier transform infrared (FTIR) spectrum
MP, EC, Eudragit RS 100 (RS 100), PVP and their combinations such as EC:PVP:MP and RS:PVP:MP are shown in Figure 1. Each component was mixed individually with KBr (infrared [IR] grade) in 100:1 ratio, which was pressurized using the hydraulic press to obtain pellets. The FTIR was performed using an FTIR spectrometer (Cary 630 FTIR Spectrometer; Agilent Technologies, Santa Clara, CA, USA) and the spectra were determined at a resolution of 4 cm−1 in the frequency range of 650–4,000 cm−1 (Figure 1).15
  | Figure 1 FTIR spectrum for individual components and combined mixtures. |
In vitro release studies
In vitro release studies were carried out in a Franz diffusion cell (EMFDC-06; Orchid Scientific, Nashik, India). A piece of circular patch (2±0.1 cm) was mounted on the receptor compartment, which was filled with freshly prepared PBS having pH 7.4. Temperature was maintained at 32°C±0.5°C. Sample (0.5 mL) was withdrawn every hour for 8 hours and replaced immediately with the same volume of PBS solution. Samples were then diluted and analyzed by high-performance liquid chromatography method as mentioned in the “Drug content” section.18
In vitro antimicrobial activity
Disk diffusion assay of transdermal matrix patch
A 0.5 McFarland standard from DensiCHEK plus standards kit (bioMerieux, Inc., Durham, NC, USA) that was equal to 1.0×108 colony forming units (CFU)/mL for bacteria was used. This was compared with inoculum preparation for a colony of S. aureus American Type Culture Collection 25923 strain prepared from 24 hours old pure agar culture and transferred into 5 mL of nutrient broth.
Mueller–Hinton Agar (MHA) was used for disk diffusion assay in determining the antibacterial activity of MP patch. This assay was performed according to the standard Clinical and Laboratory Standards Institute guidelines and the British Society of Antimicrobial Chemotherapy (BSAC) for antimicrobial susceptibility testing. S. aureus was cultured on MHA plate overnight before inoculating it in nutrient broth medium to a turbidity of 0.5 McFarland. The bacterial culture suspension was then spread onto MHA plate. Matrix patches (Table 1) A1, A2, B1 and B2 were cut into 6 mm in diameter using a sterile stainless steel paper cutter (Figure 2). The matrix patches, with and without MP, and standard antibiotic control were placed and slightly pressed onto the agar-containing lawn of S. aureus. The control matrix patch without MP was placed side by side with standard control (gentamicin) and the matrix patch with MP was placed at the bottom of the agar plate. This was done in triplicate, and all plates were in inverted position and incubated at 37°C overnight. On the next day, the diameter of the clear zone, also known as inhibition zone, was measured using a ruler and standard error was taken into account. This was to test the efficacy of the matrix patches that contained the drug.
In vivo animal studies
In vivo animal studies for bacterial count
Animal studies were used to evaluate the effect of matrix patch on the rat skin against S. aureus. The University Research Ethics Committee of Universiti Brunei Darussalam approved the procedures and animal care for the experiments undertaken in this project (Reference number: UBD/AVC-RI/1.21.6). The experimental conditions were in compliance with the requirements of the University Research Ethics Committee.
Male Sprague Dawley rats were used for animal studies. They were freely given food and water. Sprague Dawley rats (n=6) which were 5 months old and weighed 400–450 g were included in the experiment and separated into four different groups. The groups of animals used for evaluation are as follows: group 1 is negative control (matrix patch without drug); group 2 is positive control group (commercial 2% MP ointment applied on rat skin); group 3 is A1 patch (EC matrix patch with MP [1.5 cm2 area, 6.25 mg]) and group 4 is B1 patch (Eudragit RS 100-based matrix patch with MP [1.5 cm2 area, 6.25 mg]), as shown in Table 2. Animals were shaved for an area of about 4 cm2 at the back of their neck using an electrical hair clipper, and then cleaned with isopropanol wipes (70%) and left undisturbed for a day to let the skin to recover. This method had been modified from that of Mendes et al.19
Application of matrix patch on the rat’s skin
The rat was restrained by holding the rat’s head and body gently in a sterile animal room. Isopropanol wipes (70%) were used to clean the hair removal area before applying the transdermal patch. A negative control matrix patch of 1.5 cm2 area, commercial 2% MP ointment and test patches (A1 and B1) were applied on the clean skin surface. These were applied at the middle of the 4 cm2 of shaved area (Figure 3A). To ensure the stability of the matrix patch being attached strongly on the skin area, an additional adhesive membrane was used (Figure 3B). A freshly prepared S. aureus that had been suspended in 0.9% saline was adjusted to a turbidity equivalent to 0.5 McFarland standard. These bacteria were introduced on the rat’s skin by inoculating 100 μL of the suspended bacteria within 2 cm2 area around the patch and were evenly spread out (Figure 3C). Two swabs were taken around this area, which were at 1 and 2 cm, respectively (Figure 3D), before placing the rats back in their respective individual cages and observed for few minutes to prevent the patch detaching from the skin.
Bacterial swab at different intervals
Swabs were taken from the area inoculated with bacteria using sterile swabs at two different locations which were approximately 1 cm and 2 cm away (Figure 3D) from the matrix patch at 0, 5 and 24 hours. The swab taken was rolled on the skin 3 times and suspended in the sterile 0.9% saline on ice.
Plate inoculation
The swabs taken from the rats were maintained at 5°C±1°C temperature until further studies. The vial containing the swab was vortexed to ensure that the bacteria were displaced into liquid. Then, 100 μL of the suspension was plated onto MHA plate. The entire plate was covered by using L-spreader to ensure that the inoculum was evenly spread. It was incubated overnight and the colonies were counted the next day (Table 2).
|
Skin irritancy test
Skin irritancy test was performed to determine the localization of skin reaction on male Sprague Dawley rats weighing between 400 and 450 g with slight modifications to the method reported by Rajabalaya et al.20 Four groups of animals were used to evaluate skin irritancy: the first group was applied negative control patch, the second group received 2% MP ointment (positive control), the third group received an A1 patch with MP (1.5 cm2, 6.25 mg) and the fourth group received a B1 patch with MP (1.5 cm2, 6.25 mg). The optimized patches (1.5 cm2, 6.25 mg) were applied once on 4 cm2 of the shaved area on the posterior side of the dorsal region at the back of rat. The observed localized reactions were edema and erythema as shown in Table 3.
Histopathologic analysis
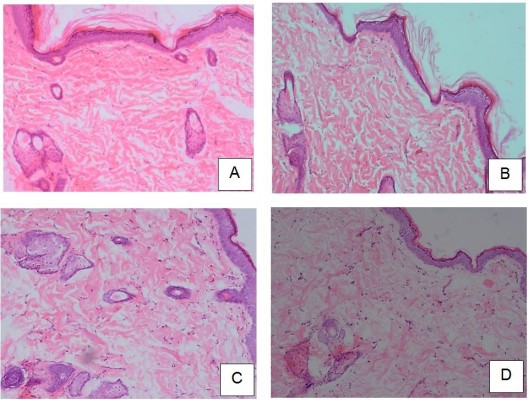
Histopathologic studies were carried out for the elucidation of the mechanism of penetration enhancement and the skin irritation potential of the investigated enhancer. In this study, the rats that had been treated with matrix patch were sacrificed after 24 hours and the skin samples were removed and transported in 0.9% saline to the laboratory.16 Each specimen was cleaned and unnecessary fat tissue was removed; the specimen was cut into cross-sectional and longitudinal sections for observational studies. These samples were stored in 10% formalin solution in PBS. Each section was dehydrated using ethanol, embedded in paraffin for fixing, sectioned into 5 μM thick sections and stained with hematoxylin and eosin. These samples were then observed under a light microscope and compared with control sample. Three different sites were scanned for observation in each skin sample (Figure 4).
Statistical analysis
Microsoft Office Excel was used to tabulate and represent the results graphically. All data are expressed as mean ± SD unless otherwise specified.
Results
Characterization of matrix patch
Thickness, tensile strength and modulus of elasticity
The mechanical properties of the formulated patches are shown in Table 1. The average thickness of all formulations was from 0.63 to 1.12 mm. However, the thicknesses of A1 and B1 (0.66 and 0.63 mm, respectively) matrix patches were lower compared to those of A2 and B2 patches (1.11 and 1.12 mm, respectively). The average tensile strength was from 5.08 to 10.08 MPa, and the modulus of elasticity ranged from 21.53 to 42.19 MPa. From the results, it was evident that the tensile strengths of A1 and B1 (5.08 and 7.50 MPa, respectively) were lower compared to those of A2 and B2 (9.06 and 10.08 MPa, respectively). Furthermore, the modulus of elasticity value was higher for lower thickness patches such as A1 and B1 (21.53 and 28.26 MPa, respectively) than higher thickness patches A2 and B2 (38.56 and 42.19 MPa, respectively). Table 1 shows clearly that there was no significant difference in mechanical properties between these two different polymer-based patches, A and B from EC and Eudragit RS 100, respectively. However, the B1 and B2 patches were superior to A1 and A2 in terms of tensile strength, modulus of elasticity and physical appearance. The physiochemical characteristics of the patches without silicone adhesive are not presented as they were not significantly different compared to the silicone adhesive patches.
Drug content, moisture content and water absorption capacities
The average drug content of all the patches was >94% of the incorporated amount. Furthermore, lower thickness patches (A1- 96.2% and B1- 97.4%) had a slightly higher percentage of drug content than the higher thickness patches (A2- 94.5% and B2- 95.8%). The lower thickness patches had lower moisture content compared to the higher thickness patches. Similarly, the water absorption capacities at both RH conditions were determined and the data are shown in Table 1. The data show that for A1 patches, the water absorption capacity was 49% higher at 93% RH than at 75% RH, while it was 124% higher for B1 patches. The water absorption capacities at 75% RH were 1.471, 3.307, 1.287 and 2.067 wt% for A1, A2, B1 and B2, respectively. Moreover, the water absorption capacity was higher at 93% RH for all matrix patches B than the matrix patches A.
All formulations contain 20% DBS plasticizer and 20 mg MP. The “Patch with silicone” formulations contain 3 mL silicone adhesive, while the “Patch without silicone” formulations do not contain silicone.
FTIR spectrum
FTIR spectrums of MP, individual polymers such as EC, Eudragit RS 100 (RS) and PVP, and combined mixtures EC:PVP:MP and RS:PVP:MP were compared to observe for spectral shifts in the mixture (Figure 1). All the peaks of the MP and polymers used in the mixture were observed in the FTIR spectra. The characteristic peaks of MP (1,643, 2,924, 2,954 and 3,437 cm−1), EC (1,047, 1,631 and 2,947 cm−1), RS 100 (1,220, 1,410, 1,750 and 2,966 cm−1) and PVP (1,360, 1,485, 1,600 and 2,968 cm−1) were observed. The FTIR spectra of the drug and combined mixtures revealed a band due to −OH stretching at 3,437 cm−1; there were bands recorded for −C−H stretching (aromatic) at 2,954 and 2,966 cm−1, while −CH3 stretching (aliphatic) resulted in bands at 2,968 and 2,924 cm−1. Furthermore, C=C stretching (aromatic) ensued 1,600 and 1,485 cm−1 bands. The peaks corresponding to either polymers or the drug did not show any significant shifts in the spectrum. However, there were few overlaps found in the characteristic peaks of the drug and the polymer.
In vitro drug release studies
The cumulative release patterns of EC and RS 100 matrix patches with silicone adhesive having lower as well as higher thickness patches after 8 hours of diffusion evaluated by plotting cumulative percentage release against time (T1/2) are shown in Figure 5. The cumulative percentage release of the drug was lower from the EC matrix patch containing silicone adhesive with lower thickness A1 (11.84%) compared to that of formulation without silicone, A1 (23.24%; Figure 5). Nevertheless, the percentage of drug release was lower from the higher thickness EC polymeric patches, irrespective of whether they had silicone adhesive or not, compared to the lower thickness patches.
However, the cumulative percentage release of the drug was lower from the Eudragit RS 100 patch containing silicone adhesive with lower thickness, B1 (20.71%) compared to the formulation without silicone B1 (27.24%). In contrast to the EC patches, in the higher thickness Eudragit RS 100 patches, the percentage of drug release was higher irrespective of whether they had silicone adhesive or not (38.49% and 47.94%, respectively).
The plots clearly depict that diffusion-controlled matrix model is followed in the drug release by both EC and RS 100 patches; furthermore, the total percentage of the released drug is proportional to the square root of time.
Disk diffusion assay of transdermal matrix patch
Commercially available disk (gentamicin, 10 μg) and matrix patch containing MP (A1, A2, B1 and B2) were used for this assay. The drug concentration of the 6 mm patch was 0.2 mg/disk. The MP matrix patches with silicone adhesive layered surface (6 mm, 0.2 mg) showed good zone of inhibition (ZOI). Gentamicin used as a standard exhibited 20 mm ZOI.
The inhibition zone of the matrix patches with silicone adhesive layered surface (A1- 14.3 mm, A2- 16.0 mm, B1- 15.3 mm and B2- 13.0 mm) showed slightly lesser ranges from 65% to 80% compared to gentamicin standard (Figure 2A), whereas for MP matrix patches without silicone adhesive layered surface (6 mm, 0.2 mg), the inhibition zone was higher (A1- 28.3 mm, A2- 26.7 mm, B1- 27.0 mm and B2- 25.0 mm) compared to gentamicin standard (Figure 2B).
It was clear from the results that the inhibition zones produced by MP matrix patches were susceptible to S. aureus growth. Data from Figure 2A show that A1 and B1 of the matrix patches without silicone adhesive layered surface produced significant inhibition of S. aureus with a larger inhibition zone than with silicone adhesive layered surface patch (Figure 2A). This can be illustrated from A1, where the former had 28.3 mm ZOI while the latter had 14.3 mm. Overall, the matrix patches A1, A2, B1 and B2 released MP by showing an inhibition zone against S. aureus.
In vivo animal studies for bacterial count
From Figure 3 and Table 2, it can be observed that the bacterial count decreased over time at two different swab locations (1 and 2 cm) from 0 to 24 hours of treatment. The bacterial counts at 0 hour of negative control patch were 4.45×103 and 3.82×103 CFU/mL at 1 and 2 cm, respectively. It can be seen that, 2% MP ointment (positive control) had bacterial counts of 3.28×103 and 5.19×103 CFU/mL at 1 and 2 cm, respectively, at 0 hour. For the test group, A1 matrix patch, the bacterial counts were 3.19×103 and 4.36×103 CFU/mL at 1 and 2 cm, respectively, at 0 hour. For B1 matrix patch, the bacterial counts were 2.95×103 and 4.69×103 CFU/mL at 1 and 2 cm, respectively.
The negative control patch exhibited bacterial counts of 2.99×103 and 2.16×103 CFU/mL at 1 and 2 cm, respectively, at 5 hours, whereas the commercial 2% MP ointment had bacterial counts of 1.5×103 and 2.17×103 CFU/mL at 1 and 2 cm, respectively. Also, A1 and B1 matrix patches had bacterial counts which were significantly reduced.
At the final 24 hours, the bacterial counts of negative control patch were 2.17×103 and 2.3×103 CFU/mL at 1 and 2 cm, respectively. In 2% MP ointment, the bacterial counts decreased from 1.5×103 to 3.2×102 CFU/mL from 5 to 24 hours respectively, at 1 cm, recording almost 370% decrease.
Likewise, both A1 and B1 patches showed a significant decrease of bacterial count, as seen in Table 2. Results of A1 and B1 MP matrix patches (1.5 cm2, 6.25 mg) show their capability to prevent the migration of S. aureus, as the bacterial counts taken at 1 and 2 cm locations from cleanly shaved live rat skin showed a decreased bacterial count over a period of time.
Skin irritancy test
The skin irritancy test was conducted to find out the safety of transdermal matrix patches (A1 and B1). The results are tabulated in Table 3. The negative control showed no signs of edema and erythema, while the positive control showed slight edema. Likewise, the patches also had insignificant erythema.
Histopathologic analysis
The histopathologic sections from the skin of the negative control (patch without drug), stained with hematoxylin and eosin, showed intact outer epidermis, which appeared to be of normal morphology and thickness. Beneath the epidermis was the dermis which showed fibrous connective tissue with adequate vascularization, and scattered appendages such as sweat glands and hair follicles (Figure 4A). The positive control group (2% MP ointment) showed intact epidermis with normal morphology and thickness. The dermis showed fibrous connective tissue with adequate vascularization and normal scattered appendages (Figure 4B). In A1 matrix patch, the histopathologic sections from the skin showed the epidermis, which appeared to be slightly thickened and showed features of mild focal acanthosis, with prominent stratum corneum layer, without any parakeratosis. There was no evidence of inflammation. The dermis showed adequate normal connective tissue. There seems to be a slight increase in the sweat glands and hair follicles, suggesting that the biopsy is from a hairy portion (Figure 4C). For B1 matrix patch, the histopathologic section from the skin showed similar features as A1. The epidermis was intact without any discontinuity, ulceration or necrosis, but thinned out in the focal area. The dermis showed adequate normal connective tissue with occasional scattered nonspecific inflammatory cells (Figure 4D).
Discussion
Characterization of matrix patch
Testing of the tensile strength is an opportunity to demonstrate the mechanical properties of the transdermal patches, such as stress–strain tolerance and stress at failure (strength). It is essential to have appropriate strength to be intact during storage and use.
Both EC and Eudragit RS 100 patches containing higher PVP concentration showed higher thickness. This is due to the higher molecular weight of DBS plasticizer which occupies the highly concentrated polymer–polymer chain spacing with higher amount of PVP.14 Furthermore, the patches with lower amount of polymer had lower tensile strength and lower modulus of elasticity than the patches with higher amount of polymer. However, the tensile strength was not increased by doubling the amount of polymers and PVP in the patches. This may be due to either the PVP interrupting the continuity of the polymer chain molecules, resulting in a decrease in the blended polymer strength, or a decrease in the internal stresses of the polymer matrices. The other phenomenon is the presence of plasticizer molecules which were entrapped within the matrix system and thus decreased the cohesive forces considerably between the chains in the matrix system.21 Consequently, there were new interactions between plasticizer and PVP polymer, after the preparation of the formulation, which led to considerable reduction in the ultimate strength of the patches.17
The results of this study demonstrate slightly improved mechanical strength of Eudragit RS 100 MP patches with 20% DBS (matrix patches B) than the EC patch (matrix patches A). Table 1 shows clearly that there was no significant difference in mechanical properties between the patches based on two different polymers (EC and Eudragit RS 100). However, Eudragit RS 100–based patches were superior to EC-based patches in terms of tensile strength, modulus of elasticity and physical appearance.
The high average drug content in the polymeric patches, especially >94%, infers that the intended amount of drug is uniformly dispersed throughout the patch, which would ensure uniform release/permeation of the drug from the matrix patches. Moisture content and water absorption capacity studies were carried out to find out whether the patches were acceptable for storage and handling as topical or transdermal patches. It is important to meet standard limits for the transdermal patches to show such properties. The moisture content and water absorption capacities of the EC and Eudragit RS 100-patches were solely dependent on the concentration of polymers used to fabricate the patches.
Hydrophilic PVP is also hygroscopic and, therefore, has an important influence on the moisture content in the patches; accordingly, the patches with higher PVP amounts had higher moisture content.22 Also, patches containing EC had a higher moisture content and water absorption capacity than RS 100 patches.22 Correspondingly, the amounts of absorption of water at both 75% and 93% RH conditions were also less significant; generally, hydrophilic PVP allows easy diffusion of water into the matrix film, while hydrophobic plasticizers such as DBS block the water absorption and moisture uptake. It was relatively difficult to hydrate patches with hydrophobic polymers, especially at 20%.23 Table 1 clearly demonstrates that the patches were intact in both RH conditions.
FTIR spectra of the drug and the components of the formulation provide valuable information on integrity of the molecules. Compatibility between the polymer and drug is important for formulation. Absence of significant shifts in the spectra is a confirmation that there is no major interaction, and indicates the compatibility of the drug with the other components of the patch.
In vitro drug release studies
From Figure 5A, it can be observed that the EC matrix patch with lower thickness exhibited higher percentage of drug release compared to higher thickness patch. The rigid polymer–polymer chain interaction in EC does not allow the drug for diffusion into the diffusion medium;14 thus, the EC matrix patch with lower thickness exhibited higher percentage of drug release compared to the higher thickness patch. However, the amine group in the polymer Eudragit RS 100, as well as the silicone adhesives are able to permeate the drug molecules easily,15 which leads to higher percentage of drug release in the higher thickness RS 100 matrix patch in comparison to lower thickness patch.
The use of higher amounts of EC polymer required more time for swelling in the presence of PVP; thus, the polymer chain relaxed slowly, resulting in lesser drug release than lesser amount of EC polymer and is the probable reason for the lower cumulative release. Additionally, silicone adhesive may block the pores of matrix, which leads to less diffusion of the drug molecules. Therefore, there are two reasons attributed to lower cumulative percentage release: firstly, lower surface diffusion due to slow hydration and swelling of polymeric matrix and secondly, the blockade of the polymer matrix pores which decreases the diffusion rate significantly.24,25
The percentage of drug release was observed to be in the following descending order approximately at 8 hours: RS 100 patches without silicone > RS 100 patches with silicone > EC matrix patch without silicone > EC matrix patch with silicone. This phenomenon is probably due to the following three reasons: firstly, absence of silicone adhesive makes the membrane pore to freely allow easy diffusion of the drug molecule, leading to greater surface diffusion; secondly, higher amount of PVP, a hydrophilic polymer, increases diffusion due to the formation of pores attributed to the presence of leached PVP in the diffusion medium; and thirdly, higher amount of amine content from RS 100 makes the membrane permeable for more diffusion of drug molecules.
Disk diffusion assay of transdermal matrix patches
MP has been used as the antibacterial of choice in eradicating S. aureus due to its effectiveness and low cost. Gentamicin can be used as a comparison to MP due to their similarity in the mode of action, that is, both of them inhibit protein synthesis inside the bacteria.26,27 This method helps to evaluate the antimicrobial activity of drugs such as MP and gentamicin against S. aureus, which leads to determination of the antimicrobial susceptibility of patches.
The inhibition zone diameters were interpreted in accordance with the BSAC for antimicrobial susceptibility testing. Standard zone diameter breakpoints of gentamicin for Staphylococci are as follows: if the inhibition zone is ≤19 mm, they are considered as resistant and if the inhibition zone is ≥20 mm, they are considered as susceptible. For MP, staphylococci are considered as resistant if the inhibition zone is ≤6 mm, intermediate if the inhibition zone is between 7 and 26 mm and susceptible if the inhibition zone is ≥27 mm.28 Table 1 shows that standard gentamicin produced inhibition zone well within the range of susceptibility, which can inhibit the growth of S. aureus. Thus, it was used as a positive control. Moreover, the MP matrix patches were susceptible toward S. aureus according to the BSAC.
As seen in the “Results” section, inhibition zone produced by lower thickness matrix patches was larger than that of higher thickness matrix patches of both polymers, as observed in patches without adhesive silicone layered surface. This may be due to the release of drug easily from the lower thickness matrix patches into the agar medium. The adhesive silicone layer decreased the drug release from the matrix pores, which attributed to a lower size of inhibition zone produced with both lower and higher thickness patches.29 This revealed that the adhesive silicone layered surface blocked the drug release from the matrix pores, resulting in lower ZOI both in EC and RS 100 patches. MP was more susceptible than gentamicin toward S. aureus. This assay also showed that the test for antimicrobial activity of transdermal MP in the matrix patches produced a significant inhibition zone toward S. aureus. Hence, MP can be an effective prophylaxis against S. aureus.7,30,31 However, this assay cannot determine whether the effects are due to bactericidal or bacteriostatic effects; although there was bacterial growth inhibition, it does not infer bacterial death and, moreover, it cannot quantify the amount of antibacterial agent diffused into the agar medium.32
In vivo animal studies
Bacterial swab test is used to show the effectiveness of the matrix patch in preventing the migration of S. aureus from the outside, which is placed at 2 cm to a nearer area. The animal hair may contain residual bacteria which could regrow; thus, the area of the animal used for the study was clean shaved. Bacteria were placed 2 cm away from the patch to simulate the growth and migration of bacteria toward the site. It was proposed that as the bacteria migrate toward the patch, the antibiotic from the patch would leach and keep them under control either by bacteriostatic or bactericidal mechanism.
The matrix patch without the drug showed a reduction of 50% of the initial bacterial count, which may be due to the release of low-molecular-weight hydrophobic plasticizer that leads to bacterial degradation and also makes the place unsuitable for the bacterial growth near the patch. However, the bacterial count was considerably high and required the drug molecule to be loaded to eradicate the bacterial growth.
The MP ointment, used as positive control, decreased the bacterial count, which showed that the positive control was effective due to its mode of action of inhibiting bacterial protein synthesis.33 However, the bacterial count was lower at 1 cm than at 2 cm, which may be due to the melting of the ointment bases by the rat skin temperature; therefore, there was a possibility of ointment bases along with the drug spreading beyond 1 cm area and thus, consequently, inhibiting the growth or multiplication of bacteria more at 2 cm area at 24 hours of treatment.
The MP-loaded EC matrix patch had lower number of bacteria over time, which was due to the capability of MP being released from the matrix patch into the skin layers, as the EC polymeric matrix has the ability to release the drug in a controlled manner.34
The significant decrease of the bacterial count in MP-loaded Eudragit RS 100 matrix patch was due to the presence of ammonium groups in RS 100, which increased the permeability, thus rapidly increasing the release of MP from the matrix.35 This was supported by the in vitro release studies which showed higher cumulative percentage of drug release, correlating with the decrease in bacterial growth at 24 hours.
The patches with EC polymer exhibited higher bacterial counts than the RS 100 polymer patches, as the latter polymer had the capability to release the drug faster than EC, thus reducing the bacterial count.35 Additionally, it is possible that the RS 100 polymer could have prevented the migration of bacteria even if it was beyond 3 cm from the patch, as the RS 100 polymer has better diffusion properties and surface tension which increase the drug release. It is of importance to note that both RS 100 and EC patches were effective in reducing the bacterial count of S. aureus even with a lower amount of drug dosage compared to the 2% MP ointment, whereas MP matrix patches loaded with 6.25 mg gave an effective prophylaxis therapy in animal studies. The cleanly shaven live rat skin showed a decreased bacterial count over the period of time, indicating prophylactic activities of the patch formulation.
In the histopathologic analysis, negative and positive control groups showed a normal morphology of skin layers. Likewise, matrix patch with RS 100 showed normal morphology. However, EC matrix patch showed mild nonspecific inflammation, which was not significant. All four groups showed no alteration in morphology, hence suggesting that the matrix patches containing MP that had been applied on the rat have no toxic or damaging effects on the skin (Figure 4).
Skin irritancy test is crucial to ascertain the biocompatibility of the patches. It is essential to have good skin compatibility without irritation for either topical or transdermal drug delivery system, as it stays on the skin for a longer period of time. According to classical in vivo skin irritancy assay test by Lehman et al,36 nonirritants to the skin have a primary irritancy index of <2. Thus, both EC and RS 100 patches were considered to be nonirritants to the skin (Table 3).
Erythema of the skin indicates the start of inflammatory reaction, while edema depicts stronger inflammatory reaction due to the ingredients present in the formulation. The formulation preparations without erythema and edema are considered safe for use. Table 3 shows that there was minimal erythema and no edema compared with the negative and positive controls. Thus, the matrix patch can be considered to be nonirritant to the skin. Therefore, the obtained results certainly indicate that the transdermal patch achieved the objectives of a biocompatible patch, such as being a nonirritant for a long duration of application. Thus, it can be stated that EC and methyl methacrylate polymer (EC and RS 100 polymer) deliver the drug into the skin layer over a longer period of time without any harmful effect to the skin.
Conclusion
Our findings suggest that both A1 and B1 matrix patches were more effective in providing prophylaxis effects. In the in vitro studies, matrix patches A1 and B1 showed better effectiveness by producing a larger inhibition zone than higher thickness patches. In vivo studies showed that both matrix patches containing MP were capable of preventing the migration of S. aureus by showing a decreased bacterial count and were found to be nonirritants with no damaging effects on the skin. However, matrix patch B1 was better than A1 due to its higher capacity to control the bacterial count on the rat skin. Moreover, matrix patch B1 had higher drug content than A1. The results also showed that 20% plasticizer with silicone adhesive was also responsible for contributing to the controlled release into the skin layer without irritation and any skin toxicity.20 Thus, this study has shown that the controlled release patches are comparable to 2% MP ointment without causing any significant difference in terms of prophylaxis effects and damage caused to the morphology of skin. These results can be used to provide a foundation for further studies of these matrix patches for high drug loading with longer duration.
Acknowledgment
This project was supported by the University Research Grant of Universiti Brunei Darussalam awarded to Dr Sheba R David, grant number UBD/PNC2/2/RG/1(321).
Disclosure
The authors report no conflicts of interest in this work.
References
Odudu A, Wilkie M. Controversies in the management of infective complications of peritoneal dialysis. Nephron Clin Pract. 2011;118(3):C301–C308. | ||
Akoh JA. Peritoneal dialysis associated infections: an update on diagnosis and management. World J Nephrol. 2012;1(4):106–122. | ||
Daugirdas JT, Blake PG, Ing TS. Peritonitis and Exit Site Infection. In: Handbook of Dialysis. 4th ed. Philadelphia, PA, USA: Lippincott Williams & Wilkins; 2012:425. | ||
de Vin F, Rutherford P, Faict D. In-depth review intraperitoneal administration of drugs in peritoneal dialysis patients: a review of compatibility and guidance for clinical use. Perit Dial Int. 2009; 29(4):5–15. | ||
Campbell DJ, Johnson DW, Mudge DW, Gallagher MP, Craig JC. Prevention of peritoneal dialysis-related infections. Nephrol Dial Transplant. 2015;30(9):1461–1472. | ||
Elshafie SS, Asim M, Ashour A, Elhiday AH, Mohsen T, Doiphode S. Campylobacter peritonitis complicating continuous ambulatory peritoneal dialysis: report of three cases and review of the literature. 2010;30(1):99–104. | ||
Xu G, Tu W, Xu C. Mupirocin for preventing exit-site infection and peritonitis in patients undergoing peritoneal dialysis. Nephrol Dial Transplant. 2010;25(2):587–592. | ||
Sharma A, Saini S, Rana C. Transdermal drug delivery system: a review. Int J Res Pharm Biomed Sci. 2013;4(1):286–292. | ||
Margetts L, Sawyer R. Transdermal drug delivery: principles and opioid therapy. Contin Educ Anaesthesia Crit Care Pain. 2007;7(5):171–176. | ||
Gaikwad AK. Transdermal drug delivery system: formulation aspects and evaluation. Compr J Pharm Sci. 2013;1(1):1–10. | ||
Rajabalaya R, Qin CH, David SRN. Development and in vitro evaluation of matrix type transdermal delivery of ondansetron hydrochloride. Int J Drug Deliv. 2012;4(1):125–133. | ||
Rajabalaya R, Khanam J, Nanda A. Design of a matrix patch formulation for long-acting permeation of diclofenac potassium. Asian J Pharm Sci. 2008;3(1):30–39. | ||
David SNR, Rajabalaya R, Zhia EU. Development and in vitro evaluation of self-adhesive matrix-type transdermal delivery system of ondansetron hydrochloride. Trop J Pharm Res. 2015;14(2):211–218. | ||
Rajabalaya R, David SRN, Khanam J, et al. Studies on the effect of plasticizer on in vitro release and ex vivo permeation from Eudragit E 100 based chlorpheniramine maleate matrix type transdermal delivery system. J Excipients Food Chem. 2010;1(2):3–12. | ||
Rajabalaya R, David SNR, Khanam J, Nanda A. Effect of platicizers on in vitro release and ex vivo permeation of chlorpheniramine maleate from ethyl cellulose polyvinyl pyrrolidone based matrix patches. Farmacia. 2013;61(5):975–990. | ||
Rajabalaya R, David SR, Chellian J, Xin Yun G, Chakravarthi S. Transdermal delivery of oxybutynin chloride proniosomal gels for the treatment of overactive bladder. Drug Deliv. 2016;23(5):1578–1587. | ||
Rajabalaya R, David SRN, Khanam J, et al. Studies on the effect of plasticizer on in vitro release and ex vivo permeation from Eudragit E 100 based chlorpheniramine maleate matrix type transdermal delivery system. J Excipients Food Chem. 2010;1(2):3–12. | ||
David SRN, Hui MS, Pin CF, et al. Formulation and in vitro evaluation of ethosomes as vesicular carrier for enhanced topical delivery of isotretinoin. Int J Drug Deliv. 2013;5(1):28–34. | ||
Mendes JJ, Leandro CI, Bonaparte DP, Pinto AL. A rat model of diabetic wound infection for the evaluation of topical antimicrobial therapies. Comp Med. 2012;62(1):37–48. | ||
Rajabalaya R, Leen G, Chellian J, Chakravarthi S, David SR. Tolterodine tartrate proniosomal gel transdermal delivery for overactive bladder. Pharmaceutics. 2016;8(3):E27. | ||
Repka MA, McGinity JW. Influence of chlorpheniramine maleate on topical hydroxypropylcellulose films produced by hot-melt extrusion. Pharm Dev Technol. 2001;6(3):297–304. | ||
Rajabalaya R, Choo HQ, David SRN. Development and in vitro evaluation of matrix type transdermal delivery of ondansetron hydrochloride. Int J Drug Deliv. 2012;4(1):125–133. | ||
Rajabalaya R, Nakka David SR, Khanam J, Nanda A. Effect of platicizers on in vitro release and ex vivo permeation of chlorpheniramine maleate from ethyl cellulose polyvinyl pyrrolidone based matrix patches. Farmacia. 2013;61(5):975–990. | ||
Kangarlou S, Hariria I, Gholipour Y. Physico-mechanical analysis of free ethyl cellulose films comprised with novel plasticizers of vitamin resources. Int J Pharm. 2008;356(1–2):153–166. | ||
Rajabalaya R, Musa MN, Kifli N, David SR. Oral and transdermal drug delivery systems: role of lipid-based lyotropic liquid crystals. Drug Des Devel Ther. 2017;11:393–406. | ||
Nnamani PO, Kenechukwul FC, Dibua EU, et al. Bioactivity of gentamicin contained in novel transdermal drug delivery systems (TDDS) formulated with biodegradable polyesters. African J Pharm Pharmacol. 2013;7(28):1987–1993. | ||
Amrutiya N, Bajaj A, Madan M. Development of microsponges for topical delivery of mupirocin. AAPS Pharm Sci Tech. 2009;10(2):402–409. | ||
Wootton M. BSAC Methods for Antimicrobial Susceptibility Testing, version 2015; British Society for Antimicrobial Chemotherapy. Available from: http://www.bsac.org.uk. Accessed January 15, 2017. | ||
Valenta C, Auner BG. The use of polymers for dermal and transdermal delivery. Eur J Pharm Biopharm. 2004;58(2):279–289. | ||
Nair R, Perencevich EN, Blevins AE, Goto M, Nelson RE, Schweizer ML. Clinical effectiveness of mupirocin for preventing Staphylococcus aureus infections in nonsurgical settings: a meta-analysis. Clin Infect Dis. 2016;62(5):618–630. | ||
Jorgensen JH, Ferraro MJ. Antimicrobial susceptibility testing: general principles and contemporary practices. Clin Infect Dis. 1998;26(4):973–980. | ||
Balouiri M, Sadiki M, Ibnsouda SK. Methods for in vitro evaluating antimicrobial activity: A review. J Pharm Anal. 2016;6(2):71–79. | ||
Sesso R, Barbosa D, Leme IL, et al. Staphylococcus using central aureus venous prophylaxis in hemodialysis catheter: effect of mupirocin patients ointment. J Am Soc Nephrol. 1998;9(6):1085–1092. | ||
Piao ZZ, Lee KH, Kim DJ, et al. Comparison of release-controlling efficiency of polymeric coating materials using matrix-type casted films and diffusion-controlled coated tablet. AAPS Pharm Sci Tech. 2010;11(2):630–636. | ||
Patra CN, Priya R, Swain S, Ghose D. Pharmaceutical significance of Eudragit: a review. Futur J Pharm Sci. 2017;3(1):33–45. | ||
Lehman AJ, Hanzlik PJ, Newman HW. Methods for the study of irritation and toxicity of substances applied to the skin and mucous membranes. J Pharmacol Exp Ther. 1944;82(3):377–390. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.