Back to Journals » ClinicoEconomics and Outcomes Research » Volume 12
Development of a Severity Classification System for Sickle Cell Disease
Authors Shah N , Beenhouwer D, Broder MS , Bronte-Hall L , De Castro LM, Gibbs SN , Gordeuk VR, Kanter J, Klings ES, Lipato T, Manwani D, Scullin B , Yermilov I , Smith WR
Received 8 August 2020
Accepted for publication 16 October 2020
Published 28 October 2020 Volume 2020:12 Pages 625—633
DOI https://doi.org/10.2147/CEOR.S276121
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Dean Smith
Nirmish Shah,1 David Beenhouwer,2 Michael S Broder,2 Lanetta Bronte-Hall,3 Laura M De Castro,4 Sarah N Gibbs,2 Victor R Gordeuk,5 Julie Kanter,6 Elizabeth S Klings,7 Thokozeni Lipato,8 Deepa Manwani,9 Brigid Scullin,10 Irina Yermilov,2 Wally R Smith8
1Department of Medicine, Duke University, Durham, NC, USA; 2Partnership for Health Analytic Research (PHAR), LLC, Beverly Hills, CA, USA; 3Foundation for Sickle Cell Disease Research, Hollywood, FL, USA; 4Department of Medicine, University of Pittsburgh Medical Center, Pittsburgh, PA, USA; 5Department of Medicine, University of Illinois at Chicago, Chicago, IL, USA; 6Department of Medicine, University of Alabama at Birmingham, Birmingham, AL, USA; 7Department of Medicine, Boston University School of Medicine, Boston, MA, USA; 8Department of Internal Medicine, Virginia Commonwealth University, Richmond, VA, USA; 9Department of Pediatrics, Albert Einstein College of Medicine, Bronx, NY, USA; 10Department of Medicine, University of North Carolina, Chapel Hill, NC, USA
Correspondence: Nirmish Shah
Duke University, Department of Medicine, Duke South, Durham, NC 27710, USA
Tel +1 (919) 668-5178
Email [email protected]
Purpose: There is no well-accepted classification system of overall sickle cell disease (SCD) severity. We sought to develop a system that could be tested as a clinical outcome predictor.
Patients and Methods: Using validated methodology (RAND/UCLA modified Delphi panel), 10 multi-disciplinary expert clinicians collaboratively developed 180 simplified patient histories and rated each on multiple axes (estimated clinician follow-up frequency, risk of complications or death, quality of life, overall disease severity). Using ratings on overall disease severity, we developed a 3-level severity classification system ranging from Class I (least severe) to Class III (most severe).
Results: The system defines patients as Class I who are 8– 40 years with no end organ damage, no chronic pain, and ≤ 4 unscheduled acute care visits due to vaso-occlusive crises (VOC) in the last year. Patients < 8 or > 40 years with no end organ damage, no chronic pain, and < 2 unscheduled acute care visits are also considered Class I. Patients any age with ≥ 5 unscheduled acute care visits and/or with severe damage to bone, retina, heart, lung, kidney, or brain are classified as Class III (except patients ≥ 25 years with severe retinopathy, no chronic pain, and 0– 1 unscheduled acute care visits, who are considered Class II). Patients not meeting these Class I or III definitions are classified as Class II.
Conclusion: This system consolidates patient characteristics into homogenous groups with respect to disease state to support clinical decision-making. The system is consistent with existing literature that increased unscheduled acute care visits and organ damage translate into clinically significant patient morbidity. Studies to further validate this system are planned.
Keywords: expert panel, disease severity, vaso-occlusive crises, organ damage, chronic pain
Introduction
Sickle cell disease (SCD) is characterized by the presence of sickle hemoglobin (HbS), chronic hemolysis, inflammation and vascular adhesion, recurrent pain episodes, irreversible multi-organ damage, and early death. The clinical course of the disease varies greatly by patient depending on age, complications, comorbidities, and psychosocial health.1 There are multiple genotypes for SCD. The HbSS and HbSβ0-thalassemia genotypes are often associated with the most severe clinical manifestations, while HbSC and HbSβ+-thalassemia are generally considered less severe. There are multiple compound heterozygous sickling genotypes that so vary in significance and clinical severity. Even within individual genotypes, there can be a broad range of disease severity.1
A national or international SCD registry that can be used to characterize and classify SCD severity currently does not exist. While several researchers have developed severity classification systems to predict outcomes and mortality in SCD,2–5 none have been widely adopted in clinical practice in part because of the large number and complexity of variables included. While the Cooperative Study of Sickle Cell Disease (CSSCD)2 and Sickle Cell Disease Assessment Instrument (SCDAI)3 models are practical because they use easily identified predictors, neither have been validated. Furthermore, the CSSCD model excludes adults and has limited contemporary validity because death, which was the second most commonly predicted severe adverse event, is now rare during childhood in high-resource nations.1
Although there is no such classification system for SCD, there are models for classification systems in other diseases which are valid, reliable, and clinically useful. For example, the New York Heart Association (NYHA) classification, the Composite Asthma Severity Index, several cancer staging systems (such as the Eastern Cooperative Oncology Group Performance Status), and other systems for acute illnesses (such as CURB-65 for pneumonia or APACHE II for sepsis) have been developed and are widely used in clinical settings. The simplicity of the NYHA classification has led to its widespread application and while it is partially subjective, it appears to correlate reasonably well with more objective and time-consuming testing.6,7
The aim of this descriptive study was to take the first step in developing a classification system for SCD using a validated expert panel process that could be implemented in a clinical setting and could be tested for its ability to predict clinical outcomes in future studies. Our goal was not to change the definition of SCD but rather to help define a conceptually sound categorization of patients with SCD.
Patients and Methods
Study Design and Participants
We used the RAND/UCLA modified Delphi panel method, which has been more fully described elsewhere.8–10 Briefly, this method is a formal group judgement process which systematically and quantitatively combines expert opinion and literature review evidence by asking panelists to rate, discuss, and then re-rate various patient scenarios. The primary steps in the process include identification of the question to be answered, a literature review of the evidence, selection of expert panelists, generation of a rating form, first round survey, an in-person meeting where panelists discuss areas of disagreement, final ratings and analysis of those ratings, and development of a written summary of areas of agreement.
Our multi-disciplinary panel included 10 expert clinicians (9 Medical Doctors and 1 Doctor of Nursing Practice) from various backgrounds (5 hematologist/oncologists, 3 internists, 1 psychiatrist/public health practitioner, and 1 pulmonologist; 8 treating adults and 2 treating children). We had representatives with diverse experience in clinical, pre-clinical, and epidemiologic research and an average of 20 years of experience caring for SCD patients. We also conducted a targeted literature review summarizing evidence from the 2014 National Heart, Lung, and Blood Institute Expert Panel Report,11 4 SCD severity scoring systems,2–5 and 2 recent systematic literature reviews,12,13 focusing on factors associated with morbidity and mortality in SCD. We referred to this evidence summary when we completed our ratings.
Rating Form
We collaboratively developed the rating form through individual phone interviews. We began by developing a list of patient characteristics that the 2014 National Heart, Lung, and Blood Institute Expert Panel Report,11 SCD severity scoring systems,2–5 and 2 recent systematic literature reviews12,13 listed as risk factors of morbidity and mortality in SCD. Early drafts included up to 34 characteristics. We narrowed down this list by grouping characteristics (eg, organ damage), eliminating those that on their own would not influence one’s broad classification of SCD severity (eg, gender), eliminating disease modifiers (eg, alpha-thalassemia), and eliminating those that are known to affect only a small group of patients (eg, patients on chronic transfusion with alloimmunization). Using the final list of characteristics, we developed 180 distinct patient scenarios (simplified patient histories) made up of all combinations of the final characteristics chosen. We used our combined clinical experience treating patients with SCD to develop these scenarios and did not use data from patient medical records.
Scenarios differed by patient age (<8, 8–15, 16–24, 25–40, or >40 years old), hemoglobin genotype (HbSS/HbSβ0 or HbSC/HbSβ+), presence/absence of chronic pain (defined as ongoing pain on most days over the past 6 months14), and the number of unscheduled acute care visits per year due to vaso-occlusive crises (VOCs) (0–1, 2–4, ≥5). Acute care included unscheduled visits to out-patient offices, the emergency department, or day hospitals, as well as in-patient hospitalizations. VOCs included episodes of acute pain, priapism (sustained, unwanted, painful penile erection lasting 4 or more hours), acute chest syndrome (acute illness with a new lung infiltrate and characterized by fever and/or respiratory symptoms resembling pneumonia), splenic sequestration (blood trapped in the spleen), and hepatic sequestration (blood trapped in the liver). In addition, the simplified patient histories included three categories of end organ damage, defined as no, mild/moderate, or severe damage to organs (Table 1).
 |
Table 1 Definitions of End Organ Damage Used in the Rating Form |
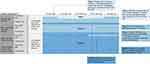
Using a 1 to 9 scale, we rated each of the 180 scenarios on multiple axes, including estimated clinician follow-up frequency, risk of additional serious complications or death in the next 5 years (for patients ≥16 years old) or 10 years (for patients <16 years old), quality of life impact, and overall disease severity (Figure 1). The final classification system was based on the responses related to overall disease severity; ratings on follow-up frequency, risk of complications, and quality of life impact were used to generate discussion about the meaning of overall disease severity. In total, 640 items were rated. We completed ratings independently before a full-day in-person meeting (first-round ratings). At the in-person meeting, we discussed the items and scenarios where our ratings differed. Ratings were completed a second time at the conclusion of the meeting (second-round ratings).
 |
Figure 1 Example rating form of patient scenarios. Each cell represents 1 patient scenario. The table is read from top to bottom and left to right. For example, the first cell in column A1 reads: “In a patient with no end organ damage, no chronic pain, 0–1 unscheduled acute care visits due to VOCs in the last year, with HbSS or HbSβ0, how often should this patient be seen by a SCD provider?” aThis table was replicated for each patient age group: Patient <8, 8–15 years old, 25–40 years old, >40 years old. Young children might have no history, so the classification system might not be as applicable. bA SCD provider includes any clinician treating SCD and its primary consequences (eg, hematologist or pulmonologist). cAdditional serious complications include end organ damage, sepsis, or other. dRisk of complications or death in the next 5 years for patients ≥16 years old and next 10 years for patients <16 years old. eChronic pain defined as ongoing pain present on most days over the past 6 months.12 fAcute care includes unscheduled office visits, ED visits, day hospital visits, and hospitalizations; VOCs include pain, priapism, acute chest syndrome, splenic sequestration, and hepatic sequestration. gRefer to Table 1 for definitions of end organ damage provided. |
Data Analysis
Median ratings were calculated for each item and grouped into 3 categories (1–3, 4–6, 7–9). Disagreement was defined as items with ≥2 individual ratings outside the category in which the median rating fell (eg, ratings of 4, 5, 6, 7, 8, 8, 8, 8, 9, 9 would be a median of 8 with disagreement because 3 ratings were outside the range 7–9). This is a modification of the definition of disagreement used widely in Delphi panels (where disagreement is defined less stringently as ≥2 individual ratings between 1 and 3 and ≥2 individual ratings between 7 and 9). Using the second-round ratings and follow-up discussions by phone and email with the panelists to resolve any remaining areas of disagreement, we identified characteristics that the panel agreed defined a patient as having more or less severe disease. We used these characteristics to develop a 3-level severity classification system, ranging from Class I (least severe) to Class III (most severe).
Results
The proportion of scenarios with disagreement on overall severity decreased from 59% to 23% from the first to second-round ratings. In the second-round ratings, we continued to disagree on some scenarios, such as with patients with 2–4 unscheduled acute care visits due to VOCs in the last year, patients 16–40 years old, and patients with severe damage to bone or retina and no chronic pain. These remaining disagreements were resolved by further rounds of discussion by phone and email following the in-person meeting. We then agreed on the classification definitions listed in Figure 2 and described below.
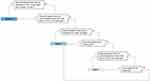
We agreed that patients 8–40 years old with no end organ damage, no chronic pain, and ≤4 unscheduled acute care visits due to VOCs in the last year should be classified as Class I (least severe disease). Patients <8 or >40 years old with no end organ damage, no chronic pain, and <2 unscheduled acute care visits due to VOCs in the last year should also be considered Class I. Patients any age with ≥5 unscheduled acute care visits due to VOCs in the last year should be considered Class III (most severe disease). Similarly, patients of any age with severe damage to bone, retina, heart, lung, kidney, or brain should be classified as Class III, with the exception of patients ≥25 years old presenting with severe retinopathy, no chronic pain, and 0–1 unscheduled acute care visits due to VOCs in the last year, who should be considered Class II (patients with severe damage to bone are unlikely to have no chronic pain and are therefore not specified in this exclusion). All other patients are classified as Class II. Figure 3 illustrates how patients could be classified into one of these classes using only a few key clinical questions.
We agreed that genotype does not affect classification. Scenarios that differed only by hemoglobin genotype were rated similarly, with mean differences in medians in the 0.0–0.4 range out of 9.
Discussion
A validated methodology was used to assist a multi-disciplinary expert panel in developing a 3-level severity classification system for SCD. The system defines patients as Class I (the lowest severity) if they have no end organ damage or chronic pain, and infrequently (≤4 times/year for patients 8–40 and <2 times/year for others) have acute care visits for VOC. The highest severity classification (Class III) is primarily reserved for patients with ≥5 acute care visits and/or severe damage to bone, retina, heart, lung, kidney, or brain. In this descriptive study, we developed the classification system; in later studies, we will test the validity of the system using patient data.
Our resulting classification system is consistent with existing literature. Patients classified by our system as having the most severe disease share characteristics demonstrated to be associated with worse outcomes and death. These include increased frequency of acute pain episodes (acute chest syndrome, hepatic and splenic sequestration, priapism, and thromboembolism), which has been associated with hospital readmissions,15 as well as the accumulation of organ damage,16 which can sometimes occur in the absence of VOCs.17
Patients with chronic pain were classified as at least Class II. Evidence supports that SCD patients with chronic pain have greater healthcare utilization, depression, anxiety, and poorer health-related quality of life.18 Greater opioid use is also associated with worse health-related quality of life among those with chronic pain.19 In addition, the oldest and youngest patients with frequent VOCs (≥2 unscheduled acute care visits) were classified as having more severe disease than patients 8–40 years old with a similar number of VOCs. Increasing age has been associated with risk of early death,13,20 increased acute care visits,21 and a higher severity score in Sebastiani et al’s network analysis model (that used a cohort of 3,380 individuals from the Cooperative Study of Sickle Cell Disease to predict the risk of death).4 We also agreed that younger patients with frequent VOCs should be classified as more severe due to their increased risk for complications earlier in life.20
Our severity classification system does not use hemoglobin genotype as an independent variable. The patient characteristics described in the scenarios accounted for the phenotypic expression of disease, regardless of genotype. This is supported by evidence that shows that there is significant phenotypic variation in disease expression and severity by genotype.1
There are concerns about the changing standards for defining disease in a way that fundamentally changes the number of people considered to have the condition.22 This classification system does not attempt to change the definition of SCD. Instead, we attempt to help define a conceptually sound categorization of patients with SCD. Our severity classification system uses clinical characteristics to consolidate patients into homogenous groups with respect to disease state. Some of these clinical characteristics are transient and may worsen or improve based on therapeutic interventions. Therefore, patients may move between severity classes over time.
Our system is made up of few patient characteristics that can be obtained during a clinical visit. For example, Figure 3 illustrates how patients are classified using only a few key clinical questions. In contrast, Sebastiani et al’s network analysis model included 14 patient characteristics and 5 laboratory values4 and the pediatric severity index consisted of 12 items.5 If our system is validated, its simplicity may improve adoption and, hence, utility. Like other classification systems, it may help clinicians determine follow-up frequency, guide aggressiveness of therapy, or estimate disease progression. For example, the NYHA classification (based on patient reported and physician assessment of cardiac symptoms) correlates with other heart failure measures and is used in clinical settings to assess patients’ functional limitation and guide therapy.23 The Centor score (made up of 4 criteria based on patient signs and symptoms) has also been widely adopted to predict group A ß-hemolytic streptococcal pharyngitis in adults and improve appropriate prescribing of antibiotics.24 However, like other classification systems, our system is not intended to supersede clinical decision-making and we do not propose treatment guidelines for the different levels.
We used the RAND/UCLA modified Delphi panel method to develop this severity system. This process has been used extensively to develop quality measures and clinical guidelines in a variety of clinical areas25 and there is evidence that the resultant measures have content, construct, and predictive validity.26 Furthermore, modified Delphi panels have been shown to generate reproducible outcomes in ways that other methods have not: three separate panels using the method in another disease developed similar clinical guidelines.27
This study had limitations. First, this study is descriptive only and the relationship between severity scores using our system and outcomes has yet to be demonstrated. Second, patient scenarios were simplified patient histories and, by design, did not use patient-reported outcomes, laboratory data, or account for the severity of acute visits (for example, if transfusions or intensive care were required). This may have resulted in scenarios that were too broad to be interpreted consistently by experts. In addition, developing a single classification system applicable to both adults and children may make it less specific for either group; for example, markers of organ damage may be different across age groups. Our system assumes patients have access to acute care and that clinicians can obtain the characteristics used to classify patients (eg, evaluate evidence of organ damage). This may not be the case in low-resource settings. Therefore, on a global scale, our system might only be useful in a minority of cases. Lastly, although the modified Delphi technique has extensive support in the literature, panels consist of a relatively small number of clinicians who bring their individual clinical judgement, expertise, and experience to the process.
Conclusion
Using this rigorous and reproducible process, we developed a straightforward classification system that could be implemented in a clinical setting. Studies to validate this classification system and further refine the tool are planned. Specifically, retrospective studies (including medical chart reviews and/or patient registries) can be conducted to identify how clinical outcomes differ in each severity class definition. Prospective studies can be conducted to test the system’s ability to predict clinical outcomes as well as explore how patients may move between severity classes. After validating this classification system, its use in a clinical setting should help support clinician decision-making and patient care by better predicting health outcomes and disease course for SCD.
Ethical Approval
This article does not describe a research study using human participants or animals, so ethical approval was not required.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
This work was funded by Novartis Pharmaceutical Corporation.
Disclosure
Nirmish Shah has received funding from Novartis (honoraria, consultancy, research funding, speaker’s bureau), Alexion (speaker’s bureau), Global Blood Therapeutics (research funding), CSL Behring and Bluebird Bio (consultant). David Beenhouwer, Michael Broder, Sarah N Gibbs, and Irina Yermilov are employees of the Partnership for Health Analytic Research (PHAR), LLC, which was paid by Novartis to conduct the research described in this manuscript. David Beenhouwer, Michael Broder, Sarah N Gibbs, and Irina Yermilov are employees of the Partnership for Health Analytic Research (PHAR), LLC, which was paid by AbbVie, Akcea, ASPC, Amgen, AstraZeneca, BMS, Boston Scientific Corporation, Celgene, Eisai, Ethicon, GRAIL, Helsinn, Illumina, Innovation and Value Initiative, Ionis, Jazz, Kite, Novartis, Otsuka, Pathnostics, PhRMA, Prothena, Sage, Verde Technologies, Genentech, Greenwich Biosciences, Mirum Pharmaceuticals, Sanofi US Services, Sunovion Pharmaceuticals, and Dompe US to conduct research outside of the submitted work. Lanetta Bronte-Hall has received funding from Novartis (honoraria, consulting), Global Blood Therapeutics (research funding), Pfizer (consultancy and research support), and BlueBird Bio (research funding). Laura M De Castro has received funding from Novartis (honoraria, membership on Board of Directors or advisory committees), Global Blood Therapeutics (membership on Board of Directors or advisory committees), and Pfizer (consultancy). She has received research support from Global Blood Therapeutics, Novartis, Pfizer, and Bayer. Victor Gordeuk has received funding from Global Blood Therapeutics (honoraria, consultancy, research funding), Emmaus (honoraria, consultancy), Novartis (honoraria, consultancy, research funding), Modus Therapeutics (honoraria, consultancy), Pfizer (research funding), Inctye (research funding), CSL Behring (honoraria, consultancy, research funding), Ironwood (research funding), and Imara (research funding). Julie Kanter has received funding from Novartis (honoraria, membership on advisory committees). She has also received funding from AstraZeneca (steering committee), Imara (honoraria), Modus Therapeutics (honoraria), and Global Blood Therapeutics (travel). Elizabeth S Klings has received funding from Novartis (honoraria). She has received research support from Actelion, Reata, Incyte, Bayer, Arena/United Therapeutics. She has received funding from Pfizer (membership on Acute Chest Syndrome adjudication committee for Rivipansel clinical trial) and Micelle (Data and Safety Monitoring Board). Thokozeni Lipato has received funding from Novartis (honoraria) and Global Blood Therapeutics (speaker’s bureau). Deepa Manwani has received funding from Novartis (honoraria, consultancy), Pfizer (consultancy), and Global Blood Therapeutics (consultancy, research funding). Brigid Scullin has received funding from Novartis (honoraria, consultancy). Wally R Smith has received funding from Novartis (honoraria, consultancy) and reports funding as an investigator for NHLBI, HRSA, PCORI, Pfizer, Novartis, Emmaus, Imara, and Shire; consultant for Novartis, Pfizer, Global Blood Therapeutics, and Emmaus. The authors report no other conflicts of interest in this work.
Selected components of this work were presented at the 2019 American Society of Hematology (ASH) Annual Meeting in Orlando, Florida, on December 8, 2019.
References
1. Quinn CT. Minireview: clinical severity in sickle cell disease: the challenges of definition and prognostication. Exp Biol Med. 2016;241(7):679–688. doi:10.1177/1535370216640385
2. Miller ST, Sleeper LA, Pegelow CH, et al. Prediction of adverse outcomes in children with sickle cell disease. N Engl J Med. 2000;342(2):83–89. doi:10.1056/NEJM200001133420203
3. Day SW. Development and evaluation of a sickle cell disease assessment instrument. Pediatr Nurs. 2004;30(6).
4. Sebastiani P, Nolan VG, Baldwin CT, et al. A network model to predict the risk of death in sickle cell disease. Blood. 2007;110(7):2727–2735. doi:10.1182/blood-2007-04-084921
5. van den Tweel XW, van der Lee JH, Heijboer H, Peters M, Fijnvandraat K. Development and validation of a pediatric severity index for sickle cell patients. Am J Hematol. 2010;85(10):746–751. doi:10.1002/ajh.21846
6. Hunt SA, Abraham WT, Chin MH, et al. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American college of cardiology/American heart association task force on practice guidelines (writing committee to update the 2001 guidelines for the evaluation and management of heart failure): developed in collaboration with the American college of chest physicians and the international society for heart and lung transplantation: endorsed by the heart rhythm society. Circulation. 2005;112(12):e154–235. doi:10.1161/CIRCULATIONAHA.105.167586
7. Lim FY, Yap J, Gao F, Teo LL, Lam CSP, Yeo KK. Correlation of the New York Heart Association classification and the cardiopulmonary exercise test: a systematic review. Int J Cardiol. 2018;263:88–93. doi:10.1016/j.ijcard.2018.04.021
8. Fink A, Kosecoff J, Chassin M, Brook RH. Consensus methods: characteristics and guidelines for use. Am J Public Health. 1984;74(9):979–983. doi:10.2105/ajph.74.9.979
9. Campbell SM, Braspenning J, Hutchinson A, Marshall M. Research methods used in developing and applying quality indicators in primary care. Qual Saf Health Care. 2002;11(4):358–364. doi:10.1136/qhc.11.4.358
10. Fitch K, ed. The Rand/UCLA Appropriateness Method User’s Manual. Rand; 2001.
11. National Institutes of Health, National Heart, Lung, and Blood Institute. Evidence-based management of sickle cell disease: expert panel report. 2014:161.
12. Meier ER, Fasano RM, Levett PR. A systematic review of the literature for severity predictors in children with sickle cell anemia. Blood Cells Mol Dis. 2017;65:86–94. doi:10.1016/j.bcmd.2017.01.014
13. Maitra P, Caughey M, Robinson L, et al. Risk factors for mortality in adult patients with sickle cell disease: a meta-analysis of studies in North America and Europe. Haematologica. 2017;102(4):626–636. doi:10.3324/haematol.2016.153791
14. Dampier C, Palermo TM, Darbari DS, Hassell K, Smith W, Zempsky W. AAPT diagnostic criteria for chronic sickle cell disease pain. J Pain. 2017;18(5):490–498. doi:10.1016/j.jpain.2016.12.016
15. Ballas SK, Lusardi M. Hospital readmission for adult acute sickle cell painful episodes: frequency, etiology, and prognostic significance. Am J Hematol. 2005;79(1):17–25. doi:10.1002/ajh.20336
16. Ballas SK, Lieff S, Benjamin LJ, et al. Definitions of the phenotypic manifestations of sickle cell disease. Am J Hematol. 2010;85:6–13. doi:10.1002/ajh.21550
17. van Beers EJ, van Tuijn CFJ, Mac Gillavry MR, et al. Sickle cell disease-related organ damage occurs irrespective of pain rate: implications for clinical practice. Haematologica. 2008;93(5):757–760. doi:10.3324/haematol.12152
18. Sogutlu A, Levenson JL, McClish DK, Rosef SD, Smith WR. Somatic symptom burden in adults with sickle cell disease predicts pain, depression, anxiety, health care utilization, and quality of life: the PiSCES project. Psychosomatics. 2011;52(3):272–279. doi:10.1016/j.psym.2011.01.010
19. Karafin MS, Singavi A, Hussain J, et al. Predictive factors of daily opioid use and quality of life in adults with sickle cell disease. Hematology. 2018;23(10):856–863. doi:10.1080/10245332.2018.1479997
20. Platt OS, Brambilla DJ, Rosse WF, et al. Mortality in sickle cell disease. Life expectancy and risk factors for early death. N Engl J Med. 1994;330(23):1639–1644. doi:10.1056/NEJM199406093302303
21. Lanzkron S, Little J, Field J, et al. Increased acute care utilization in a prospective cohort of adults with sickle cell disease. Blood Adv. 2018;2(18):2412–2417. doi:10.1182/bloodadvances.2018018382
22. Doust JA, Bell KJL, Glasziou PP. Potential consequences of changing disease classifications. JAMA. 2020;323(10):921–922. doi:10.1001/jama.2019.22373
23. Yap J, Lim FY, Gao F, Teo LL, Lam CSP, Yeo KK. Correlation of the New York heart association classification and the 6-minute walk distance: a systematic review: correlation of NYHA and 6MWD. Clin Cardiol. 2015;38(10):621–628. doi:10.1002/clc.22468
24. Aalbers J, O’Brien KK, Chan W-S, et al. Predicting streptococcal pharyngitis in adults in primary care: a systematic review of the diagnostic accuracy of symptoms and signs and validation of the centor score. BMC Med. 2011;9(1):67. doi:10.1186/1741-7015-9-67
25. Boulkedid R, Abdoul H, Loustau M, Sibony O, Alberti C. Using and reporting the delphi method for selecting healthcare quality indicators: a systematic review. PLoS One. 2011;6(6):e20476. doi:10.1371/journal.pone.0020476
26. Kravitz RL, Laouri M, Kahan JP, et al. Validity of criteria used for detecting underuse of coronary revascularization. JAMA. 1995;274(8):632–638. doi:10.1001/jama.1995.03530080048040
27. Shekelle PG, Kahan JP, Bernstein SJ, Leape LL, Kamberg CJ, Park RE. The reproducibility of a method to identify the overuse and underuse of medical procedures. N Engl J Med. 1998;338(26):1888–1895. doi:10.1056/NEJM199806253382607
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.