Back to Journals » Journal of Pain Research » Volume 10
Development of a novel algorithm to determine adherence to chronic pain treatment guidelines using administrative claims
Authors Margolis JM , Princic N, Smith DM, Abraham L, Cappelleri JC , Shah SN, Park PW
Received 27 July 2016
Accepted for publication 16 November 2016
Published 8 February 2017 Volume 2017:10 Pages 327—339
DOI https://doi.org/10.2147/JPR.S118248
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Michael Schatman
Jay M Margolis,1 Nicole Princic,2 David M Smith,2 Lucy Abraham,3 Joseph C Cappelleri,4 Sonali N Shah,5 Peter W Park5
1Truven Health Analytics, Bethesda, MD, 2Truven Health Analytics, Cambridge, MA, USA; 3Pfizer Ltd, Tadworth, UK; 4Pfizer Inc, Groton, CT, 5Pfizer Inc, New York, NY, USA
Objective: To develop a claims-based algorithm for identifying patients who are adherent versus nonadherent to published guidelines for chronic pain management.
Methods: Using medical and pharmacy health care claims from the MarketScan® Commercial and Medicare Supplemental Databases, patients were selected during July 1, 2010, to June 30, 2012, with the following chronic pain conditions: osteoarthritis (OA), gout (GT), painful diabetic peripheral neuropathy (pDPN), post-herpetic neuralgia (PHN), and fibromyalgia (FM). Patients newly diagnosed with 12 months of continuous medical and pharmacy benefits both before and after initial diagnosis (index date) were categorized as adherent, nonadherent, or unsure according to the guidelines-based algorithm using disease-specific pain medication classes grouped as first-line, later-line, or not recommended. Descriptive and multivariate analyses compared patient outcomes with algorithm-derived categorization endpoints.
Results: A total of 441,465 OA patients, 76,361 GT patients, 10,645 pDPN, 4,010 PHN patients, and 150,321 FM patients were included in the development of the algorithm. Patients found adherent to guidelines included 51.1% for OA, 25% for GT, 59.5% for pDPN, 54.9% for PHN, and 33.5% for FM. The majority (~90%) of patients adherent to the guidelines initiated therapy with prescriptions for first-line pain medications written for a minimum of 30 days. Patients found nonadherent to guidelines included 30.7% for OA, 6.8% for GT, 34.9% for pDPN, 23.1% for PHN, and 34.7% for FM.
Conclusion: This novel algorithm used real-world pharmacotherapy treatment patterns to evaluate adherence to pain management guidelines in five chronic pain conditions. Findings suggest that one-third to one-half of patients are managed according to guidelines. This method may have valuable applications for health care payers and providers analyzing treatment guideline adherence.
Keywords: chronic pain, drug therapy, practice guidelines, adherence, algorithm
Abbreviations
AAN, American Academy of Neurology; ACR, American College of Rheumatology; CPT-4®, Current Procedural Technology, 4th edition; DCI, Deyo-Charlson Comorbidity Index date; EFNS, European Federation of Neurological Societies; EULAR, European League Against Rheumatism; FL, first-line; FM, fibromyalgia; GLMs, generalized linear models; GT, gout; HCPCS, Healthcare Common Procedure Coding System; ICD-9-CM, International Classification of Diseases, 9th Revision, Clinical Modification; LL, later-line; NDC, National Drug Code (United States); NR, not recommended; OA, osteoarthritis; OARSI, Osteoarthritis Research Society International; OTC, over-the-counter; pDPN, painful diabetic peripheral neuropathy; PHN, postherpetic neuralgia; SD, standard deviation; US, United States
Introduction
In recent decades, the focus of patient care has been shifting toward disease management and quality improvement. Clinical practice guidelines, according to the 2011 definition developed by the Institute of Medicine, are disease management recommendations intended to optimize patient care, “informed by systematic review of evidence and an assessment of the benefits and harms of alternative care options”.1 Objectives common to numerous clinical practice guidelines available from the US National Guideline Clearinghouse (Agency for Healthcare Research and Quality, US Department of Health and Humans Services) include improving the quality of care, treatment outcomes, appropriateness and effectiveness of care, and providing an optimal ratio of costs to benefits.2 While most physicians are aware of their practice’s relevant guidelines, numerous studies have found gaps in clinician adherence to guidelines, leading to suboptimal outcomes of morbidity and mortality and significant financial costs estimated at $100 billion annually in the US.3–7
Guidelines should help physicians prescribe the best treatment management pathway for most patients. Some disease-specific guidelines are very clear and straightforward with their recommendations, such as Hypertension Guidelines linked to metrics (eg, blood pressure) to help guide when to incorporate adjunctive therapy.5,7 Other conditions are more complex, with many different treatment pathways one could prescribe, and one gold standard for guidelines may not exist, such as with chronic pain conditions. Guidelines for chronic pain conditions vary for FL treatments and adjunctive therapy based on the nature and level of pain. Measuring the level of pain is typically a subject matter that may vary for different patients and pain etiologies, making management even more complex. Furthermore, most patients have multiple pain comorbidities, making it hard to pinpoint the pain condition to be treated. Another challenge with pain conditions is the option for treatment with opioids, for which there is currently considerable controversy surrounding opioid use and abuse. Chronic pain guidelines typically layer treatment recommendations to deal with these factors, with treatment choices that may be complex and/or confusing.8–10
Measuring compliance with treatment guidelines has been studied in numerous disease areas, including asthma, heart failure, coronary artery disease, depression, diabetes, hyperlipidemia, hypertension, migraine, chronic obstructive pulmonary disease, hypertension, ischemic heart disease, stroke, and osteoporosis.3,4,6,7,11–14 Methods ranged from examining medical and pharmacy health care claims for specific characteristics, such as prescriptions for selected drugs, to examination of electronic medical record (EMR) evidence, medical chart reviews, and physician or patient surveys. The use of administrative health care claims for guideline adherence studies leverages the potential longitudinality and large sample sizes afforded by “big data” coupled with documented evidence of actual care or treatments received in real-world practice settings and therefore of great potential utility for conducting such studies.4 However, despite the prevalence, expense, and numerous guidelines for the treatment of various types of pain, and especially in view of current controversies surrounding opioid use/abuse, we found a paucity of studies utilizing big data to measure adherence to chronic pain treatment guidelines.15 A deterrent to such studies may be the challenges in determining guideline adherence given the complexity of treatment pathways.
The goal of this study was to formulate a method for analyzing pain pharmacotherapies received in the real-world setting by patients with newly diagnosed chronic pain conditions by comparing the medications taken for the disease/condition with the existing pain management guidelines and measuring the effects of adherence and nonadherence to treatment guidelines. This study undertook a novel approach by devising a unique algorithm that systematically categorizes adherence based on practices common to the pain management guidelines and then using administrative health care claims to create and analyze longitudinal treatment patterns before and after the index date diagnosis for each patient to compare against the guideline adherence algorithm. The following five chronic pain conditions were evaluated using this new algorithm: OA, GT, pDPN, PHN, and FM.
Methods
Study design and data sources
Adherence to pain management guidelines was measured using prescription claims for evaluating treatment of the chronic disease pain, as well as the disease guidelines of focus. Study variables were derived from administrative medical and pharmacy claims data from the Truven Health Analytics’s MarketScan® Commercial Claims and Encounters (Commercial) and Medicare Supplemental (Medicare) Databases using enrollment records, service dates, ICD-9-CM codes, CPT-4® codes, HCPCS codes, and NDCs, to identify diagnoses and services provided.
The process involved two main steps. First, we identified all relevant guidelines through a review of literature gathering and comparing published pain guidelines for the selected conditions, then evaluated different layers of recommendations and levels of evidence, assessed consistency among available publications, and developed categorizations for the medications. The following are the guidelines used for each of the five chronic pain conditions (note that more recent versions of these guidelines may be available):
GT: ACR 2012 guidelines for GT (part 2 – flare ups),18 EULAR 2006 gout guidelines,19 and British Society for Rheumatology – Treatment Guidelines for Gout 2007.20
Neuropathic pain (used for both PHN and pDPN): EFNS 2010,21 AAN Guidelines 2011,22 Neuropathic Pain and Pharmacological Treatment,23 and 2007 Pain – Dworkin.24
FM: Canadian Guidelines 2012.25
Based on the guidelines for each condition, pain medications were grouped as FL for those drug classes recommended as the best choice/highest efficacy, as LL for drug classes with limited evidence of effectiveness or considered an alternative when there is an inadequate response to FL, and as NR for treatments without evidence of efficacy, not evaluated by the guidelines or deemed inappropriate for the condition (Table 1). (All FL, LL, and NR medications by chronic pain condition may be found in Table S1.)
The MarketScan® Commercial Claims and Encounters and Medicare Supplemental databases are fully compliant with the Health Insurance Portability and Accountability Act (HIPAA), and have been certified to satisfy conditions set forth in Sections 164.514 (a)-(b)1ii of the HIPAA privacy rule regarding the determination and documentation of statistically de-identified data. Therefore because this study used only de-identified patient records and did not involve the collection, use, or transmittal of individually identifiable data, Institutional Review Board (IRB) approval to conduct this study and patient consent were not required.
Algorithm development
The second step was developing the algorithm to link patients’ patterns of medication use to the guidelines. All the possible pathways of treatment that were occurring in these newly diagnosed patient populations had to be considered. Ideally, the goal was to group all patients according to their clinicians’ pain medication prescribing patterns as either adherent or nonadherent to the pain management guidelines. It became apparent early in the process that the variability found in observational real-world prescription data, and especially for the large sample sizes available, required a third category, referred to as the “unsure” cohort, that could not be categorized definitively due to unclear or unexplainable treatment patterns (ie, no pain medications or inconsistent medication patterns). These patients were grouped separately to reduce misclassification error in guideline adherent and nonadherent cohorts. Figure 1 pictorially depicts all of the treatment pathways and decision tree rules leading to the assignment of a patient’s pain medication pattern as adherent, nonadherent, or unsure.
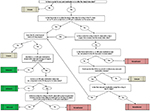  | Figure 1 Adherence algorithm treatment pathways and decision tree. Abbreviations: FL, first-line; LL, later-line; NR, not recommended. |
The algorithm assessed timing on the receipt of pain medications and the days’ supply (as stated in the prescription claim) relative to their index date diagnosis date up to 90 days before (preindex) or prescribed during the 12-month period following the index date diagnosis (postindex) relative to guidelines for each pain condition and using the above categorizations of drugs as FL, LL, or NR. The first pain medication class received postindex was placed in context with all other pain medications received up to 90 days preindex.
A patient’s clinicians’ pain prescribing was determined to be adherent to the treatment guidelines if there was at least 30 days of continuous drug supply of an FL drug class during the postindex period prior to having any new prescriptions for an LL or NR drug class. If the FL prescription claim occurred prior to the index date date and the days supply overlapped the index date, then this was considered acceptable. Prescriptions for LL or NR classes in the 90 days prior to index date were assumed to be for the treatment of a comorbid condition and therefore did not impact adherence classification.
A patient’s pain prescription pattern was identified as nonadherent to treatment guidelines if the first prescription during the postindex period was for a new treatment (ie, one not previously prescribed during the 90 days prior to index date) in LL or NR classes prior to receiving 30 days of continuous drug supply of an FL treatment.
A patient was grouped into the unsure category if they had no pharmacotherapy of any FL, LL, or NR drug during the postindex period, had no FL medications and no new LL or NR treatments (ie, continued on LL or NR treatments already prescribed during the 90 days prior to index date), or had <30 days of continuous drug supply on any FL treatment (ie, insufficient initial trial of FL treatments).
Patient selection
Patients were initially identified, considered newly diagnosed, for a pain cohort if they had ≥1 inpatient or ≥2 outpatient claims at least 30 days apart with an ICD-9-CM diagnosis code for the chronic pain condition (OA 715.xx, GT 274.xx, pDPN 357.2x, PHN 053.1x, and FM 729.1x) during the period from July 1, 2006 to June 30, 2013, and were aged ≥18 years on the date of the first such diagnosis found (index date). In addition, to confirm that the diagnosis was painful, patients in the pDPN cohort were required to have a prescription neuropathic pain medication during the first 90 days. Patients were then excluded if they had a claim anytime during the study period with a diagnosis or procedure code for pregnancy, transplant surgery, or malignant cancer (except for basal cell and squamous cell skin cancers and benign neoplasms). All patients were required to have continuous medical and pharmacy benefit eligibility for a minimum of 365 days before the index date (preindex period) with no diagnoses for the chronic pain condition of interest (proxy for newly diagnosed). Continuous medical and pharmacy benefits were also required for at least 365 days following the index date diagnosis date (postindex period).
Outcome measurements
Each of the five chronic pain condition cohorts was analyzed separately to evaluate the adherent, nonadherent, and unsure adherence cohorts according to the adherence algorithm and drug treatment guideline classifications. Each algorithm endpoint (ie, reason and pathway in the decision tree) was identified and reported.
Health care cost and health care resource utilization were also measured for patients during the preindex and postindex periods for each of the five pain condition cohorts and compared by guideline adherence status for adherent versus nonadherent to guideline cohorts. The patients classified into the unsure group were not included in the direct comparison of these outcomes because a clear status of adherence could not be determined. The detailed outcome analyses are the focus of and reported in a separately (to be) published article on this topic.
Key covariates
Demographic characteristics (age, gender, geographic region, urban residence, primary payer [commercial or Medicare], and insurance plan type) were measured on the index date for each patient in each of the five pain condition cohorts. Clinical characteristics measured in both pre- and postindex periods included comorbid conditions, pain-related symptoms, pain-related procedures (eg, physical therapy), and pain medication use. General health status (relative comorbidity) was also measured using the DCI,26 the number of unique three-digit ICD-9 codes, and the number of unique drugs received.27
Statistical analysis
Patient demographics, clinical characteristics, algorithm outcomes, and health care expenditure outcomes were summarized separately for each pain condition in a series of Microsoft Excel tables, with counts and percentages of patients in each category for categorical variables and with the mean and SD for continuous variables. GLMs28 were estimated to identify the impact of guideline adherence versus nonadherence on health care utilization and costs for each chronic condition, controlling for potential confounding bias due to differences in patient demographics and preindex clinical characteristics. Details about the specific models conducted and results of all descriptive and multivariate analyses for the outcomes are provided in depth in the accompanying article that is focused on cost and utilization outcomes comparing patients classified as adherent versus nonadherent to the respective treatment guidelines.
Results
Study population
There were 441,465 OA patients, 76,361 GT patients, 10,645 pDPN patients, 4,010 PHN patients, and 150,321 FM patients, meeting all eligibility criteria. The mean ages ranged from 48.6 years for FM patients to 65.8 years for PHN. Females represented the majority of patients in the OA, PHN, and FM cohorts. The pDPN cohort had approximately equal percentages of females and males. GT patients were predominantly males. Patients in the pDPN cohort had the highest comorbidity burden prior to diagnosis as measured by DCI score (3.0 versus <1.0 for all other cohorts) and higher mean counts of both unique ICD-9-CM diagnosis codes and unique drugs prior to index date compared to all other conditions. (Patient demographics and clinical characteristics may be viewed in Table S2.)
Algorithm outcomes
Following implementation of the categorization algorithm to each of the five cohorts, the proportion of patients whose prescribing pattern was found to be adherent to the guidelines according to the algorithm ranged from approximately a quarter to slightly more than half depending on the pain condition (51.1% of OA patients, 25% of GT patients, 59.5% of pDPN patients, 54.9% of PHN patients, and 33.5% of FM patients). The pain medication prescribing patterns of the remaining patients from each pain cohort were either identified as being nonadherent to the guidelines or placed into the unsure category (Table 2).
Adherent
The majority (~90%) of patients with prescribing patterns adherent to the guidelines were identified by one of the following two pathways:
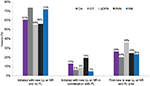
In one pathway, the first prescription for a pain medication following the index date was from the FL class. These patients then continued on FL for a minimum of 30 days prior any prescription for additional pain medications. Clinician’s prescribing adherence in this group was the most straightforward to identify as they were not prescribing any pain medications in combination with the FL treatment.
In the other pathway, the first prescription for a pain medication following the index date was for an FL class and patients then continued on FL for a minimum of 30 days, but these patients also received prescription(s) for drugs in the LL or NR classes that were not new (present within 90 days preindex). Clinician’s prescribing adherence in this group was more difficult to identify because there were pain medications for preexisting conditions in combination with the FL treatment for the newly diagnosed disease.
The results for patients whose prescribing patterns were adherent to guidelines is shown in Figure 2.
Nonadherent
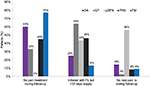
The majority (~90%) of clinician’s prescribing patterns found nonadherent to the guidelines were identified by one of the following two pathways:
1. The first prescription following the index date was for a new initiated LL or NR class not found preindex, and no prior FL drugs overlapping or after the index date.
2. The first prescription following the index date was for an LL or NR treatment found preindex, and the first new prescription not found preindex was for a newly initiated LL or NR class, without no prescriptions for drugs in the FL class.
Only 5%–13% of the clinician’s prescribing patterns in the nonadherent group were categorized as nonadherent because they initiated treatment on combination therapy (FL concomitant with a new LL or NR).
The results for patients whose prescribing pattern was nonadherent to guidelines are shown in Figure 3.
Unsure
A patient was grouped into the unsure category if the treatment pathways could not be clearly identified as being adherent or nonadherent to the guidelines. The reasons for categorizing prescribing patterns into the unsure group, as shown in Figure 3, varied over the five pain conditions. For the OA cohort and the FM cohort, 60% and 77% of the unsure groups, respectively, were patients with no claims for any pain medications. It is possible that some patients in this group were taking OTC medications or other treatments not usually found in claims data (eg, acupuncture). By definition, patients with a “painful” condition such as pDPN required treatment, although is was found that 56% of patients in the unsure group received treatments only in the postperiod who were already present preindex. For both the pDPN cohort and the PHN cohort, 43.6% and 46.3%, respectively, did not have a full 30-day supply of their FL medication.
The results for patients for whom it was unsure if they followed guidelines are shown in Figure 4.
Discussion
In this study, a novel algorithm was systematically designed for using big data to categorize the extent of clinicians’ prescribing adherence to chronic pain management guidelines based on each patient’s real-world treatment pattern over a period of 90 days before and up to 12 months after their initial diagnosis. This algorithm and the applied analytical methods accounted for a number of previously identified problems when using claims data to analyze adherence: 1) accounting for clinicians failing to prescribe an indicated drug or prescribing a contraindicated drug; 2) taking measures to minimize incomplete data (eg, including patients continuously enrolled in a health plan as the source of claims data, assessing patient eligibility for pharmacotherapy over the entire assessment period); 3) using methods that adjust for early prescription refills and/or medication adjustments within a drug class (eg, dose adjustment or drug switching); and 4) requiring positive disease identification (ie, ≥1 inpatient or >2 outpatient claims at least 30 days apart with the ICD-9-CM diagnosis code for condition, not including provisional diagnostic claims such as laboratory testing and diagnostic radiology) to identify patients requiring therapy.4
It is important to note that our methods also allowed for patients who have multiple pain conditions, as is most often the case in the real world, and so more generally applicable. The data source, administrative claims, does not account for prescriptions written but never filled, and so the adherence categorizations may underestimate clinician’s guideline adherence. Integration of administrative claims with electronic medical records has the potential to fill that analytic gap, although that was not done for this study. Our study methods are in agreement with the 10-step method for assessing both clinician’s evidence-based adherence and patients’ medication adherence identified by Kawamoto et al4 and have gone one step further to explicitly document the algorithm used for determining guideline adherence.
Despite differences in pain therapy recommendations for the five chronic pain conditions, the guideline adherence algorithm was applied to each condition without disease-specific exceptions. Slightly over half of OA, pDPN, and PHN patients were found to have had pain therapy adherent with guidelines. These percentages are consistent with findings from other studies of adherence to treatment in evidence-based practice guidelines that also found adherence in an average of ~50% of patients across several chronic conditions (asthma, chronic heart failure, coronary artery disease, depression, diabetes, hyperlipidemia, hypertension, and migraine).3,29 The results for FM showing only 33.5% of patients receiving guideline-adherent therapy, while differing from the 50% adherence in other conditions, were not surprising based on the difficulty of diagnosing this syndrome, the broad array of symptoms, and the often lengthy period of rule-out diagnoses resulting in numerous treatment trials preceding a positive diagnosis.30
The adherence algorithm did not, however, appear to be an ideal fit for the analysis of GT. Of the 68% of GT patients put into the unsure category, approximately one-third were found to have received no prescription GT medications and two-thirds received <30 days of an FL GT medication. In practice, it is known that many patients find relief using OTC NSAIDs, which would not be detected in claims data, and some find relief using dietary restrictions alone. Furthermore, colchicine dosing for the relief of GT flares (two tablets at flare onset, one tablet 1 hour later, and then one to two tablets daily until the flare subsides) may involve less than a 30-day supply,31 and the most common oral steroid dosing is typically a 1–2-week dosage regimen32 and also <30-day supplies.
Our approach to developing this algorithm and analytical method may be used for developing similar algorithm-based guideline adherence analyses in other chronic disease areas. Overall, this algorithm is particularly beneficial to use when the sample size is large and the condition is managed with prescription medications. It may present difficulty or require further refinements when the targeted condition has a small sample size and treatment involves medications not covered by insurance or uses short courses of therapy. Furthermore, the administrative claims databases used for this analysis were very large, diverse, and geographically dispersed. Investigators attempting to use other prescription claims databases need to be aware of their specific formulary restrictions that could limit or bias results from real-world settings.
While this analysis was done using only prescription claims, we believe that significant benefit could be gleaned by using EMR or survey data in combination with the real-world claims data. The claims data provide evidence of the pharmacotherapy that patients received. EMR or survey data may complement that with patient history and clinical data unavailable in the claims, potentially adding more details to drive better definition of adherent and nonadherent prescribing patterns and fewer in the unsure group. Importantly, OTC treatments that cannot be captured in claims data may be noted in EMRs. OTC treatments, particularly NSAIDs for OA, were part of FL therapy and therefore may result in patients being more appropriately classified as “adherent”.
Certainly this algorithm and methodology needs to undergo further scrutiny to refine the definitions of guideline adherence and nonadherence, to reduce the percentage of unsure categorizations, and to account for influences of comorbid pain conditions. It would also be beneficial to adapt the algorithm so that it could address longer term guideline adherence (beyond the 30-day requirement) to assess outcomes of that therapy. Nonetheless, we believe that further use and revision of the algorithm and analytical methodology, including validation of guideline adherence using EMRs or medical charts and expert clinician input or inclusion of alternative treatments such as physical therapy into the algorithm, can enable broader use, perhaps with other disease states beyond pain, and provide another tool for health care managers in improving patient outcomes.
Limitations
There were several limitations to this analysis that should be identified. This was a first attempt (that we know of) to develop a claims-based algorithm to determine the adherence of guidelines for chronic pain using administrative claims. This was challenging due to the lack of evidence related to chronic pain outcomes in secondary data sources. We also acknowledge that clinical judgment and individualizing treatment to patients may warrant deviation from chronic pain guidelines that may often be based on expert opinion rather than strong evidence. Pain pharmacotherapies may require trial, titration, and managing adverse effects, any of which may cause departure from guidelines, regardless of the degree of flexibility in the categorization algorithm. The data have not been cross-validated with patient charts or physician notes and are thus subject to misclassification of prescribing patterns in the adherent versus nonadherent groups. Grouping patients with questionable treatment pathways into a third unsure group removed those patients from the comparisons who had questionable prescription patterns or no treatment; however, the absence of additional information not available in claims (ie, pain intensity, full patient history, and OTC drug use) leaves open the possibility for further misclassification. In addition, patients’ pain medications may have been grouped as nonadherent or unsure adherence due to the patient having contraindicated conditions or allergies resulting in their inability to take an FL agent.
This was an observational retrospective cohort study using administrative claims data, which are not collected for research purposes and therefore are subject to measurement error, which could be introduced by inaccuracy in medical coding (under- or overcoding for pain conditions), and direct information from physician’s notes and/or patient charts was not available. Some of the chronic conditions, such as FM and OA, may take years to diagnose, may be treated based on provisional diagnoses, and may not get a diagnosis at every office visit; thus, some patients may have appeared to be newly diagnosed who actually were not. Prescription data were used as the principal measure of guideline adherence, and the complexity of weaving the wide range of individual patient characteristics into the treatment algorithms was beyond the scope of this study. Furthermore, although associations can be made to describe the relationship of treatment adherence with outcomes, causality cannot be inferred in claims data. MarketScan health care claims databases are US-based convenience samples of commercially insured employees, retirees, and their dependents, and therefore the results may not be generalizable to other populations in the US or abroad.
Conclusion
To our knowledge, this is the first study to develop a prescription claims-based algorithm using treatment patterns in “real-world” data to evaluate adherence to guidelines for management of chronic pain. The methods developed in this study allowed researchers to objectively assess adherence to guidelines, finding that only about one-third to one-half of patients across the five conditions that span the three areas of chronic pain are being treated according to recommendations. Further research is needed to improve and test this algorithm in other conditions. We believe that the approach used for developing and using the algorithm may be applied to other conditions and thus could provide a valuable tool for health care systems and managed care organizations to use for optimizing care.
Acknowledgments
The authors acknowledge the key contributions of Boris Ivanov for his work in programming analytics and technical support of the research study. This study was sponsored by Pfizer.
Disclosure
Jay M Margolis, Nicole Princic, and David M Smith are employees of Truven Health Analytics, which was paid by Pfizer Inc in connection with the research and development of this manuscript. Lucy Abraham, Joseph C Cappelleri, Sonali N Shah, and Peter W Park are employees and shareholders of Pfizer Inc. The authors report no other conflicts of interest in this work.
References
Institute of Medicine, Graham R, Mancher M, Wolman DM, Greenfield S, Steinberg E, editors. Clinical Practice Guidelines We Can Trust. Washington, DC: National Academies Press; 2011:2. Available from: https://www.nap.edu/catalog/13058/clinical-practice-guidelines-we-can-trust. Accessed January 17, 2015. | ||
National Guideline Clearinghouse, Agency for Healthcare Research and Quality, United States Department of Health and Humans Services. Available from: http://www.guideline.gov/index.aspx. Accessed September 21, 2015. | ||
Thier SL, Yu-Isenberg KS, Leas BF, et al. In chronic disease, nationwide data show poor adherence by patients to medication and by physicians to guidelines. Manag Care. 2008;17(2):48–57. | ||
Kawamoto K, Allen LaPointe NM, Silvey GM, Anstrom KJ, Eisenstein EL, Lobach DF. Development and evaluation of an improved methodology for assessing adherence to evidence-based drug therapy guidelines using claims data. AMIA Annu Symp Proc. Epub 2007:394–398. | ||
Milchak JL, Carter BL, James PA, Ardery G. Measuring adherence to practice guidelines for the management of hypertension: an evaluation of the literature. Hypertension. 2004;44(5):602–608. | ||
Kim JY, Kim HJ, Jung SY, et al. Utilization of evidence-based treatment in elderly patients with chronic heart failure: using Korean Health Insurance claims database. BMC Cardiovasc Disord. 2012;12:60. | ||
Theodorou M, Stafylas P, Kourlaba G, Kaitelidou D, Maniadakis N, Papademetriou V. Physicians’ perceptions and adherence to guidelines for the management of hypertension: a national, multicentre, prospective study. Int J Hypertens. 2012;2012:503821. | ||
Berger JE. The ongoing challenge of pain medications. Am J Pharm Benefits. 2014;6:68–69. | ||
Chronic Pain Medical Treatment Guidelines Medical Treatment Utilization Schedule. American Academy of Pain Medicine. 2008. Available from: http://www.painmed.org/files/medical-treatment-utilization-schedule-proposed-regulations.pdf. Accessed January 17, 2015. | ||
O’Rorke JE, Chen I, Genao I, Panda M, Cykert S. Physicians’ comfort in caring for patients with chronic nonmalignant pain. Am J Med Sci. 2007;333(2):93–100. | ||
Baker CL, Zou KH, Su J. Long-acting bronchodilator use after hospitalization for COPD: an observational study of health insurance claims data. Int J Chron Obstruct Pulmon Dis. 2014;9:431–439. | ||
DiMartino LD, Shea AM, Hernandez AF, et al. Use of guideline-recommended therapies for heart failure in the Medicare population. Clin Cardiol. 2010;33(7):400–405. | ||
Kirigaya D, Nakayama T, Ishizaki T, Ikeda S, Satoh T. Management and treatment of osteoporosis in patients receiving long-term glucocorticoid treatment: current status of adherence to clinical guidelines and related factors. Intern Med. 2011;50(22):2793–2800. | ||
Milchak JL, Carter BL, Ardery G, et al. Physician adherence to blood pressure guidelines and its effect on seniors. Pharmacotherapy. 2008;28:843–851. | ||
Broekmans S, Dobbels F, Milisen K, Morlion B, Vanderschueren S. Medication adherence in patients with chronic non-malignant pain: is there a problem? Eur J Pain. 2009;13(2):115–123. | ||
Zhang W, Nuki G, Moskowitz RW. OARSI recommendations for the management of hip and knee osteoarthritis Part III: changes in evidence following systematic cumulative update of research published through January 2009. Osteoarthritis Cartilage. 2010;18(4):476–499. | ||
Hochberg M, Altman R, April K, et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken). 2012;64(4):465–474. | ||
Khanna D, Fitzgerald JD, Khanna PP, et al. 2012 American College of Rheumatology Guidelines for Management of Gout Part I: systematic non-pharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res (Hoboken). 2012;64:1431–1446. | ||
Zhang W, Doherty M, Bardin T, et al. EULAR evidence based recommendations for gout. Part II: management. Report of a task force of the EULAR Standing Committee For International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis. 2006;65(10):1312–1324. | ||
Jordan KM, Cameron JS, Snaith M, et al. British Society for Rheumatology and British Health Professionals in Rheumatology Guideline for the Management of Gout. Rheumatology. 2007;46(8):1372–1374. | ||
Attal N, Cruccu G, Baron R, et al. EFNS guidelines on the pharmacological treatment of neuropathic pain: 2010 revision. Eur J Neurol. 2010;17(9):1113–e88. | ||
American Academy of Neurology. Treatment of Painful Diabetic Peripheral Neuropathy. 2011. Available from: www.aan.com. Accessed September 21, 2015. | ||
Jongen JL, Hans G, Benzon HT. Neuropathic pain and pharmacological treatment. Pain Pract. 2014;14:1–13. | ||
Dworkin RH, O’Conner AB, Backonja M, et al. Pharmacologic management of neuropathic pain: evidence based medicine. Pain. 2007;132(3):237–251. | ||
Fitzcharles MA, Ste-Marie PA, Golenberg DL, et al. 2012 Canadian Guidelines for the diagnosis and management of fibromyalgia syndrome: executive summary. Pain Res Manag. 2013;18(3):119–126. | ||
Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619. | ||
Farley JF, Harley CR, Devine JW. A comparison of comorbidity measurements to predict healthcare expenditures. Am J Manag Care. 2006;12(2):110–117. | ||
Dobson A, Barnett A. An Introduction to Generalized Linear Models. 3rd ed. London: Chapman & Hall; 2008. | ||
McGlynn E, Asch SM, Adams J, et al. The quality of health care delivered to adults in the United States. N Engl J Med. 2003;348(26):2635–2645. | ||
Fitzcharles MA, Galimova L, Jovey RD, et al. Update: clinical challenges in the diagnosis and management of fibromyalgia. Pract Pain Manage. 2011:11(3). | ||
Colcrys™ (colchicine, USP) tablets [prescribing information]. USA: Takeda Pharmaceuticals, Inc; 2012. 32. PrMedrol* (methylprednisone, USP) tablets [prescribing information]. Canada: Pfizer Inc; 2015. |
Supplementary materials
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.