Back to Journals » Clinical Interventions in Aging » Volume 18
Development of a Brief Cognitive Screening Tool for Predicting Postoperative Delirium in Patients with Parkinson’s Disease: A Secondary Analysis
Authors Zhou Y, Wang X, Li Z, Ma Y, Yu C, Chen Y, Ding J, Yu J, Zhou R, Yang N, Liu T, Guo X, Fan T, Shi C
Received 26 April 2023
Accepted for publication 4 September 2023
Published 14 September 2023 Volume 2023:18 Pages 1555—1564
DOI https://doi.org/10.2147/CIA.S410687
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Nandu Goswami
Yongde Zhou,1,* Xiaoxiao Wang,2,3,* Zhengqian Li,2,4 Yu Ma,5 Cuiping Yu,1 Yao Chen,1 Jian Ding,1 Jianfeng Yu,1 Rongsong Zhou,5 Ning Yang,2,4 Taotao Liu,2,4 Xiangyang Guo,2,4 Ting Fan,1 Chengmei Shi2,4
1Department of Anesthesiology, Tsinghua University Yuquan Hospital, Beijing, 100040, People’s Republic of China; 2Department of Anesthesiology, Peking University Third Hospital, Beijing, 100191, People’s Republic of China; 3Research Center of Clinical Epidemiology, Peking University Third Hospital, Beijing, 100191, People’s Republic of China; 4Beijing Center of Quality Control and Improvement on Clinical Anesthesia, Beijing, 100191, People’s Republic of China; 5Department of Neurosurgery, Tsinghua University Yuquan Hospital, Beijing, 100040, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Chengmei Shi, Department of Anesthesiology, Peking University Third Hospital, Beijing, 100191, People’s Republic of China, Tel +86-13811592813, Email [email protected] Ting Fan, Department of Anesthesiology, Tsinghua University Yuquan Hospital, Beijing, 100040, People’s Republic of China, Tel +86-13681100715, Email [email protected]
Background: A simple, rapid, and effective cognitive screening test appropriate for fast-paced settings with limited resources and staff is essential, especially preoperatively. This study aimed to develop and validate the short versions of Mini-Mental State Examination (MMSE) and Montreal Cognitive Assessment (MoCA) for predicting postoperative delirium (POD) in patients with Parkinson’s disease (PD) who were scheduled for surgery.
Methods: The current study was a secondary analysis of data collected from 128 inpatients scheduled for deep brain stimulation of the subthalamic nuclei (STN-DBS) lasting > 60 min, at Tsinghua University Yuquan Hospital, China. Preoperative cognitive screening was performed during the preoperative visit using the MMSE and MoCA. The optimal MMSE and MoCA cut-off scores for detecting PD-MCI was 27 and 23 respectively. The POD was assessed twice a day on the first postoperative day until discharge by the confusion assessment method. The backward conditional logistic regression analysis was used to organize the reduced versions of the MMSE or MoCA. Also, the areas under the receiver operating characteristic curves (AUCs) were examined using the DeLong test.
Results: 125/128 PD patients were included in the analysis, and 27 (21.6%) developed POD. The MMSE reduced version (orientation to time, attention and calculation, and comprehension) demonstrated performance similar to the original MMSE in predicting POD (z=0.820, p=0.412). The AUC of the original MoCA and the short MoCA (visuospatial and executive attention and orientation) were 0.808 and 0.826, respectively. There was no significantly difference in the AUC values between the tests (z=0.561, p=0.575).
Conclusion: Our simplified MMSE and MoCA could be efficiently used to identify patients at risk for POD. Also, short cognitive tests could be considered while predicting POD in fast-paced preoperative settings with limited resources and staff.
Plain Language Summary: Routine preoperative cognitive screening is very important in predicting postoperative delirium.MMSE and MoCA are the most commonly used cognitive testing method which normally takes a long time.The short variant of MMSE and MoCA show a predictive performance similar to the original tests.
Keywords: Parkinson’s disease, preoperative cognitive impairment, mini-mental state examination, Montreal Cognitive Assessment, postoperative delirium
Introduction
Millions of people undergo life-saving surgery worldwide each year to regain health. However, many surgical patients frequently develop postoperative delirium (POD), which is characterized by acute changes in attention and consciousness and is associated with increased morbidity and mortality.1 Preoperative cognitive impairment (CI) also be reported significantly increases the risk of POD.2 The Mini-Mental State Examination (MMSE)3 and Montreal Cognitive Assessment (MoCA)4 are the most commonly used scales to detect CI and have been commonly used in preoperative settings. Preoperative MMSE or MoCA scores are negatively associated with high incidences and severity of POD.2,5
The MMSE takes 10 min, MoCA takes 15 min,6 and the time required for elders might be prolonged. These tests require a substantial amount of time when implemented for the anesthesiologists during the preoperative visit and may not be practical for routine preoperative cognitive screening. On the other hand, some patients are not able to complete the standard MMSE or MoCA due to discomfort caused by illness (fractures, weakness, and pain) or are limited by education level, as well as other clinical conditions: for example, Parkinson’s disease (PD) with muscular/neurological deficits. Therefore, a simple, rapid, and effective cognitive screening test appropriate for fast-paced settings with limited resources and staff is essential, especially preoperatively.
Notably, not all MMSE or MoCA subtests are required to identify CI,7,8 and redundant items could cost time and human resources. Therefore, less useful items were excluded, and those with a high discriminative value were used to construct the adapted versions of the cognitive tests. Brief cognitive screening instruments have been developed and validated in non-surgical populations.9,10 Herein, we aimed to develop and test a short cognitive test for predicting POD in patients with Parkinson’s disease (PD) undergoing elective surgery.
Materials and Methods
Study Design and Population
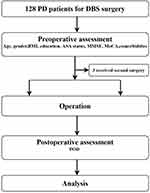
This study was approved by the Ethics Committee of Yuquan Hospital of Tsinghua University (20190014). All patients enrolled have signed informed consent. The clinical trial registration number was ChiCTR1900027210.
This study comprised patients with PD scheduled for deep brain stimulation of the subthalamic nuclei (STN-DBS) between November 2019 and October 2020. The inclusion criteria were patients meeting the criteria and elected to receive DBS surgery (primary PD, hereditary PD or various genotypes PD, responds well to compound levodopa; drug efficacy has decreased significantly or obvious motor complications affect the patient’s quality of life; adverse drug reactions that cannot be tolerated and affect the efficacy of drugs; and there are tremors that cannot be controlled by drugs.). The exclusion criteria were pre-existing significant CI (dementia), severe (refractory) depression, anxiety, schizophrenia, other mental diseases, and medical comorbidities that affect surgery or survival.
The baseline characteristics, including age, sex, body mass index, the highest level of education, American Society of Anesthesiologists (ASA) functional status, and preoperative complications, were collected. Bilateral STN-DBS treatment was initiated according to the Movement Disorders Society guidelines.11
Preoperative Cognitive Evaluation
All participants were Chinese speaking and were screened using the Chinese version of the MMSE12 and a revised Chinese version of the MoCA (the Peking Union Medical College Hospital version of the MoCA).8 The MMSE and MoCA were administered by a trained geriatrician during the preoperative visit.
The MMSE is a 30-point scale that assesses 11 cognitive domains: orientation to time (range 0–5), orientation to place (range 0–5), registration (range 0–3), attention and calculation (range 0–5), recall (range 0–3), naming (range 0–2), repetition (range 0–1), comprehension (range 0–3), reading (range 0–1), writing (range 0–1), and drawing (range 0–1).12 The MoCA is a 15-min, 30-point cognitive screening test designed to detect CI. It encompasses seven cognitive domains: visuospatial and executive function (range 0–5), naming (range 0–3), attention (range 0–6), language (range 0–3), abstraction (range 0–2), recall (range 0–5), and orientation (range 0–6).8 The optimal MMSE and MoCA cut-off scores for detecting PD-MCI was 27 and 23 respectively.10,13–15
POD Assessment
POD was assessed twice a day on the first postoperative day until discharge, once in the morning and once in the evening, by research members trained before the study and blinded to patient’s preoperative cognitive function. It was identified using the confusion assessment method (CAM).16
Sample Size Calculation
A sample size of 36 (18 POD and 18 non-POD) provides precision on a 95% confidence interval (CI) of the area under the curve (AUC) to be approximately ±0.15 if a discrimination magnitude of approximately 0.80 is assumed. Given an anticipated dropout rate of 20%, 23 POD patients are required. A previous study showed that approximately 20% of PD patients developed POD,17 and thus, a minimum sample size of 115 PD patients scheduled for STN-DBS is required.
Statistical Analysis
Statistical analyses were performed with SPSS 24 (IBM Corp., Armonk, NY, USA). Categorical variables are presented as n (%). Normally and non-normally distributed continuous data are expressed as mean±standard deviation (SD) or as median with first and third quartiles respectively. χ2 test or Fisher’s exact test were used to compare categorical data between patients with and without POD. Unpaired Student’s t-test or Mann–Whitney U-test were used to compare continuous data.
In order to organize the reduced versions of the MMSE or MoCA, we performed a backward conditional logistic regression analysis with all MMSE or MoCA items used as independent variables and POD diagnosis as a dependent variable;18 the probability to be removed from the model was set at 0.05. The variables retained in the model were used to compose the reduced versions of the cognitive screening tests.
Pearson’s correlation coefficient was used to evaluate the correlation between the short cognitive screening tests and the original tests. Receiver operating characteristic (ROC) curve analysis was used to determine the ability of the original and short cognitive tests to discriminate between POD and non-POD individuals. Next, the DeLong test was used to examine the difference between the AUCs. AUC values closer to 1 representing a high predictive accuracy. Logistic regression was used for developing the models for POD prediction. The c-statistic (equivalent to the AUC) is a standard measure of the predictive accuracy of a logistic regression model. The level of significance was set at α=0.05.
Results
Subject Characteristics
A total of 128 consecutive PD patients who underwent bilateral STN-DBS fulfilled the inclusion criteria. Three patients were excluded due to reoperation. Among the remaining 125 patients included in this analysis, 27 (21.6%) developed POD (Figure 1, flow diagram). No statistically significant group differences were detected in demographic data or baseline characteristics other than age and education (Table 1). Compared to individuals without POD, those with POD were older, with a mean age of 57.7±9.4 and 65.6±6.5, respectively. POD patients had lower education than those without POD, with the mean value of 6.9±3.1 and 11.4±2.8, respectively.
 |
Table 1 Demographic and Baseline Characteristics of Patients |
MMSE Scores and MMSE Reduced Version
POD patients had lower MMSE scores than those without POD (Table 2). The registration, naming, repetition, and reading did not differ between the groups. All the remaining MMSE subtests helped to discriminate between the POD and non-POD groups. The backward conditional logistic regression excluded some of the redundant items. Thus, orientation to time, attention and calculation, and comprehension comprised the MMSE reduced version. A strong positive correlation was established between the brief version and the standard MMSE (Pearson r=0.910, p<0.001).
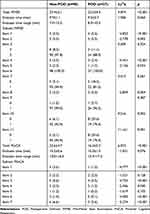 |
Table 2 Total and Subtest MMSE or MoCA Scores of Patients with and without POD |
MoCA Scores and the MoCA Reduced Version
The POD group presented significantly lower scores than the non-POD group regarding MoCA (Table 2). All MoCA subtests but naming and abstraction could discriminate between POD and non-POD individuals. After the backward conditional logistic regression, the following subtests in the model were used to construct the MoCA reduced version: visuospatial and executive functioning, attention, and orientation. A strong positive correlation was established between the MoCA reduced version and the original MoCA (Pearson r=0.884, p<0.001).
Ability of Short Cognitive Tests to Predict Postoperative Delirium
Compared to non-POD individuals, those with POD had lower scores in the MMSE reduced version (t= 5.574, p<0.001), with the mean values of 11.8±1.6 and 9.1±2.4, respectively. Moreover, POD patients had lower scores on the MoCA reduced version than those without POD (t= 6.007, p<0.001), with mean values of 10.3±3.5 and 14.5±1.9, respectively.
As shown in Table 3, the original cognitive and short tests were associated with POD. All the cognitive tests showed good performance in the prediction of POD (AUC: 0.800–0.826; sensitivity: 0.70–0.78; specificity: 0.68–0.90). The short MMSE demonstrated performance (0.818, 95% CI: 0.722–0.915) similar to the original MMSE (0.800, 95% CI: 0.693–0.906) (Figure 2A, z=0.820, p=0.412). Using the cut-off for a MMSE-R of 11.5 (total score 13), sensitivity was 0.778 and the specificity 0.684. The AUCs of the original and the short MoCAs were 0.808 (95% CI: 0.717–0.899) and 0.826 (95% CI: 0.728–0.924), respectively. The differences in the AUC values between the tests were not significant (Figure 2B, z=0.561, p=0.575). A MoCA-R cutoff of 12.5 (total score 17) had a sensitivity of 0.704, and sensitivity was 0.898.
 |
Table 3 Ability of the Original and Short Cognitive Tests to Predict POD |
Risk Prediction Models for Postoperative Delirium
Table 4 summarizes the results of the four models to predict POD. Age, education, and total MMSE scores were independent predictors in the multivariate model (c-statistic: 0.922). The c-statistic of the model (age, education, and short MMSE scores) were 0.930. The comparison of the two predictive models did not show a significant difference (Figure 3A, z=1.270, p=0.204).
 |
Table 4 Risk Prediction Models for Postoperative Delirium |
Moreover, age, education, and total MoCA scores predicted the POD (c-statistic: 0.907). The c-statistic of the model (age, education, and short MoCA scores) was 0.921. The prediction performance of the two models was similar (Figure 3B, z=1.750, p=0.080).
Discussion
In this secondary analysis, we developed and examined the accuracy of a short variant of MMSE and a short form MoCA in the prediction of POD. The short cognitive tests were short and showed a predictive performance similar to the original tests.
The prevalence of PD in people over 65 years of age is approximately 1.0–3.0%.19 STN-DBS is a effective treatment for advanced PD patients.20 However, POD is a common complication after DBS surgery, and 27/125 (21.6%) of our study population developed POD, which was consistent with the previous report.17
As very common in the elderly, POD is a serious, costly, under-recognized and often fatal complication.16 Acute onset, fluctuating symptoms, inattention, impaired consciousness, and disturbance of cognition (for example, disorientation, memory impairment, and alteration in language) are all key diagnostic features of POD.21 PD patients need special attention for increased risk of delirium.22 An accurate and timely preoperative identification of high-risk patients is an effective and easy method for the prevention of POD.
Preoperative CI is a risk factor of POD.23–25 CI includes mild CI (minimally affects the activities of daily living) and dementia or major neurocognitive disorder where ADLs are compromised.26 CI is defined as abnormal scores on cognitive screening tests, such as MMSE and MoCA.2
Cognitive impairment is a common non-motor symptom of PD.27 Cognitively impaired patients are at increased risk of adverse outcomes following surgery.2 High proportion of CI or dementia patients were unrecognized preoperatively,28 which could be because surgeons do not focus on preoperative CI. On the other hand, it is also a fact that the existing cognitive screening scales require large amounts of human resources and time,6 thus limiting the routine preoperative cognitive screening. Therefore, a simple, fast, and effective cognitive screening test is required.
In this study, we found that some subtests can have a high predictive diagnostic value in predicting POD and proposed a simplified MMSE and a reduced version of MoCA. Our simplified MMSE is composed of time orientation, attention/calculation, and verbal comprehension, and the simplified MoCA contains executive/visuospatial, attention, and orientation domains. These tests measured the cognitive dimensions most prevalent in PD patients with mild CI (PD-MCI).10 Also, an overlap was detected between these domains and the key features of POD (acute changes in attention and consciousness, disturbance of orientation, and alteration in language).21 These phenomena supported the rationality of our simplified MMSE or MoCA.
Our simplified MMSE or MoCA contained three domains each, which were greatly reduced in length compared to the original tests. These short tests require approximately 5 min, and implementation of routine preoperative cognitive screening using these short tests is feasible. Notably, the short cognitive tests showed good performance in the prediction of POD. The AUC values of the short MMSE and MoCA were 0.818 and 0.826, similar to the original tests. The prediction models for POD containing the short tests performed similarly to the models containing the original tests. Therefore, these tests were short and effective, which helps to identify risk patients and implement appropriate management strategies to reduce adverse postoperative outcomes.
At present, age as an important independent risk factor for POD has become a consensus in the field of neurocognitive research.29,30 Education years might be negatively associated with postoperative delirium.31 The presumed reason is hippocampal atrophy in people engaged in complex mental activities are much less.32 This study also showed age and education could also predicted the POD.
To the best of our knowledge, this is the first study to develop a short and effective cognitive screening tool appropriate for preoperative settings. Our simplified MMSE and MoCA perform adequately in the prediction of POD and may serve as a viable alternative for detecting CI and predicting POD when the original tests could not be feasibly administered in busy clinical services. Moreover, the short cognitive tests were created in PD patients scheduled for surgery; the application of these short forms in other surgical patients needs further study.
Nevertheless, the present study had several limitations. Firstly, it was a single-center study with a small sample size as only a few hospitals currently perform the DBS surgery. Secondly, similar to the development of many abbreviated test versions, the short cognitive screening tests were also derived from the standard version,8,18 and the predictive performance of these short cognitive tests requires further validation.
Conclusion
Our simplified MMSE and MoCA could be used to predict POD efficiently in PD patients scheduled for surgery. To the best of our knowledge, this is the first study to develop short and effective instruments for predicting POD appropriate for fast-paced preoperative settings with limited resources and staff. Nonetheless, additional studies are required to validate our findings in various surgical patients.
Abbreviations
POD, Postoperative Delirium; CAM, Confusion Assessment Method; PD, Parkinson’s Disease; STN-DBS, Deep Brain Stimulation of the Subthalamic Nuclei; CI, Cognitive Impairment; MMSE, Mini-Mental State Examination; MMSE-R: The MMSE Reduced Version; MoCA, Montreal Cognitive Assessment; MoCA -R: The MoCA Reduced Version; ROC, receiver operating characteristic curve; AUC, area under the ROC curve; ASA, American Society of Anesthesiologists.
Data Sharing Statement
The data that support the findings of this study are available on request from the corresponding author Chengmei Shi. The data are not publicly available due to privacy or ethical restrictions.
Ethics Approval and Consent to Participate
This study have been performed in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of Yuquan Hospital of Tsinghua University (IRB #20190014, Date of approved by ethic committee: 2019-10-21). Written informed consent was obtained from all subjects participating in the study.
Acknowledgments
We thank Nan Li and Hua Zhang (Research Center of Clinical Epidemiology, Peking University Third Hospital, Beijing 100191, China) for their help with the data analysis. Their contributions are sincerely appreciated.
Funding
This study was supported by the National Natural Science Foundation of China (CN) (82101264, 81801070) and the Scientific and Technological Innovation 2030 under Grant 2021ZD0204300.
Disclosure
The authors declare no conflicts of interest.
References
1. Jin Z, Hu J, Ma D. Postoperative delirium: perioperative assessment, risk reduction, and management. Br J Anaesth. 2020;125(4):492–504. doi:10.1016/j.bja.2020.06.063
2. Chen L, Au E, Saripella A, et al. Postoperative outcomes in older surgical patients with preoperative cognitive impairment: a systematic review and meta-analysis. J Clin Anesth. 2022;80:110883. doi:10.1016/j.jclinane.2022.110883
3. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. J Psychiatr Res. 1975;12(3):189–198. doi:10.1016/0022-3956(75)90026-6
4. Nasreddine ZS, Phillips NA, Bã©dirian V, et al. The montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. doi:10.1111/j.1532-5415.2005.53221.x
5. Arias F, Wiggins M, Urman RD, et al. Rapid in-person cognitive screening in the preoperative setting: test considerations and recommendations from the Society for Perioperative Assessment and Quality Improvement (SPAQI). J Clin Anesth. 2020;62:109724. doi:10.1016/j.jclinane.2020.109724
6. Zhuang L, Yang Y, Gao J. Cognitive assessment tools for mild cognitive impairment screening. J Neurol. 2021;268(5):1615–1622. doi:10.1007/s00415-019-09506-7
7. Stein J, Luppa M, Kaduszkiewicz H, et al. Is the short form of the Mini-Mental State Examination (MMSE) a better screening instrument for dementia in older primary care patients than the original MMSE? Results of the German study on ageing, cognition, and dementia in primary care patients (AgeCoDe). Psychol Assess. 2015;27(3):895–904. doi:10.1037/pas0000076
8. Tan JP, Wang X, Zhang S, et al. Accuracy of the short-form montreal cognitive assessment Chinese versions. Front Aging Neurosci. 2021;13:687824. doi:10.3389/fnagi.2021.687824
9. Liew TM. The optimal short version of montreal cognitive assessment in diagnosing mild cognitive impairment and dementia. J Am Med Dir Assoc. 2019;20(8):
10. Yu YW, Tan CH, Su HC, et al. A new instrument combines cognitive and social functioning items for detecting mild cognitive impairment and dementia in Parkinson’s disease. Front Aging Neurosci. 2022;14:913958. doi:10.3389/fnagi.2022.913958
11. Fox SH, Katzenschlager R, Lim SY, et al. International Parkinson and movement disorder society evidence-based medicine review: update on treatments for the motor symptoms of Parkinson’s disease. Mov Disord. 2018;33(8):1248–1266. doi:10.1002/mds.27372
12. Tan JP, Wang X, Lan X, et al. The epoch effect on cognitive function requires regular updating of cognitive screening tests. J Alzheimers Dis. 2020;77(2):667–674. doi:10.3233/JAD-200112
13. Hoops S, Nazem S, Siderowf AD, et al. Validity of the MoCA and MMSE in the detection of MCI and dementia in Parkinson disease. Neurology. 2009;73(21):1738–1745. doi:10.1212/WNL.0b013e3181c34b47
14. Uysal-Cantürk P, Hanağası HA, Bilgiç B, Gürvit H, Emre M. An assessment of movement disorder society task force diagnostic criteria for mild cognitive impairment in Parkinson’s disease. Eur J Neurol. 2018;25(1):148–153. doi:10.1111/ene.13467
15. Badrkhahan SZ, Sikaroodi H, Sharifi F, Kouti L, Noroozian M. Validity and reliability of the Persian version of the Montreal Cognitive Assessment (MoCA-P) scale among subjects with Parkinson’s disease. Appl Neuropsychol Adult. 2020;27(5):431–439. doi:10.1080/23279095.2019.1565762
16. Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911–922. doi:10.1016/S0140-6736(13)60688-1
17. Li H, Han S, Feng J. Delirium after deep brain stimulation in Parkinson’s disease. Parkinsons Dis. 2021;2021:8885386. doi:10.1155/2021/8885386
18. Cecato JF, Martinelli JE, Izbicki R, Yassuda MS, Aprahamian I. A subtest analysis of The Montreal Cognitive Assessment (MoCA): which subtests can best discriminate between healthy controls, mild cognitive impairment and Alzheimer’s disease? Int Psychogeriatr. 2017;29(4):701. doi:10.1017/S104161021600212X
19. Pringsheim T, Jette N, Frolkis A, Steeves TD. The prevalence of Parkinson’s disease: a systematic review and meta-analysis. Mov Disord. 2014;29(13):1583–1590. doi:10.1002/mds.25945
20. Georgiev D, Mencinger M, Rajnar R, et al. Long-term effect of bilateral STN-DBS on non-motor symptoms in Parkinson’s disease: a four-year observational, prospective study. Parkinsonism Relat Disord. 2021;89:13–16. doi:10.1016/j.parkreldis.2021.06.017
21. Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113(12):941–948. doi:10.7326/0003-4819-113-12-941
22. Ebersbach G, Ip CW, Klebe S, et al. Management of delirium in Parkinson’s disease. J Neural Transm. 2019;126(7):905–912. doi:10.1007/s00702-019-01980-7
23. Adogwa O, Elsamadicy AA, Vuong VD, et al. Association between baseline cognitive impairment and postoperative delirium in elderly patients undergoing surgery for adult spinal deformity. J Neurosurg Spine. 2018;28(1):103–108. doi:10.3171/2017.5.SPINE161244
24. Knaak C, Brockhaus WR, Spies C, et al. Presurgical cognitive impairment is associated with postoperative delirium and postoperative cognitive dysfunction. Minerva Anestesiol. 2020;86(4):394–403. doi:10.23736/S0375-9393.20.13903-8
25. Susano MJ, Grasfield RH, Friese M, et al. Brief preoperative screening for frailty and cognitive impairment predicts delirium after spine surgery. Anesthesiology. 2020;133(6):1184–1191. doi:10.1097/ALN.0000000000003523
26. Owens DK, Davidson KW. US Preventive Services Task Force. Screening for cognitive impairment in older adults: US preventive services task force recommendation statement. JAMA. 2020;323(8):757–763. doi:10.1001/jama.2020.0435
27. Kehagia AA, Barker RA, Robbins TW. Neuropsychological and clinical heterogeneity of cognitive impairment and dementia in patients with Parkinson’s disease. Lancet Neurol. 2010;9(12):1200–1213. doi:10.1016/S1474-4422(10)70212-X
28. Kapoor P, Chen L, Saripella A, et al. Prevalence of preoperative cognitive impairment in older surgical patients.: a systematic review and meta-analysis. J Clin Anesth. 2022;76:110574. doi:10.1016/j.jclinane.2021.110574
29. American Geriatrics Society Expert Panel on Postoperative Delirium in Older Adults. Postoperative delirium in older adults: best practice statement from the American Geriatrics Society. J Am Coll Surg. 2015;220(2):136–48.e1. doi:10.1016/j.jamcollsurg.2014.10.019
30. Ishihara A, Tanaka S, Ueno M, et al. Preoperative risk assessment for Delirium after hepatic resection in the elderly: a prospective multicenter study. J Gastrointest Surg. 2021;25(1):134–144. doi:10.1007/s11605-020-04562-1
31. Ordóñez-Velasco LM, Hernández-Leiva E. Factors associated with delirium after cardiac surgery: a prospective cohort study. Ann Card Anaesth. 2021;24(2):183–189. doi:10.4103/aca.ACA_43_20
32. He R, Wang F, Shen H, Zeng Y, Zhang L. Association between increased neutrophil-to-lymphocyte ratio and postoperative delirium in elderly patients with total Hip arthroplasty for Hip fracture. BMC Psychiatry. 2020;20(1):496. doi:10.1186/s12888-020-02908-2
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.