Back to Journals » Clinical Interventions in Aging » Volume 18
Development and Internal Validation of a Nomogram for Predicting Postoperative Cardiac Events in Elderly Hip Fracture Patients
Received 13 August 2023
Accepted for publication 1 December 2023
Published 12 December 2023 Volume 2023:18 Pages 2063—2078
DOI https://doi.org/10.2147/CIA.S435264
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Nandu Goswami
Yuanmei Liu, Huilin Liu,* Fuchun Zhang*
Department of Geriatrics, Peking University Third Hospital, Beijing, 100191, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Huilin Liu; Fuchun Zhang, Department of Geriatrics, Peking University Third Hospital, Beijing, 100191, People’s Republic of China, Email [email protected]; [email protected]
Purpose: Postoperative cardiac events (PCEs) are among the main adverse events after hip fracture surgery in the elderly. Existing cardiac risk assessment tools have some limitations and are not specifically designed for elderly patients undergoing hip fracture surgery. This study aimed to develop and internally validate a nomogram for prediction of PCEs in these patients.
Patients and Methods: We performed a retrospective study of 992 patients aged ≥ 65 years undergoing hip fracture surgery in our hospital from July 2015 to December 2021. Patients’ demographics and clinical data were collected. Least Absolute Shrinkage and Selection Operator (LASSO) regression was used to select predictors, and multivariate logistic regression was employed to construct a nomogram. Internal validation was performed by bootstrapping. The discriminatory ability of the model was determined by the area under the receiver operating characteristic curve (AUC). The calibration and clinical utility of the model were assessed. The predictive power and clinical benefit of the nomogram were compared with the Revised Cardiac Risk Index (RCRI).
Results: The nomogram was constructed including seven variables: general anesthesia, the American Society of Anesthesiologists (ASA) classification, history of heart failure, history of severe arrhythmia, history of coronary artery disease, preoperative platelet count, and serum creatinine. The nomogram had an excellent predictive ability (AUC = 0.875, 95% confidence interval [CI]: 0.828– 0.918). Satisfactory calibration was shown by calibration plots and the Hosmer-Lemeshow goodness-of-fit test (P = 0.520). Clinical usefulness was confirmed by decision curve analysis and clinical impact curve. The predictive power and clinical utility of the nomogram were superior to RCRI.
Conclusion: We developed an easy-to-use nomogram for prediction of PCEs in elderly hip fracture patients. This prediction model could effectively identify patients at high risk of PCEs and may be useful for perioperative management optimization.
Plain Language Summary: Postoperative cardiac events (PCEs) are serious cardiac complications of hip fracture surgery in patients aged ≥ 65 years. There is a lack of accurate cardiac risk assessment tools specifically designed for such patients with hip fractures.
This study aimed to develop a specific prediction model for the prediction of PCEs in elderly hip fracture patients and evaluate its performance.
We performed a study of 992 patients aged ≥ 65 years undergoing hip fracture surgery. Forty-eight routine clinical data were collected as potential risk factors. These were put into statistical models to screen significant predictors and establish a nomogram prediction model (a simple visualized two-dimensional diagram). Then, seven predictors were identified, namely general anesthesia, the American Society of Anesthesiologists (ASA) classification, history of heart failure, history of severe arrhythmia, history of coronary artery disease, preoperative platelet (PLT) count, and serum creatinine (Scr). The nomogram was developed based on these predictors and performed well in this unique population. It could easily and accurately identify those patients at high risk of PCEs and might be useful for improving patient outcomes.
Keywords: prediction model, nomogram, postoperative cardiac events, hip fracture, elderly
Introduction
Hip fractures are one of the most common and serious fractures with high morbidity, mortality, and healthcare costs, mainly occurring in elderly patients. As the population ages, the number of hip fractures has increased rapidly, and it has become a major public health problem worldwide.1,2 Operative treatment is recommended for most hip fractures.3 Despite the positive surgical outcomes, hip fracture surgery is significantly associated with multiple postoperative complications, including various postoperative cardiac adverse events such as myocardial infarction, new-onset arrhythmia, development or exacerbation of heart failure, and cardiac arrest.
The incidence of postoperative cardiac events (PCEs) varies widely, ranging from 2.2% to 8.0%, due to the different definitions of PCEs in previous studies.4–6 Despite the relatively low incidence rate, PCEs are one of the most serious postoperative complications associated with poor prognosis.5,7–9 Geriatric patients are at increased risk for perioperative cardiac adverse events due to their poorer health status and higher cardiovascular comorbidity rate.10,11 Previous studies identified multiple risk factors for PCEs, including patient-related and surgery-specific factors such as old age,12 male sex,12 low body mass index (BMI),13 elevated creatine,14 elevated preoperative N-terminal pro-B type natriuretic peptide (NT-proBNP)14–16 or troponin,16,17 high American Society of Anesthesiologists (ASA) classification grade,18 preoperative red blood cell transfusion,14 frailty19 and preoperative comorbidities such as cardiac disease,4,12,20,21 hypertension,12 peripheral artery disease,4,12 and diabetes mellitus (DM).12,22 However, these factors alone are insufficient for predicting the risk of cardiac events. Preoperative cardiac examinations such as echocardiography and coronary computed tomography angiography are expensive and time-consuming, limiting their use in clinical practice. To date, several cardiac risk assessment tools have been developed for predicting perioperative cardiac events after non-cardiac surgery, such as the Revised Cardiac Risk Index (RCRI),23 Gupta myocardial infarction or cardiac arrest (MICA) risk index,24 American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) surgical risk calculator,25 and Geriatric-Sensitive Perioperative Cardiac Risk Index (GSCRI).26 RCRI is the most widely used risk tool, using six clinical data, and is easy-to-use. However, it was developed over two decades and has only moderate discriminative ability. MICA is a cardiac risk calculator developed from the NSQIP database, which predictive performance surpasses that of the RCRI, but it is limited to predicting the probability of perioperative myocardial infarction or cardiac arrest. The 21-component ACS NSQIP surgical risk calculator has superior predictive performance, but it is complex and less manageable. GSCRI is a prediction model specifically designed for geriatric non-cardiac surgery patients; however, it has not been validated in elderly hip fracture patients. Therefore, it is desirable to develop a specific model to predict PCEs in elderly hip fracture patients easily and accurately.
The purpose of this study was to identify significant predictors from routine clinical data and establish an easy-to-use nomogram for the prediction of PCEs in elderly hip fracture patients.
Materials and Methods
Study Population
A retrospective cohort study was conducted to identify key predictors and develop a nomogram prediction model for predicting PCEs in elderly hip fracture patients. Patients aged 65 years or older who were diagnosed with a hip fracture (including femoral neck, intertrochanteric, and subtrochanteric fractures) and underwent surgery between July 2015 and December 2021 in Peking University Third Hospital were initially deemed eligible. Exclusion criteria were as follows: (1) multiple fractures; (2) pathological fractures; (3) old fractures (>21 days from injury); (4) repeat admissions or duplicated medical records; (5) incomplete medical records. A flowchart of the study design is shown in Figure 1.
 |
Figure 1 Flowchart of the study design. Abbreviation: RCRI, Revised Cardiac Risk Index. |
This study was performed according to the Declaration of Helsinki and approved by the ethics committee of Peking University Third Hospital (No. M2023256). Written informed consent was waived due to the retrospective nature of the study with minimal risk.
Data Collection
All the data were obtained from the electronic medical record system and a radiology information system. The electronic medical records were screened by trained clinical reviewers. Patients who met the inclusion/exclusion criteria were identified. Based on previous studies12–14,18–22 and other cardiac risk indices currently in use,23–26 48 pre- and intraoperative potential predictive clinical variables were retrospectively collected. Preoperative data that were investigated included demographics (age, sex, and BMI); preoperative comorbidities comprising heart failure, coronary artery disease (including percutaneous coronary intervention or stenting, myocardial infarction, angina, and surgical coronary revascularization), severe arrhythmia (including atrial fibrillation, atrial flutter, and ventricular tachycardia), DM, hypertension, cerebrovascular disease, pulmonary disease (defined as pneumonia, history of chronic obstructive pulmonary disease, or interstitial lung disease), renal insufficiency, liver disease (defined as a history of hepatitis or cirrhosis), and active cancer; injury-related characteristics (type of fracture, time from injury to admission, and time from injury to surgery); physical examination data (heart rate, systolic blood pressure, and diastolic blood pressure at admission); the ASA classification and preoperative blood transfusion. Preoperative laboratory examinations including white blood cells (WBCs), hemoglobin (HGB), neutrophil, lymphocyte, monocyte, and platelet (PLT) count, prothrombin time (PT), activated partial thromboplastin time (APTT), fibrinogen (FIB), alanine transaminase (ALT), total bilirubin (TBIL); potassium, sodium, urea nitrogen (BUN), serum creatinine (Scr), and uric acid (UA) were evaluated. The neutrophil-to-lymphocyte ratio, monocyte-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and systemic immune-inflammation index (platelet × neutrophil/ lymphocyte), which demonstrated a possible association with PCEs in some studies,27–29 were also analyzed to explore the potential relationship between novel inflammatory markers and PCEs. Intraoperative data included the type and duration of surgery and anesthesia, intraoperative blood loss, intraoperative infusion volume, and intraoperative blood transfusion.
Study Outcomes and Definitions
PCEs of elderly hip fracture patients consisted of myocardial infarction (MI), congestive heart failure (CHF), new-onset severe arrhythmia, cardiac arrest, and cardiac death during hospitalization. If a patient suffered from more than one cardiac event during the observation period, the cardiac event was counted once for the outcome event.
MI was defined as elevated cardiac troponin values with at least one value above the 99th percentile upper reference limit, accompanied by chest pain, new abnormal Q wave, persistent significant ST elevation or depression, or new-onset left bundle branch block on electrocardiogram, and ventricular wall motion abnormalities on echocardiography. CHF was diagnosed based on clinical manifestations and examinations of heart failure, including dyspnea, orthopnea, peripheral edema, jugular venous distention, rales, third heart sound, elevated NT-proBNP, or chest X-ray with pulmonary congestion or edema, requiring treatment with diuretics and vasodilators. New-onset severe arrhythmia was defined as new ventricular tachycardia, atrial flutter, atrial fibrillation, and paroxysmal supraventricular tachycardia requiring medication or electrical conversion during the postoperative period. Cardiac arrest was defined as the absence of cardiac rhythm or the presence of abnormal cardiac rhythm, including pulseless ventricular tachycardia or ventricular fibrillation and pulseless electrical activity or asystole, which required the initiation of cardiopulmonary resuscitation. Cardiac death was defined as death with known fatal cardiac conditions.
Statistical Analysis
All patients who met the inclusion/exclusion criteria were included for variable selection and the development of the prediction model. A Shapiro–Wilk test was applied first to test the normality of continuous variable data. Normally distributed variables were presented as the mean ± standard deviation and compared by independent sample t-test between two groups. Non-normally distributed variables were reported as median and interquartile range and analyzed by the Mann–Whitney U-test. Categorical variables were presented as the frequency and percentage and tested by the Pearson chi-square and Yates’s correction for continuity.
Predictor Selection and Development of the Nomogram
Least Absolute Shrinkage and Selection Operator (LASSO) regression analysis was initially used to select optimal predictors with a strong correlation with the outcome from candidate predictive variables. In the variable selection process, the LASSO regression adds a penalty parameter lambda (λ) to regression coefficients, which causes the coefficients of less contributing variables to shrink to zero, and the remaining variables with a non-zero coefficient are ultimately selected. Ten-fold cross-validation was adopted to find the optimal parameter lambda (λ). Lambda.1se was chosen as the best lambda to get the simplest model with good accuracy in this study. Subsequently, the variables selected in LASSO regression analysis were entered into a multivariate logistic regression analysis to further identify independent variables and construct the nomogram following a backward step-wise selection process.
Assessment of the Nomogram
The receiver operating characteristic (ROC) curve was used to assess the discriminating ability of the prediction model. The calibration plot and Hosmer-Lemeshow goodness-of-fit test were performed to evaluate the calibration. Decision curve analysis (DCA) and clinical impact curve (CIC) were utilized to assess the clinical utility. Considering the limited study population, the bootstrapping method (1000 resamples) was employed for internal validation to further verify the discriminative ability and calibration of the prediction model. The area under the curve (AUC) of ROC and DCA of the nomogram were compared to those of the RCRI. The DeLong test was used to compare the difference in AUC between the two ROC curves.
All statistical analyses were performed by Statistical Package for Social Sciences program (SPSS) 26.0 (IBM, Armonk, New York, USA) and R software (version 4.2.1R Foundation for Statistical Computing, Vienna, Austria) with glmnet package for LASSO regression analysis, rms package for nomogram diagram, pROC package for ROC, rms package for calibration plot, HLtest package for Hosmer-Lemeshow test, rmda package for DCA and CIC, ROCR package for the comparison of AUC, and Dcurves package for the comparison of DCA between RCRI and the nomogram. All tests were two-tailed. A P value < 0.05 was considered statistically significant.
The development and validation of this prediction model was in accordance with the Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis statement.30
Results
Baseline Demographic and Clinical Characteristics
All 1153 elderly hip fracture patients (aged ≥ 65 years) undergoing surgery in our hospital were screened, and 161 of them were ruled out according to exclusion criteria, including 15 patients with multiple fractures, 2 patients with pathological fractures, 113 patients with old fractures, 18 patients with repeat admissions or duplicated medical records, and 13 patients with incomplete medical records. A total of 992 patients were eventually enrolled in the study (Figure 1). The patients were aged between 65 and 99 years, with a median age of 81 years. There were 717 (72.3%) women and 275 (27.7%) men. The patients’ baseline characteristics are shown in Table 1
 |
Table 1 Baseline Characteristics Between Patients with and without Postoperative Cardiac Events |
A total of 75 patients developed PCEs during hospitalization, with an incidence rate of 7.6%. Among these patients, 10 (13.3%) patients had MI, 56 (74.7%) patients had CHF, 33 (44.0%) patients had new-onset severe arrhythmia, 1 (1.3%) patients had cardiac arrest, and 2 (2.7%) patients had cardiac death. 51 (68.0%) patients suffered from only one PCE, 22 (29.3%) patients had two PCEs, and 2 (2.7%) patients had three PCEs. Details regarding PCEs are shown in Table 2.
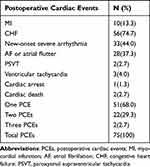 |
Table 2 Distribution and Number of Postoperative Cardiac Events |
Compared to patients without PCEs, patients with PCEs were older, had a higher prevalence of cardiovascular comorbidities including heart failure, severe arrhythmia, and coronary artery disease, higher prevalence of renal insufficiency, hypertension, cerebrovascular disease, and pulmonary disease, had a higher ASA classification grade, higher proportion of hemiarthroplasty, general anesthesia, and preoperative and intraoperative blood transfusion. Meanwhile, preoperative WBCs, monocytes, BUN, Scr, and UA were significantly higher, and levels of HGB, ALT, and sodium were lower among patients with PCEs (all P < 0.05). There was no significant difference in sex, BMI, type of fracture, prevalence of liver disease, DM, and active cancer, time from injury to admission, time from injury to surgery, preoperative heart rate, preoperative systolic and diastolic blood pressure, duration of anesthesia and surgery, intraoperative blood loss, amount of intraoperative fluid infusion, counts of PLTs, neutrophils, and lymphocytes, PT, APTT, FIB, TBIL, potassium, and inflammatory markers neutrophil-to-lymphocyte ratio, monocyte-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and systemic immune-inflammation index between patients with and without PCEs. Baseline characteristics between patients with and without PCEs are shown in Table 1.
Predictor Selection and Development of the Nomogram
Forty-eight potential predictive variables (Table 1) were incorporated in LASSO regression for predictor selection. Nine predictive variables with non-zero coefficients were selected, including general anesthesia, the ASA classification, history of heart failure, history of severe arrhythmia, history of coronary artery disease, PLT, Scr, BUN, and UA (Figure 2A and B). These variables were incorporated into multivariate logistic regression analysis, and seven independent predictors, namely general anesthesia, the ASA classification, history of heart failure, history of severe arrhythmia, history of coronary artery disease, PLT, and Scr, were eventually determined and included in the prediction model (Table 3). Based on the logistic regression model, we constructed a nomogram to predict the risk of PCEs (Figure 3).
 |
Table 3 Multivariate Logistic Regression Analysis of Predictors Screened for Constructing the Nomogram Based on LASSO Regression |
Apparent Performance and Clinical Use of the Nomogram
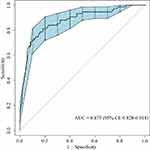
The AUC of the nomogram was 0.875 (95% confidence interval [CI]: 0.828–0.918) after 1000 bootstrap resampling internal validations, indicating that the model had good discrimination ability (Figure 4). The calibration plot displayed good concordance between predicted and actual outcomes (Figure 5). The P value of the Hosmer-Lemeshow test was 0.520, also illustrating no significant difference between predicted and actual probabilities. DCA demonstrated that the utility of the nomogram for predicting PCEs added net clinical benefit compared to treat-all or treat-none strategies when the threshold probability was between 0.05 and 0.95 (Figure 6). Similarly, CIC showed that the nomogram predicted the occurrence of PCEs in general agreement with the occurrence of true PCEs in a wide range of threshold probabilities from 0.2 to 1.0, indicating that the nomogram was clinically useful (Figure 7). The ROC curve of the nomogram showed a greater AUC than that of RCRI (0.875 vs 0.717, DeLong test: P < 0.05), demonstrating that the nomogram had better discrimination ability (Figure 8). Additionally, the comparison of DCA between the two models showed that the nomogram had more net clinical benefit (Figure 9).
 |
Figure 9 Comparison of decision curve analysis of the nomogram and RCRI for the clinical utility. Abbreviation: RCRI, Revised Cardiac Risk Index. |
Some steps are required to use the nomogram. First, a vertical line to the top points axis is drawn to get points for each variable. Second, the points of each variable are added together. Third, the total score on the total points axis is marked, and a vertical line is drawn down to the predicted probability axis to get the risk of PCEs. For example, for a 70-year-old hip fracture patient receiving general anesthesia surgery with a history of atrial fibrillation and coronary heart disease, the ASA classification III, PLT count of 350×109/L, and Scr of 100 μmol/L, the total score is 118 points, and the patient’s predicted probability of PCEs is 94%.
Discussion
In the present study, we developed and internally validated a nomogram to predict PCEs in elderly hip fracture patients for the first time. We screened out seven significant predictors, namely general anesthesia, the ASA classification, history of heart failure, history of severe arrhythmia, history of coronary artery disease, preoperative PLT count, and Scr, from 48 pre- and intraoperative routine clinical variables and established the easy-to-use nomogram with excellent predictive ability and satisfactory clinical benefit using LASSO regression and multivariate logistic regression analysis. The prediction model can be used to predict the risk of PCEs in elderly hip fracture patients and help clinicians with cardiac risk evaluation and perioperative management optimization.
Many risk factors associated with PCEs in hip fracture patients have been found in the past decades. In the present study, we considered a wide variety of pre- and intraoperative risk factors for the prediction of PCEs. Six patient-related factors, including a history of heart failure, severe arrhythmia, and coronary artery disease, the ASA classification, preoperative PLT count, and Scr, and one surgery-specific factor, namely general anesthesia, were identified as independent predictors and eventually incorporated into the nomogram. Some of them are also components of existing cardiac risk models. History of ischemic heart disease, history of CHF, and preoperative serum creatinine (>2.0 mg/dL) were three important variables in the RCRI. The ASA class and abnormal creatinine (>1.5 mg/dL) were among the five predictors of the Gupta MICA risk index. The ASA class, acute renal failure, CHF, and previous cardiac events were significant components in the ACS NSQIP surgical risk calculator. General anesthesia was one of the seven parameters in the HASBLAD score, which was established for predicting perioperative major adverse cardiovascular events in Chinese non-cardiac surgery patients.31 These significant predictors were consistently proven and incorporated in many cardiac risk models. Additionally, history of CHF, the ASA classification, and creatinine were similar predictors both in our model and the GSCRI, a seven-variable cardiac risk assessment tool specifically designed for non-cardiac surgery patients aged ≥65 years, indicating their important predictive ability for PCEs in geriatric patients. Severe arrhythmia, as one of cardiac comorbidities, was seldom studied as an independent predictor alone. It was not a component of previous models but was proven to be predictive in our study. Preoperative platelet count was also a unique predictor in our prediction model, which was rarely explored in previous studies.
History of cardiac disease has been well proven as the significant risk factor of postoperative adverse cardiac events of non-cardiac surgeries and included in many cardiac risk models.23–26 Previous studies showed that hip fracture patients with a history of cardiac disease also had a higher risk of postoperative cardiovascular events than those without a history of cardiac disease. In a retrospective cohort study of 27,441 patients with hip fractures, Sathiyakumar et al found that 594 (2.2%) patients had cardiac complications (cardiac arrest or MI) within 30 days after surgery, and cardiac disease, which was defined as a history of angina, CHF, MI, and/or percutaneous coronary intervention/stenting, was significantly predictive of cardiac complications (OR: 1.55, P = 0.017).4 In another study evaluating the association between pre-existing cardiovascular disease and the risk of postoperative cardiovascular events among hip fracture patients aged ≥65 years, pre-existing cardiovascular disease, defined as having coronary heart disease, cerebrovascular event, peripheral artery disease, heart failure, arrhythmia, valvular heart disease, or pulmonary heart disease at admission, were identified as a strong risk factor of developing postoperative cardiovascular events with adjusted odds ratio (OR) of 2.850 (95% CI, 1.318–7.139). For each specific postoperative cardiovascular event (MI, stroke, arrhythmia, and heart failure), most patients had at least one pre-existing cardiovascular disease.21 Wahlsten et al retrospectively analyzed 124,660 Danish patients aged ≥60 years and undergoing first-time hip fracture surgery and found that a history of heart failure was the risk factor of MI occurring 90 days after surgery besides peripheral artery disease, hypertension, and DM.12 Consistently, we found that hip fracture patients with a history of cardiac disease, including heart failure, severe arrhythmia, and coronary artery disease, were more likely to experience PCEs, confirming the predictive role of those pre-existing cardiac diseases for PCEs in elderly hip fracture patients.
Renal dysfunction was associated with PCEs in different non-cardiac surgeries. Jiang et al found that the risk of postoperative cardiovascular events in hip fracture patients with chronic kidney disease (estimated glomerular filtration rate < 60 mL/min/1.73 m 2) was almost twice as much as in those without chronic kidney disease.32 In the study evaluating the risk of postoperative cardiovascular events in adult patients (≥18 years) who underwent general and vascular surgery, Acheampong et al identified that preoperative creatinine > 1.2 mg/dL was the significant predictor of MI or cardiac arrest within the 30-day postoperative period (OR: 5.1, P = 0.01).33 Several widely used risk models, such as RCRI, MICA, and GSCRI, were constructed incorporating creatinine, suggesting that creatinine level was an important indicator for predicting PCEs. In our study, we also found that increased Scr was significantly associated with an increased risk of PCEs in elderly hip fracture patients and confirmed the similar predictive ability of creatinine for PCEs.
The relationship between baseline PLT count and PCEs has been scarcely reported. In a retrospective cohort of 3,884,400 adult patients who underwent elective, non-cardiac surgery, preoperative thrombocytosis was associated with a higher incidence of 30-day perioperative complications, including cardiac complications (cardiac arrest or MI).34 In another study to assess the predictive ability of preoperative laboratory values for postoperative cardiac and septic complications in orthopedic trauma and hip fracture patients, Lakomkin et al found that abnormal preoperative PLT values significantly increased the risk of cardiac arrest by 11 times in orthopedic trauma patients (OR: 11.107, P = 0.036).35 In our study, we found that higher preoperative PLT count was associated with a higher incidence of PCEs, and PLT count could be a powerful predictor of PCEs in elderly hip fracture patients. Platelets are acute phase reactants, which can be elevated in response to acute inflammatory states, such as trauma, infection, and other pathologic conditions. The preoperative elevated platelet level in geriatric hip fracture patients might play a role in the aggravation of inflammation, atherosclerotic plaque destabilization, and platelet activation and contribute to a high risk of PCEs. More research is required to confirm the relationship between elevated PLT and PCEs in hip fracture patients.
The ASA classification groups patients into categories according to their overall health status and has important implications in predicting perioperative risk. The ability of the ASA classification to predict postoperative cardiac complications was observed in many non-cardiac surgeries. In a retrospective cohort study of 161,177 patients aged ≥ 65 years and undergoing elective or urgent/emergent non-cardiac surgery, Yap et al observed that a higher ASA classification grade was related to a higher risk of PCEs, especially among urgent/emergent patients (OR: 2.46, 95% CI: 1.54–3.92 in the ASA classification III; OR: 5.72, 95% CI: 3.50–9.35 in the ASA classification IV–V).36 The ASA classification also showed dose-response associations with postoperative cardiovascular complications, including heart failure and MI, during the hospital stay and 1 year after hip fracture surgery, suggesting its good predictive ability for postoperative cardiovascular complications in hip fracture patients.18 In a large retrospective cohort study, Woo et al developed a 9-variable predictive model for stroke, major cardiac complications (MI or cardiac arrest), and mortality after non-cardiac surgery, incorporating the ASA classification in the predictive model, which demonstrated excellent predictive performance.37 Similarly, some well-established cardiac risk models such as MICA, ACS NSQIP surgical risk calculator, and GSCRI also incorporated this significant predictor. In our study, we confirmed the predictive role of the ASA classification for PCEs in elderly hip fracture patients as well. This consistency of predictive validity of the ASA classification, both in our study and previous research, supports its use as a component of risk prediction models for PCEs.
The influence of anesthetic methods on PCEs in hip fracture patients remains controversial. Most studies showed that there were no significant differences in postoperative adverse complications, including cardiac complications, between general and regional anesthesia in hip fracture patients.38,39 However, in a NSQIP database analysis of 16,600 geriatric femoral neck fracture patients undergoing hip hemiarthroplasty from 2015 to 2020, Chowdary et al found that patients who received general anesthesia had higher rates of 30-day postoperative cardiac arrest compared to those who received spinal anesthesia (0.97% vs 0.53%, P = 0.011).40 A retrospective study of 40,527 patients aged ≥ 50 years and undergoing hip fracture surgery demonstrated that general anesthesia was associated with a higher incidence of combined 30-day stroke, MI, or death compared with spinal anesthesia (OR 1.219, P = 0.002).41 The inconsistent findings might partly be attributable to the heterogeneity of participants and outcome definitions among studies. In a large Chinese single-center retrospective study, Zhao et al identified general anesthesia as a significant predictor and constructed a HASBLAD score for major adverse cardiovascular events during the perioperative period of non-cardiac surgery.31 Similarly, we found that general anesthesia was related to the higher incidence of PCEs in elderly hip fracture patients and could predict the risk of PCEs.
Although a number of preoperative cardiac risk assessment tools have been developed for patients undergoing non-cardiac surgery over the past decades, they are either simple but less accurate or effective but complex, and none of them have been derived or validated in elderly hip fracture patients. Our model had the advantage of being developed on recent data, specially for elderly hip fracture patients undergoing surgery. In our study, heart failure was the most common PCE, followed by new-onset severe arrhythmia (mainly atrial fibrillation) and MI, which was similar with previous studies.42,43 Therefore, we defined the composite outcome of MI, CHF, new-onset severe arrhythmia, cardiac arrest, and cardiac death to achieve a more comprehensive evaluation of PCEs in elderly hip fracture patients. We utilized LASSO regression,44 a shrinkage, and variable selection method appropriate for high-dimensional multicollinearity data to select the most significant predictors and reduce the overfitting of the model. Additionally, we developed a quantitative nomogram that allows for accurate, individualized risk prediction for PCEs. Our prediction model had an AUC of 0.875, demonstrating an excellent predictive ability. The nomogram also had good calibration, clinical utility, and satisfactory internal validity. Compared with the widely used RCRI model, the nomogram demonstrated superior predictive discrimination and net clinical benefit, confirming its clinical application value.
Our study had several limitations. First, it was a single-center retrospective study with a relatively small sample size and a lack of external validation that might have weakened its accuracy and generalizability. Second, the incidence of PCEs might have been underestimated due to the lack of routine postoperative surveillance with cardiac biomarkers such as troponin, NT-pro BNP, and electrocardiogram, especially for MI, which often occurred asymptomatically.45 Additionally, the outcome of our study was limited to in-hospital PCEs and did not include cardiac complications occurring in the longer period after surgery, which could have also underestimated the risk of PCEs. Third, some significant variables associated with PCEs in non-cardiac surgeries, such as troponin16,17 and NT-pro BNP,14–16 functional status,26 and frailty indices,19 were not included in our analysis because they were not measured for all patients in this study. Therefore, large multicenter prospective studies involving a larger sample of patients and a more comprehensive list of factors are needed to further refine and externally validate the model. Meanwhile, some patients with high-risk cardiac conditions that are considered contraindications to non-cardiac surgery,3 including acute coronary syndrome, decompensated heart failure, tachyarrhythmia or bradyarrhythmia associated with hypotension or requiring urgent medical attention, and symptomatic severe aortic stenosis, usually did not receive surgical treatment, which could have resulted in a low proportion of these cardiac diseases in this study. Hence, the actual risk of these high-risk cardiac conditions might have been underestimated and require additional special evaluation.
Conclusion
Cardiac risk assessment tools are useful and recommended by clinical practice guidelines for predicting perioperative cardiac risk. In this study, we developed an easy-to-use nomogram based on routine clinical factors to predict PCEs specifically for elderly hip fracture patients. Our prediction model showed remarkable predictive accuracy in this unique population and could provide clinicians with guidance for better perioperative intervention to reduce postoperative cardiac risk and improve prognosis.
Abbreviations
ALT, alanine transaminase; APTT, activated partial thromboplastin time; ASA, American Society of Anesthesiologists; AUC, area under the curve; BMI, body mass index; BUN, urea nitrogen; CHF, congestive heart failure; CI, confidence interval; CIC, clinical impact curve; DCA, decision curve analysis; DM, diabetes mellitus; FIB, fibrinogen; GSCRI, Geriatric-Sensitive Perioperative Cardiac Risk Index; LASSO, Least Absolute Shrinkage and Selection Operator; MI, myocardial infarction; MICA, Gupta myocardial infarction or cardiac arrest risk index; NSQIP, National Surgical Quality Improvement Program; NT-proBNP, N-terminal pro-B type natriuretic peptide; OR, odds ratio; PCE, postoperative cardiac event; PLT, platelet; PT, prothrombin time; RCRI, Revised Cardiac Risk Index; ROC, receiver operating characteristic; Scr, serum creatinine; TBIL, total bilirubin; UA, uric acid; WBC, white blood cell.
Acknowledgments
We would like to acknowledge Yicheng Fu for her help in data collection and also thank Liyuan Tao for assistance with data analysis. This manuscript was edited for English Language by Charlesworth Author Services (www.cwauthors.com).
Funding
This study was supported by grants from National Key R&D Program of China (Grant Nos. 2020YFC2008804) and the Key Clinical Program of Peking University Third Hospital (Grant Nos.BYSYDL2021022).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Zhang C, Feng J, Wang S, et al. Incidence of and trends in Hip fracture among adults in urban China: a nationwide retrospective cohort study. PLoS Med. 2020;17(8):e1003180. doi:10.1371/journal.pmed.1003180
2. Piscitelli P, Neglia C, Feola M, et al. Updated incidence and costs of Hip fractures in elderly Italian population. Aging Clin Exp Res. 2020;32(12):2587–2593. doi:10.1007/s40520-020-01497-0
3. Bhandari M, Swiontkowski M. Management of acute hip fracture. N Engl J Med. 2017;377(21):2053–2062. doi:10.1056/NEJMcp1611090
4. Sathiyakumar V, Avilucea FR, Whiting PS, et al. Risk factors for adverse cardiac events in Hip fracture patients: an analysis of NSQIP data. Int Orthop. 2016;40(3):439–445. doi:10.1007/s00264-015-2832-5
5. Lawrence VA, Hilsenbeck SG, Noveck H, Poses RM, Carson JL. Medical complications and outcomes after Hip fracture repair. Arch Intern Med. 2002;162(18):2053–2057. doi:10.1001/archinte.162.18.2053
6. Chen X, Ma Y, Deng Z, Li Q, Liao J, Zheng Q. Prediction of early postoperative major cardiac events and in-hospital mortality in elderly hip fracture patients: the role of different types of preoperative cardiac abnormalities on echocardiography report. Clin Interv Aging. 2020;15:755–762. doi:10.2147/CIA.S250620
7. Varon B, Kandel L, Rivkin G, Leibowitz D. New-onset perioperative atrial fibrillation is associated with increased all-cause mortality in elderly patients undergoing total knee and hip replacements. Gerontology. 2021;67(6):681–686. doi:10.1159/000514482
8. Cha YH, Ha YC, Ryu HJ, et al. Effect of heart failure on postoperative short and long-term mortality in elderly patients with Hip fracture. Injury. 2020;51(3):694–698. doi:10.1016/j.injury.2020.01.004
9. Gupta BP, Huddleston JM, Kirkland LL, et al. Clinical presentation and outcome of perioperative myocardial infarction in the very elderly following Hip fracture surgery. J Hosp Med. 2012;7(9):713–716. doi:10.1002/jhm.1967
10. Liu Z, Xu G, Xu L, Zhang Y, Huang Y. Perioperative cardiac complications in patients over 80 years of age with coronary artery disease undergoing noncardiac surgery: the incidence and risk factors. Clin Interv Aging. 2020;15:1181–1191. doi:10.2147/CIA.S252160
11. Hansen PW, Gislason GH, Jørgensen ME, et al. Influence of age on perioperative major adverse cardiovascular events and mortality risks in elective non-cardiac surgery. Eur J Intern Med. 2016;35:55–59. doi:10.1016/j.ejim.2016.05.028
12. Wahlsten LR, Zareini B, Smedegaard L, Gislason GH, Palm H, Brorson S. A medical history of arterial thrombosis is a strong predictor of post-operative myocardial infarction and stroke in patients with Hip fractures-a nationwide cohort study. Age Ageing. 2021;50(4):1252–1260. doi:10.1093/ageing/afaa279
13. Batsis JA, Huddleston JM, LJt M, et al. Body mass index and risk of adverse cardiac events in elderly patients with Hip fracture: a population-based study. J Am Geriatr Soc. 2009;57(3):419–426. doi:10.1111/j.1532-5415.2008.02141.x
14. Araguas MA, Herrera A, Garrido I, Mateo J, Mayoral AP, Muñoz M. Risk factors for major adverse cardiovascular events after osteoporotic Hip fracture repair surgery. Injury. 2020;51(Suppl 1):S30–S36. doi:10.1016/j.injury.2020.03.052
15. Ushirozako H, Ohishi T, Fujita T, et al. Does N-terminal pro-brain type natriuretic peptide predict cardiac complications after hip fracture surgery? Clin Orthop Relat Res. 2017;475(6):1730–1736. doi:10.1007/s11999-017-5245-5
16. Perrone MA, Aimo A, Bernardini S, Clerico A. Natriuretic peptides and troponins to predict cardiovascular events in patients undergoing major non-cardiac surgery. Int J Environ Res Public Health. 2022;19(9):5182. doi:10.3390/ijerph19095182
17. Lowe MJ, Lightfoot NJ. The prognostic implication of perioperative cardiac enzyme elevation in patients with fractured neck of femur: a systematic review and meta-analysis. Injury. 2020;51(2):164–173. doi:10.1016/j.injury.2019.12.012
18. Meyer AC, Eklund H, Hedstrom M, Modig K. The ASA score predicts infections, cardiovascular complications, and hospital readmissions after Hip fracture - A nationwide cohort study. Osteoporos Int. 2021;32(11):2185–2192. doi:10.1007/s00198-021-05956-w
19. Siddiqui E, Banco D, Berger JS, Smilowitz NR. Frailty assessment and perioperative major adverse cardiovascular events after non-cardiac surgery. Am J Med. 2023;136(4):372–379.e5. doi:10.1016/j.amjmed.2022.12.033
20. Ekström W, Samuelsson B, Ponzer S, Cederholm T, Thorngren KG, Hedström M. Sex effects on short-term complications after Hip fracture: a prospective cohort study. Clin Interv Aging. 2015;10:1259–1266. doi:10.2147/CIA.S80100
21. Luo Y, Jiang Y, Xu H, et al. Risk of post-operative cardiovascular event in elderly patients with pre-existing cardiovascular disease who are undergoing Hip fracture surgery. Int Orthop. 2021;45(12):3045–3053. doi:10.1007/s00264-021-05227-7
22. Norris R, Parker M. Diabetes mellitus and Hip fracture: a study of 5966 cases. Injury. 2011;42(11):1313–1316. doi:10.1016/j.injury.2011.03.021
23. Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999;100(10):1043–1049. doi:10.1161/01.CIR.100.10.1043
24. Gupta PK, Gupta H, Sundaram A, et al. Development and validation of a risk calculator for prediction of cardiac risk after surgery. Circulation. 2011;124(4):381–387. doi:10.1161/CIRCULATIONAHA.110.015701
25. Bilimoria KY, Liu Y, Paruch JL, et al. Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aid and informed consent tool for patients and surgeons. J Am Coll Surg. 2013;217(5):833–842.e3. doi:10.1016/j.jamcollsurg.2013.07.385
26. Alrezk R, Jackson N, Al Rezk M, et al. Derivation and validation of a geriatric-sensitive perioperative cardiac risk index. J Am Heart Assoc. 2017;6(11):e006648. doi:10.1161/JAHA.117.006648
27. Larmann J, Handke J, Scholz AS, et al. Preoperative neutrophil to lymphocyte ratio and platelet to lymphocyte ratio are associated with major adverse cardiovascular and cerebrovascular events in coronary heart disease patients undergoing non-cardiac surgery. BMC Cardiovasc Disord. 2020;20(1):230. doi:10.1186/s12872-020-01500-6
28. Forget P, Moreau N, Engel H, et al. The neutrophil-to-lymphocyte ratio (NLR) after surgery for Hip fracture (HF). Arch Gerontol Geriatr. 2015;60(2):366–371. doi:10.1016/j.archger.2014.11.008
29. Fisher A, Srikusalanukul W, Fisher L, Smith P. The neutrophil to lymphocyte ratio on admission and short-term outcomes in orthogeriatric patients. Int J Med Sci. 2016;13(8):588–602. doi:10.7150/ijms.15445
30. Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): the TRIPOD statement. Ann Intern Med. 2015;162(1):55–63. doi:10.7326/M14-0697
31. Zhao M, Shang Z, Cai J, et al. Development and validation of predictive model-HASBLAD score-for major adverse cardiovascular events during perioperative period of non-cardiac surgery: a single center experience in China. Front Cardiovasc Med. 2022;9:774191. doi:10.3389/fcvm.2022.774191
32. Jiang Y, Luo Y, Li J, et al. Chronic kidney disease and risk of postoperative cardiovascular events in elderly patients receiving Hip fracture surgery. Injury. 2022;53(2):596–602. doi:10.1016/j.injury.2021.12.032
33. Acheampong D, Guerrier S, Lavarias V, et al. Risk factors contributing to cardiac events following general and vascular surgery. Ann Med Surg. 2018;33:16–23. doi:10.1016/j.amsu.2018.08.001
34. Weil IA, Kumar P, Seicean S, Neuhauser D, Seicean A, Ballotta A. Platelet count abnormalities and peri-operative outcomes in adults undergoing elective, non-cardiac surgery. PLoS One. 2019;14(2):e0212191. doi:10.1371/journal.pone.0212191
35. Lakomkin N, Sathiyakumar V, Dodd AC, et al. Pre-operative labs: wasted dollars or predictors of post-operative cardiac and septic events in orthopaedic trauma patients? Injury. 2016;47(6):1217–1221. doi:10.1016/j.injury.2016.03.004
36. Yap EN, Dusendang JR, Ng KP, et al. Risk of cardiac events after elective versus urgent or emergent noncardiac surgery: implications for quality measurement and improvement. J Clin Anesth. 2023;84:110994. doi:10.1016/j.jclinane.2022.110994
37. Woo SH, Marhefka GD, Cowan SW, Ackermann L. Development and validation of a prediction model for stroke, cardiac, and mortality risk after non-cardiac surgery. J Am Heart Assoc. 2021;10(4):e018013. doi:10.1161/JAHA.120.018013
38. Chen X, Li H, Li S, et al. Comparison of risk of complication between neuraxial anaesthesia and general anaesthesia for Hip fracture surgery: a systematic review and meta-analysis. Int J Surg. 2023;109(3):458–468. doi:10.1097/JS9.0000000000000291
39. Malhas L, Perlas A, Tierney S, Chan VWS, Beattie S. The effect of anesthetic technique on mortality and major morbidity after Hip fracture surgery: a retrospective, propensity-score matched-pairs cohort study. Reg Anesth Pain Med. 2019;44(9):847–853. doi:10.1136/rapm-2019-100417
40. Chowdary AR, Beale J, Martinez J, Aggarwal V, Mounasamy V, Sambandam S. Postoperative complications of spinal vs general anesthesia in elderly patients undergoing Hip hemiarthroplasty. Arch Orthop Trauma Surg. 2023;143(9):5615–5621. doi:10.1007/s00402-023-04876-0
41. Weinstein ER, Boyer RB, White RS, et al. Improved outcomes for spinal versus general anesthesia for Hip fracture surgery: a retrospective cohort study of the national surgical quality improvement program. Reg Anesth Pain Med;2023. 104217. doi:10.1136/rapm-2022-104217
42. Folbert EC, Hegeman JH, Gierveld R, et al. Complications during hospitalization and risk factors in elderly patients with Hip fracture following integrated orthogeriatric treatment. Arch Orthop Trauma Surg. 2017;137(4):507–515. doi:10.1007/s00402-017-2646-6
43. Roche JJ, Wenn RT, Sahota O, Moran CG. Effect of comorbidities and postoperative complications on mortality after Hip fracture in elderly people: prospective observational cohort study. BMJ. 2005;331(7529):1374. doi:10.1136/bmj.38643.663843.55
44. Friedman J, Hastie T, Tibshirani R. Regularization paths for generalized linear models via coordinate descent. J Stat Softw. 2010;33(1):1–22. doi:10.18637/jss.v033.i01
45. Hietala P, Strandberg M, Strandberg N, et al. Perioperative myocardial infarctions are common and often unrecognized in patients undergoing Hip fracture surgery. J Trauma Acute Care Surg. 2013;74(4):1087–1091. doi:10.1097/TA.0b013e3182827322
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.