Back to Journals » Risk Management and Healthcare Policy » Volume 14
Development and Evaluation of a New Predictive Nomogram for Predicting Risk of Herpes Zoster Infection in a Chinese Population with Type 2 Diabetes Mellitus
Authors Zeng N , Li Y, Wang Q, Chen Y, Zhang Y, Zhang L, Jiang F , Yuan W, Luo D
Received 12 April 2021
Accepted for publication 20 October 2021
Published 27 November 2021 Volume 2021:14 Pages 4789—4797
DOI https://doi.org/10.2147/RMHP.S310938
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Natasha Hodgkinson
Ni Zeng,1,2 Yueyue Li,2 Qian Wang,3 Yihe Chen,2 Yan Zhang,1 Lanfang Zhang,1 Feng Jiang,4,5 Wei Yuan,1 Dan Luo2
1Department of Dermatology, Affiliated Hospital of Zunyi Medical University,149 Dalian Road, Huichuan District, Guizhou, 563003, People’s Republic of China; 2Department of Dermatology, The First Affiliated Hospital of Nanjing Medical University, Nanjing, 210000, People’s Republic of China; 3Department of Endocrinology, Affiliated Hospital of Zunyi Medical University, Guizhou, 563003, People’s Republic of China; 4Neonatal Department, Obstetrics and Gynecology Hospital of Fudan University, Shanghai, 200011, People’s Republic of China; 5Department of Pediatrics, The First Affiliated Hospital of Nanjing Medical University, Nanjing, 21000, People’s Republic of China
Correspondence: Wei Yuan
Department of Dermatology, Affiliated Hospital of Zunyi Medical University, 149 Dalian Road, Huichuan District, Guizhou, 563003, People’s Republic of China
Tel/Fax +86 13655187928
Email [email protected]
Dan Luo
Department of Dermatology, The First Affiliated Hospital of Nanjing Medical University, Nanjing, 210000, People’s Republic of China
Tel/Fax +86 13655187928
Email [email protected]
Purpose: To identify potential risk factors for herpes zoster infection in type 2 diabetes mellitus in southeast Chinese population.
Patients and Methods: We built a model involving 266 herpes zoster patients collecting data from January 2018 to December 2019. The least absolute shrinkage and selection operator (Lasso) predictive model was used to test herpes zoster virus risk using the patient data. Multivariate regression was conducted to decide which variable would be the strongest to decrease the Lasso penalty. The predictive model was tested using the C-index, a calibration plot, and decision curve study. External validity was verified by bootstrapping by counting probabilities.
Results: In the prediction nomogram, the prediction variables included age, sex, weight, length of hospital stay, infection, and blood pressure. The C-index of 0.844 (0.798– 0.896) indicated substantial variability and thus the model was adjusted appropriately. A score of 0.825 was achieved somewhere in the above interval. Examination of the decision curve estimated that herpes zoster nomogram was useful when the intervention was determined at the 16 percent of the herpes zoster infection potential threshold.
Conclusion: The herpes zoster nomogram combines age, weight, position of the rash, 2-hour plasma glucose, glycosuria, serum creatinine, length of the hospital stay, and hypertension. This calculator can be used to assess the individual herpes zoster risks in patients diagnosed with type 2 diabetes mellitus.
Keywords: glycemic status, herpes zoster, nomogram, type 2 diabetes mellitus, infection
Introduction
The reactivation of the varicella-zoster virus (VZV) causes shingles and that the virus localizes to the spinal cord and cranial sensory ganglia during the primary infection in children. The virus may persist latently in the sensory ganglia after the primary infection. The most common complication is post-herpetic neuralgia(PHN), which is chronic nerve pain after the rash disappears, that occurs in 20% of herpes zoster (HZ) patients.1 It may persist for several years and can significantly affect the quality of life of affected individuals.2 Studies have established risk factors correlated with the reactivation of VZV linked to a reduction in T-cell immunity, such as aging and immunosuppression.3–5 Both HZ and PHN have been associated with higher health care costs.6
Numerous clinical trials have found that individuals with diabetes and weakened immune systems are at an elevated risk of acquiring HZ. The research suggested that diabetes raises the risk and duration of HZ episodes and that HZ may contribute to a worsening of diabetes, contributing to increased need for public healthcare. In type 2 diabetes mellitus (T2DM), it is well established that the activity of cells that take part in the innate and adaptive immune responses are compromised. This decrease in specific immunity may be responsible for the reactivation of HZ, rendering diabetes a risk factor for shingles.7–9
Predictive factors for the incidence of HZ in T2DM patients are required, considering the current risk of HZ arising in patients with malignant melanoma and several associated risk factors.10 However, to date, no research has been conducted on the topic. This study aimed to establish a model capable of predicting the probability of HZ infection among patients with T2DM.
Patients and Methods
We conducted a retrospective longitudinal study at the Affiliated Hospital of Zunyi Medical University, a large teaching hospital in southwest China from January 2018 to December 2019. Informed consent was obtained from all patients for inclusion in this study.
HZ was diagnosed by the dermatologists based on clinical features. Data relative to diagnosis were collected from electronic medical records of the hospital using the ICD 10. We assessed the onset of HZ based on coding by dermatologist, and not on laboratory-based diagnostic codes, such as antigen titers.8 If participants experienced more than one HZ episode during the study period, only the first episode was considered for this study. Patient characteristics gathered from medical records included age, sex, weight, and length of hospital stay. Patients who had a previous history of autoimmune diseases were excluded from the study as receiving current glucocorticoid use could affect glycemic status. Our primary outcome was HZ incidence. We evaluated the outcome in relationship controlling for demographic features, glycemic status, and other comorbidities.
Statistical Analysis
The percent values of outcomes relative to age, sex, weight, the length of hospital stay, fasting plasma glucose, 2 hours plasma glucose (2hPG) levels, the location of skin rash, glycosuria, serum creatinine, hypertension, and infection are measured. The data used was analyzed using R statistics tools. The least absolute shrinkage and selection operator (Lasso) was used to identify risk factors predictive of HZ infection.
A high dimensional selection operator is used, and data compression was added to the output. In the cable regression model, the non-zero coefficient was selected. A prediction model was then constructed using multivariate logistic regression analysis, which considered the cable regression model features.11 These unique properties are known as risks. The analysis findings are reported as 95% confidence intervals (CI) and relative P values.12 Two-sided tests were determined to be statistically significant. Variables with a P-value <0.05 were used in the model, and those with higher P values were omitted.13 From the cohort, all variables were included in developing a prediction model with appropriate adjustments and graphical representations to predict HZ infection.
Suitable test statistics indicate the robustness of the model.14,15 We used the Harrell index to evaluate the accuracy of the HZ nomogram. Through bootstrapping, the relative corrected C-index was determined.16 The decision curve approach was used to measure the net benefits of different threshold ratios in the HZ population to assess the therapeutic effectiveness of the T2DM nomogram. Net benefits were determined by extracting from the proportion of actual positive people the proportion of all perceived positive individuals and calculating the relative risk of rejecting interventions against unsuccessful interventions’ harmful consequences.
Results
Patient Characteristics
In total, 266 patients diagnosed with herpes zoster were analyzed. The patients were grouped into diabetes and non-diabetes.The mean age of patients was 61.56 ± 12.54 years (range 18–89 years). Patient characteristics, including age, sex, weight, length of hospital stay, and fasting plasma glucose level is provided in Table 1.
 |
Table 1 Differences Between Demographic and Clinical Characteristics of T2DM and Non-T2DM Groups |
Selection of Variables
In demographics, age, sex, weight, length of hospital stay, the location of skin rash, serum creatine, glycosuria, 2hPG, hypertension, and an additional 4 characteristics were evaluated for all 266 patients included in the study (Table 1). Age, weight, the length of hospital stay, the location of skin rash, glycosuria, serum creatine, and 2hPG were found to be relevant in the Lasso model (Figure 1A and B).
An Individualized Prediction Model Was Constructed to Predict HZ Infection
Age, weight, length of hospital stay, location of the skin rash, glycosuria, serum creatine, 2hPG, and hypertension, were inserted into a logistic regression model; the data are shown in Table 2. A model was built including variables with independent variables found to be significant for HZ infection (Figure 2).
 |
Table 2 Prediction Factors for HZ in T2DM Patients |
 |
Figure 2 Development of the HZ nomogram. The chart is made from the data based on gender, age, length of hospital stay, weight, 2 hour PG, creatinine, location of skin rash, and hypertension. |
Accuracy of the HZ Exposure Nomogram in the Patient Cohort
A calibration curve of the HZ risk nomogram designed for patients with T2DM presented a very high degree of accuracy (Figure 3). The C-index for the model was 0.858 (95% CI 0.811 to 0.904), which indicated a good fit of the model. Based on the model’s C-statistic, it appears that the risk assessment nomogram had a high accuracy in its predictive capacity.
Application in the Clinic
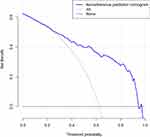
The HZ nomogram contains scales of different of variables that can be used to calculate the probability of an outcome. The judgment curve indicates that if patients’ and doctors’ thresholds are higher than 18% and lower than 65%, respectively, the non-adherence nomogram’s usage raises the estimated probability of HZ infection. This analysis compiles the overlaps to guarantee that the net benefit is equal (Figure 4).
Discussion
The most modern nomograms have been utilized to predict outcome of cancer diagnosis and the effect of cancer therapies.16 A user-friendly prognosis estimator with improved precision and a more straightforward understanding of the prognosis, will allow clinicians to be more informed on predicting patient outcomes.17 The study explores a new, risk-based HZ detection approach for patients with T2DM.
We developed a novel nomogram that correctly identifies risk of HZ virus infection in patients with T2DM. The integration of age and disease signs into a handy instrument enables to assess of HZ infection among patients with T2DM. By providing a tool for the diagnosis of patients with HZ, clinicians would be able to recognize a high-risk patient population, and this would contribute to achieve a timeline for clinical evaluation and treatment. For the prediction of HZ recurrence in patients with T2DM, our constructed nomogram was shown to be very reliable. The internal analysis shows essential internal and external calibration; in turn, the strong C-index in interval validation indicates that the nomogram could be used extensively and accurately given its huge sample size. As in previous studies, approximately 60 percentage of patients with HZ had T2DM in our study sample.12,18–20 The prevalence of HZ in T2DM is associated with age, weight, duration of hospital stay, the location of skin rash, levels of glycosuria, serum creatine, and 2hPG, and blood pressure. Of these eight risk factors associated with HZ infection, 2hPG, glycosuria, and creatine are associated with T2DM. In addition, skin rash and age are also associated with T2DM. Several clinical trials have examined the relationship with hemoglobin A1c levels.8,12 A longitudinal analysis showed that individuals with the lowest (<5.0%) hemoglobin A1c levels have a slightly greater chance of contracting HZ (shingles) after adjusting for covariates. Patients with diseases like diabetes and immune system disorders are at a higher risk of contracting HZ.21 The reactivation is attributed to a decreased VZV-specific cell-mediated immunity (VZV-CMI), which is typically found in individuals who have a weakened immune system.22 Patients aged sixty years or older are more likely to experience HZ, which may be attributed to VZV reactivation.23
In our study, hypertension was also a contributing factor for HZ infection in T2DM patients. It is universally understood that cardiovascular disease is the leading cause of death in individuals with T2DM;24 and our study indicated that hypertension was a concomitant factor in the majority of patients with T2DM, and was significantly more common among individuals with HZ infection.25 Epidemiological trials have described the importance of blood vessel pathophysiology in the development of the vascular disease and the occurrence of microvascular disease is of prognostic importance in predicting CVD.24
The goal of contemporary epidemiological research is to determine how the pathology of HZ is linked to risk factors associated with T2DM and if these risk factors may represent therapeutic goals for HZ. These findings have added microvascular events to the body of work on HZ infection and have prompted the introduction of mechanistic studies of vascular disease.24 However, currently, it is not possible to reliably predict whether a patient will experience HZ. Our aim was to develop a model that could accurately predict the onset of HZ in patients with T2DM.
Our analysis has several drawbacks. First, the data we gathered was biased as the study enrolled only patients diagnosed with HZ and may not be indicative of all Chinese VZV-CMI patients. Secondly, the data presented did not provide details regarding the impact of vaccination against HZ, which could have influenced the results. However, the HZ vaccine became available in 2019 in China, and only a small number of individuals will have been vaccinated, any therapeutic efficacy of the vaccine in the present study would be limited. The recommended plan of action is that individuals over the age of 50 should receive the HZ vaccine. In addition, the possible factors that lead to T2DM were not taken into consideration. Factors affecting resistance are also unknown, including any impact of social assistance might have had. While the bootstrap test accurately calculated the precision of our nomogram, it might not be generalizable to HZ infections to all patient groups around the world. The findings of this study requires analysis in different centers and geographical areas to be validated.
Conclusion
A modern risk assessment nomogram was created to help clinicians classify the risk of HZ in patients with T2DM. Physicians and patients should take a more customized approach to illness risk reduction via lifestyle and medicinal intervention monitoring. The nomogram based on these factors could be used to predict the probability of HZ for patients with T2DM and to develop an individualized care plan to achieve optimal outcome for patients with T2DM by minimizing the risk of HZ.
Ethical Approval
All research studies on humans (individuals, samples or data) performed in accordance with the principles stated in the Declaration of Helsinki. The Ethical Committee Institutional Review Board approved this study at the affiliated Zunyi Medical University Hospital (approval no [KLL-2020-288]).
Acknowledgments
Thanks to our fellow physicians from the Affiliated Hospital of Zunyi Medical University. I would like to thank very much Feng Jiang for his consideration and great help to me in both bioinformatics study and life.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Saadatian-Elahi M, Bauduceau B, Del-Signore C, et al. Diabetes as a risk factor for herpes zoster in adults: a synthetic literature review. Diabetes Res Clin PR. 2020;159:107983. doi:10.1016/j.diabres.2019.107983
2. Johnson RW, Rice AS, Solomon CG. Clinical practice. Postherpetic neuralgia. N Engl J Med. 2014;371(16):1526–1533. doi:10.1056/NEJMcp1403062
3. El KM, Wifi MN, Mahdy R, et al. Herpes Zoster reactivation in patients with chronic hepatitis C under treatment with directly acting antiviral agents: a case series. Arab J Gastroenterol. 2017;18(1):39–41. doi:10.1016/j.ajg.2017.02.003
4. Bonanni P, Breuer J, Gershon A, et al. Varicella vaccination in Europe - taking the practical approach. BMC Med. 2009;7:26. doi:10.1186/1741-7015-7-26
5. Lal H, Cunningham AL, Godeaux O, et al. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med. 2015;372(22):2087–2096. doi:10.1056/NEJMoa1501184
6. Gater A, Uhart M, McCool R, et al. The humanistic, economic and societal burden of herpes zoster in Europe: a critical review. BMC Public Health. 2015;15:193. doi:10.1186/s12889-015-1514-y
7. Carey IM, Critchley JA, DeWilde S, et al. Risk of infection in type 1 and type 2 diabetes compared with the general population: a matched cohort study. Diabetes Care. 2018;41(3):513–521. doi:10.2337/dc17-2131
8. Kobayashi D, Shimbo T, Noto H, et al. Low level of hemoglobin A1c and the increased incidence of herpes zoster: longitudinal study. Eur J Clin Microbiol. 2019;38(8):1539–1545. doi:10.1007/s10096-019-03584-1
9. Muller LM, Gorter KJ, Hak E, et al. Increased risk of common infections in patients with type 1 and type 2 diabetes mellitus. Clin Infect Dis. 2005;41(3):281–288. doi:10.1086/431587
10. Xiong Y, Ling QH, Han F, et al. An efficient gene selection method for microarray data based on LASSO and BPSO. BMC Bioinform. 2019;20(Suppl 22):715. doi:10.1186/s12859-019-3228-0
11. Ami R, Hatta W, Iijima K, et al. Factors associated with metachronous gastric cancer development after endoscopic submucosal dissection for early gastric cancer. J Clin Gastroenterol. 2017;51(6):494–499. doi:10.1097/MCG.0000000000000620
12. Munoz-Quiles C, Lopez-Lacort M, Ampudia-Blasco FJ, et al. Risk and impact of herpes zoster on patients with diabetes: a population-based study, 2009–2014. Hum Vaccin Immunother. 2017;13(11):2606–2611. doi:10.1080/21645515.2017.1368600
13. Kidd AC, McGettrick M, Tsim S, et al. Survival prediction in mesothelioma using a scalable Lasso regression model: instructions for use and initial performance using clinical predictors. BMJ Open Respir Res. 2018;5(1):e240. doi:10.1136/bmjresp-2017-000240
14. Kramer AA, Zimmerman JE. Assessing the calibration of mortality benchmarks in critical care: the Hosmer-Lemeshow test revisited. Crit Care Med. 2007;35(9):2052–2056. doi:10.1097/01.CCM.0000275267.64078.B0
15. Vickers AJ, Cronin AM, Elkin EB, et al. Extensions to decision curve analysis, a novel method for evaluating diagnostic tests, prediction models and molecular markers. BMC Med Inform Decis Mak. 2008;8:53. doi:10.1186/1472-6947-8-53
16. Wang H, Zhang L, Liu Z, et al. Predicting medication nonadherence risk in a Chinese inflammatory rheumatic disease population: development and assessment of a new predictive nomogram. Patient Prefer Adherence. 2018;12:1757–1765. doi:10.2147/PPA.S159293
17. Jiang F, Wei K, Lyu W, et al. Predicting risk of insulin resistance in a Chinese population with polycystic ovary syndrome: designing and testing a new predictive nomogram. Biomed Res Int. 2020;2020:1–9.
18. Chen HH, Lin CL, Yeh SY, et al. Short-term dipeptidyl peptidase-4 inhibitor use increases the risk of herpes zoster infection in Asian patients with diabetes. QJM. 2016;109(2):91–95. doi:10.1093/qjmed/hcv096
19. Guignard AP, Greenberg M, Lu C, et al. Risk of herpes zoster among diabetics: a matched cohort study in a US insurance claim database before introduction of vaccination, 1997–2006. Infection. 2014;42(4):729–735. doi:10.1007/s15010-014-0645-x
20. Yun H, Yang S, Chen L, et al. Risk of herpes zoster in autoimmune and inflammatory diseases: implications for vaccination. Arthritis Rheumatol. 2016;68(9):2328–2337. doi:10.1002/art.39670
21. Kawai K, Yawn BP. Risk factors for herpes zoster: a systematic review and meta-analysis. Mayo Clin Proc. 2017;92(12):1806–1821. doi:10.1016/j.mayocp.2017.10.009
22. Ogunjimi B, Buntinx F, Bartholomeeusen S, et al. Herpes zoster is associated with herpes simplex and other infections in under 60 year-olds. J Infect. 2015;70(2):171–177. doi:10.1016/j.jinf.2014.08.016
23. Kawai K, Gebremeskel BG, Acosta CJ. Systematic review of incidence and complications of herpes zoster: towards a global perspective. BMJ Open. 2014;4(6):e4833. doi:10.1136/bmjopen-2014-004833
24. Madonna R, Balistreri CR, Geng YJ, et al. Diabetic microangiopathy: pathogenetic insights and novel therapeutic approaches. Vascul Pharmacol. 2017;90:1–7. doi:10.1016/j.vph.2017.01.004
25. Strain WD, Paldanius PM. Diabetes, cardiovascular disease and the microcirculation. Cardiovasc Diabetol. 2018;17(1):57. doi:10.1186/s12933-018-0703-2
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.