Back to Journals » Transplant Research and Risk Management » Volume 16
Devastating Invasive Aspergillus Infection Following Commercial Kidney Transplantation: Case Report, Review of Literature, and Ethical Issues
Authors Basok A, Romanjuk E, Rogachev B, Haviv YS, Shaco Halevy R, Vorobiov M
Received 21 June 2023
Accepted for publication 4 January 2024
Published 8 February 2024 Volume 2024:16 Pages 1—6
DOI https://doi.org/10.2147/TRRM.S425072
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Qing Yi
Anna Basok,1 Elvira Romanjuk,1 Boris Rogachev,1 Yosef Shmuel Haviv,1 Ruth Shaco Halevy,2 Marina Vorobiov1
1Nephrology Department, Ben Gurion University Medical Center, Beer Sheva, Israel; 2Pathology Department, Ben Gurion University Medical Center, Beer Sheva, Israel
Correspondence: Anna Basok, Nephrology Department, Ben Gurion University Medical Center, Uchmanit 13, Beer-Sheva, 8485283, Israel, Email [email protected]
Abstract: We described an extraordinary case of invasive aspergillosis (IA) after commercial kidney transplantation. Massive infestation by angiotrophic fungi with morphological features of Aspergillus caused fungal arteritis of renal blood vessels with detachment of the kidney graft from the iliac artery with ipsilateral leg ischemia, necessitating graft removal and recurrent vascular surgery to restore the blood supply to the leg. A literature review comprising various features of invasive aspergillosis and ethical aspects of commercial kidney transplantations is elucidated in this paper.
Keywords: invasive aspergillosis, IA, transplant tourism, TT, galactomannan
Introduction
Aspergillus hyphae invade pulmonary arterioles and lung parenchyma, leading to ischemic necrosis and subsequent hematogenous dissemination and angioinvasion of distant organs (kidneys, liver, spleen, sinuses, and central nervous system). The latter is a rare but devastating infection that occurs mostly in immunocompromised patients treated with chemotherapy, with prolonged neutropenia, or after solid organ transplantation. This life-threatening complication in solid organ transplant recipients is characterized by very high mortality rates and graft dysfunction. Mortality due to invasive fungal infections was significantly lower in lung transplant recipients (14.8%) than in all other transplants (globally: 48.6%; kidney 52.0%, liver 58.3%, heart 31.2%, and combined 42.9%).1
The diagnosis of IA based on clinical, radiological, and microbiological studies is challenging, with approximately 30% of cases remaining undiagnosed and untreated until death.2
We present the case of a 39-year-old female who underwent commercial living unrelated donor kidney transplantation with IA and Aspergillus arteritis causing life-threatening vascular complications, caused by contaminated graft. Ethical considerations were also discussed in this study.
Case Report
A 39-year-old female, married with three children (gravida 3) suffered from chronic kidney disease (CKD) due to mesangial proliferative glomerulonephritis. Her last pregnancy was complicated by gestational hypertension/preeclampsia with serum creatinine above 7 mg/dL, and the pregnancy was terminated on the 31st week by a cesarean section. A central catheter was placed and hemodialysis treatment was initiated. After one year of hemodialysis therapy, she traveled to a neighboring country for a commercial living unrelated to donor kidney transplantation.
After transplantation, the patient returned home on postoperative day 4 (POD 4) and was hospitalized for observation and further treatment. On admission, the patient’s kidney graft function was excellent. The patient was treated with corticosteroids, tacrolimus, mycophenolate mofetil (MMF), valganciclovir, and trimethoprim/sulfamethoxazole. Ultrasound examination performed two days later demonstrated a kidney graft 11.2 cm in length with normal perfusion and a ureteric stent. A small collection of 4.5 cm2 diameter was evident beneath the lower pole of the kidney graft.
On POD 10, the patient complained of right lower quadrant abdominal pain accompanied by high fever. Klebsiella ESBL and E coli were detected in the blood and urine cultures; therefore, carbapenem treatment was initiated.
On POD 18, the medical staff was summoned urgently for abrupt abdominal swelling, severe abdominal pain and signs of hemorrhagic shock with distal right leg ischemia. Rupture of the anastomosis of the kidney graft was suspected.
During urgent surgery, the renal artery of the transplant was found to be mutilated and detached from the iliac artery, and the graft had a bluish color, with necrotic areas requiring excision. As the external iliac artery was also dismembered with no possibility of restoring the anastomosis, a Goretex bypass of the right internal iliac artery to the proximal external iliac artery was created, and the defect in the common iliac artery was repaired. During surgery, six units of packed red blood cells and two units of fresh frozen plasma were transfused. During the postoperative period, the patient restarted chronic hemodialysis.
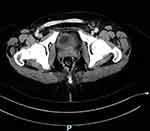
On POD 25, she was urgently readmitted for reoperation for critical right leg ischemia and bleeding between the sutures of the surgical abdominal wound and fascia. A large hematoma was observed in the abdominal wound and retroperitoneum. After the removal of the blood clots, acute bleeding from the perforated iliac arteries was observed. A segment of the iliac artery, with brittle walls 4–5 cm in length, was revealed between the sutures of the first operation. Hemostasis with ligation of the iliac artery above and beneath the area of the previous surgery was performed within healthy margins. The first Gore-Tex graft to the ipsilateral iliac artery fell apart, necessitating a new anastomosis. A new Gore-Tex anastomosis was created between both femoral arteries side-to-end, as demonstrated by the computerized tomography (CT) scan (Figure 1). At the end of the surgery, satisfactory pulses of both legs were palpated and measured using Doppler study.
The wound cultures obtained after graft nephrectomy were positive for Enterococcus faecium, Stenotrophomonas maltophilia and Klebsiella ESBL, the latter was also found in blood and urine cultures requiring treatment with carbapenem and colistin because of the resistance profile of the bacteria.
Gross pathology demonstrated necrotic graft with mutilated blood vessels. Pathological examination of the external iliac artery fragment revealed massive infestation by angiotrophic fungi with morphological features of Aspergillus (Figure 2), which caused fungal arteritis with focal necrosis and inflammation of the vascular wall with disruption of blood vessels necessitating recurrent vascular surgery. The blood cultures and graft pathology did not reveal the fungus.
As the fungal invasion of the blood vessel morphology was consistent with Aspergillus, treatment with voriconazole was started intravenously, followed by oral therapy for three months. Since then, our patient resumed hemodialysis with a poor outlook for another transplantation due to the absence of vascular access.
Discussion
Invasive aspergillosis (IA) typically occurs in immunocompromised patients with grim prognosis. Aspergillus species include A. fumigatus (approximately 90% of cases), A. flavus, A. niger, A. terreus, and A. nidulans, mostly involving the pulmonary parenchyma with bronchopulmonary aspergillosis, although hyphal invasion into the blood vessels with hematogenous spread and systemic dissemination may also occur.2 Impaired phagocytosis and alteration of calcium-calcineurin-nuclear factor of activated T cells (NFAT) by calcineurin inhibitors after solid organ transplantation or the use of tyrosine kinase inhibitors (ibrutinib) in patients with lymphoproliferative malignancies are presumed mechanisms of dissemination in patients with IA.3 Antifungal prophylaxis in transplant recipients remains problematic owing to insufficient data, significant drug interactions between azoles and calcineurin inhibitors, and risk of pathogen resistance.4
In a Swiss cohort during the first-year post-transplantation among 263 fungal infections, Candida species (60%) prevailed in transplant recipients. Aspergillus fumigatus is responsible for 1.4% of all infections, particularly in liver transplant recipients, and is associated with a high mortality rate.5
Risk factors for IA in kidney transplant patients studied according to the United States Renal Data System (USRDS) between 2005 and 2008 included certain comorbidities and immunosuppressive medications as well as organism-specific factors. The risk for any IA increased with age above 65 years, diabetes (RR = 1.71), bacterial pneumonia (RR = 1.62), and UTI (RR = 1.4) and were the top clinical risk factors for infection, with aspergillosis prevalence of 22% among invasive fungal diseases.6
The revision and Update of the Consensus Definitions of Invasive Fungal Disease (IDF) update Consortium by J Peter Donnelly et al, published in 2020, classified IFD as “proven”, “probable”, and “possible”. In adult patients group, risk factors for IFD include neutropenia, hematological malignancies, prolonged use of immunosuppressants, and inherited severe immunodeficiency.
Radiographic findings such as the halo sign, air crescent formation, cavitation, multiple pulmonary nodules, bronchopneumonia, pleural effusions, ground-glass opacities, tree-in-bud opacities, and atelectasis.7,8
The diagnostic approach is based on clinical, radiological, and microbiological studies, including histopathology, galactomannan, and1,3-β-D glucan (BDG), which are components of the fungal cell wall, and PCR in serum, plasma, and bronchoalveolar lavage (BAL). A positive serum serological assay for galactomannan (GM) can be detected 7–14 days before other diagnostic clues become apparent. Previous treatment with antifungal medications compromises the reliability of the GM test; thus, it should be interpreted with caution after treatment with antimold drugs.7,8
However, a definitive diagnosis of IA requires histopathology of specimens by Gomori’s methenamine silver stain or periodic acid–Schiff (PAS) stains or fungal-positive culture. Tissue diagnosis with fungal hyphae in formalin-fixed paraffin-embedded samples may prove fungal disease but does not distinguish the fungus involved. If fungal elements are observed on histopathology, amplification of fungal DNA by PCR combined with DNA sequencing is recommended.7,8
Treatment options for aspergillosis include anti-mold triazoles, voriconazole, and caspofungin. Increasing reports of multitriazole resistance among Aspergillus species are due to a TR34/L98H mutation, intrinsic azole resistance, and resistance to amphotericin B. Antifungal susceptibility testing (AST) in vitro or by mass spectrometry is optional.4
The prognosis is unfavorable. In a single-center study that included kidney transplant recipient, Santos et al identified 45 cases of IFD. Mortality rates were above 45%, and patients who survived lost their grafts and started regular hemodialysis.8
Invasive fungal infections occurred in nineteen cases of 17 patients, resulting in graft loss or death in 13/17 (76%) of patients and an overall mortality of 59% (10/17), as described by Shoham et al.9
Ethical Issues
The shortage of organs, long waiting lists, and uncertainties in obtaining a kidney from a family member force many patients to seek commercial kidney transplantation.10 Persistent disparities in access to kidney transplantation in highly sensitized patients and the disadvantages of racial/ethnic minorities explain the delay in access to transplantation in these groups.11
According to Akoh,12 commercial transplantation accounts for 5–10% (3500–7000) of kidney transplants performed annually worldwide, and is becoming a billion-dollar industry that exploits vulnerable donors.
Transplant tourism (TT) primarily occurs in developing countries. There is no officially regulated bilateral or multilateral organ-sharing programs based on a reciprocated organ-sharing program among authorities.13 According to Ambagtsheer et al,14 among patients who travel for transplantation, only some reported having paid for their transplants, and payments for organs in their native countries were also unreported. The Declaration of Istanbul (2008) proclaimed that optimal care of organ donors and transplant recipients should be the primary goal of transplant policies and programs and clarified the terms “organ trafficking”, “transplant commercialization”, and particularly “transplant tourism”, by introducing the term “travel for transplantation”.15
Global Financial Integrity ranks trade among the top 10 of ten world’s most profitable crimes, with an estimated annual illegal profit up to $1.2 billion. Commercial dealers such as traders, brokers, organ vendors, hospitals, and physicians in organ-selling countries are involved in this industry. As a result, poor evaluation of the donors, lack of clear consensus for donation, and the acceptance of minors as donors comprise the ethical and medical problems of commercial kidney transplantation.16
TT is associated with a high incidence of surgical complications, acute rejection, and invasive infections, all of which cause major morbidity and mortality.17 Commercial transplantations were associated with primary graft non-function, reduced kidney allograft survival, delayed graft function, decreased eGFR, hepatitis B or C seroconversion with active hepatitis and/or fulminant hepatic failure, pulmonary tuberculosis, fungal sepsis, urosepsis with antibiotic-resistant bacteria, wound infection, urine leaks, surgical complications, and malignancy.
Lessons from the Case
Invasive fungal infections may cause high mortality and morbidity in kidney transplant recipients, reinforcing the need for a high index of suspicion and prompt treatment. Commercial kidney transplantations pose the greatest risk for multiple complications, including fungal infections. In our patient, disseminated aspergillosis involving the abdominal cavity and blood vessels (Aspergillus arteritis) eventually caused life-threatening bleeding from a detached kidney graft. In addition to graft loss, the patient required repetitive vascular surgery to rescue the ischemic leg, with irredeemable complications that may preclude the option for forthcoming kidney transplantation due to loss of available blood vessel access.
Ethics and Consent Statements
- The patient has signed an informed consent including the consent to publish images (including CT scan and pathology).
- The policy of our institution does not require permission for publication of a case report.
- Organ source: Our article is dedicated to the theme of “organ trade” and “transplant tourism (TT)”. Our patient traveled abroad for commercial living unrelated to donor kidney transplantation. The source of donated organ is by organ trade and not in accordance with the declaration of Istanbul as discussed in our article with serious complication of Aspergillus arteritis caused by infected kidney graft.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The authors declare no funding was received for this work.
Disclosure
Professor Yosef Shmuel Haviv reports personal fees from Nephrocare Israel, outside the submitted work. The authors declare no other conflicts of interest in this work.
References
1. Gioia F, Filigheddu E, Corbella L, Fernández-Ruiz M, et al. Invasive aspergillosis in solid organ transplantation: diagnostic challenges and differences in outcome in a Spanish national cohort (Diaspersot study). Mycoses. 2021;64(11):1334–1345. doi:10.1111/myc.13298
2. Schubert M, Spiegel H, Schillberg S, Nolke G. Aspergillus-specific antibodies - Targets and applications. Biotechnol Adv. 2018;36(4):1167–1184. doi:10.1016/j.biotechadv.2018.03.016
3. Ceesay MM, Mehra V, Pagliuca A. Aspergillus Infections. N Engl J Med. 2022;386(2):198.
4. Neofytos D, Garcia-Vidal C, Lamoth F, Lichtenstern C, Perrella A, Vehreschild JJ. Invasive aspergillosis in solid organ transplant patients: diagnosis, prophylaxis, treatment, and assessment of response. BMC Infect Dis. 2021;21(1):296. doi:10.1186/s12879-021-05958-3
5. van Delden C, Stampf S, Hirsch HH, et al. Burden and timeline of infectious diseases in the first year after solid organ transplantation in the Swiss Transplant Cohort Study. Clin Infect Dis. 2020;71(7):e159–e69. doi:10.1093/cid/ciz1113
6. Leitheiser S, Harner A, Waller JL, et al. Risk factors associated with invasive fungal infections in kidney transplant patients. Am J Med Sci. 2020;359(2):108–116. doi:10.1016/j.amjms.2019.10.008
7. Donnelly JP, Chen SC, Kauffman CA, et al. Revision and update of the consensus definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin Infect Dis. 2020;71(6):1367–1376. doi:10.1093/cid/ciz1008
8. Santos T, Aguiar B, Santos L, et al. Invasive fungal infections after kidney transplantation: a single-center experience. Transplant Proc. 2015;47(4):971–975. doi:10.1016/j.transproceed.2015.03.040
9. Shoham S, Hinestrosa F, Moore J, O’Donnell S, Ruiz M, Light J. Invasive filamentous fungal infections associated with renal transplant tourism. Transpl Infect Dis. 2010;12(4):371–374. doi:10.1111/j.1399-3062.2010.00498.x
10. Mazaris E, Papalois VE. Ethical issues in living donor kidney transplantation. Exp Clin Transplant. 2006;4(2):485–497.
11. Petrini C. Preemptive kidney transplantation: an ethical challenge for organ allocation policies. Clin Ter. 2017;168(3):e192–e3. doi:10.7417/T.2017.2004
12. Akoh JA. Key issues in transplant tourism. World J Transplant. 2012;2(1):9. doi:10.5500/wjt.v2.i1.9
13. Budiani-Saberi DA, Delmonico FL. Organ trafficking and transplant tourism: a commentary on the global realities. Am J Transplant. 2008;8(5):925–929. doi:10.1111/j.1600-6143.2008.02200.x
14. Ambagtsheer F, de Jong J, Bramer WM, Weimar W. On patients who purchase organ transplants abroad. Am J Transplant. 2016;16(10):2800–2815. doi:10.1111/ajt.13766
15. International Summit on Transplant T, Organ T. The declaration of Istanbul on organ trafficking and transplant tourism. Clin J Am Soc Nephrol. 2008;3(5):1227–1231. doi:10.2215/CJN.03320708
16. Prasad GVR, Ananth S, Palepu S, Huang M, Nash MM, Zaltzman JS. Commercial kidney transplantation is an important risk factor in long-term kidney allograft survival. Kidney Int. 2016;89(5):1119–1124. doi:10.1016/j.kint.2015.12.047
17. Epstein M. Sociological and ethical issues in transplant commercialism. Curr Opin Organ Transplant. 2009;14(2):134–139. doi:10.1097/MOT.0b013e3283219d8e
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.