Back to Journals » Pediatric Health, Medicine and Therapeutics » Volume 12
Determining Anterior Fontanel Size and Associated Factors Among Term Neonates on the First Day of Life Born at Jimma University Medical Center (JUMC), Southwest Ethiopia: A Linear Regression Model
Authors Sheleme M, Nigatu TA , Gebremariam T, Etefa T , Birhanu A
Received 5 January 2021
Accepted for publication 7 May 2021
Published 1 June 2021 Volume 2021:12 Pages 269—278
DOI https://doi.org/10.2147/PHMT.S300399
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Roosy Aulakh
Merga Sheleme,1 Tilahun Alemayehu Nigatu,2 Teka Gebremariam,2 Tesema Etefa,2 Abdi Birhanu1
1School of Medicine, College of Health and Medical Science, Haramaya University, Harar, Ethiopia; 2Department of Biomedical Sciences, College of Medical Sciences, Institute of Health Sciences, Jimma University, Jimma, Ethiopia
Correspondence: Merga Sheleme
School of Medicine, College of Health and Medical Science, Haramaya University, P.O. Box, 235, Harar, Ethiopia
Tel +251920963059
Email [email protected]
Background: Knowledge of the normal variation in AF size may be helpful to cue early diagnosis of congenital hypothyroidism, hyperthyroidism, cardiac disease, meningitis, degree of dehydration or provide a clue to disorders of neural and skeletal development. However, the data is scarce. Therefore, this study was aimed to determine AF size and associated factors among term neonates on the first day of life born in Jimma University Medical Center (JUMC), Southwest Ethiopia.
Methodology: An institution-based cross-sectional study design was used to consecutively sample term and health newborns. Descriptive statistics, one-way ANOVA, independent samples t-test and correlation were implemented. Finally, multiple Linear regressions were used to see the association of the dependent and independent variables at 95% confidence interval. The significance level was declared at < 0.05 p-value.
Results: The mean AF size of the study population was 3.018 cm with standard deviation (±SD) of 0.909 cm (range 0.4– 5.50cm). A multiple linear regression analysis revealed that neonatal birth weight (B=0.001, 95% CI: 0.000– 0.001, p=0.000), crown heel length (B=0.048, 95% CI, 0.018– 0.078, p=0.002), labor duration (B= − 0.028, p=0.001, 95% CI: − 0.45; − 0.012), and gender of the neonates (B=− 0.275, 95% CI: − .441; − .109, p=0.001) were statistically significantly associated with AF size. In a multiple linear regression analysis AF size was explained by independent variables by 54.3%.
Conclusions: AF size of the study population was 3.018 cm with a standard deviation (±SD) of 0.909 cm. Birth weight, crown heel length, duration of labor, and gender of the neonate were significantly associated with AF size.
Keywords: anterior fontanel, associated factors, linear regression, term neonate, Ethiopia
Introduction
The fontanels are area of calvarium that appear as membrane-covered gaps between adjoined cranial bones. It is located where more than two cranial bones are juxta-posed.1,2 Anterior fontanel (AF) is the most prominent, variable and largest fontanelles, measures approximately 4cm in anteroposterior and 2.5cm in transverse dimension.3 The variation in size, shape and closure time is a key feature of normal AF.4,5
Assessing the size of anterior fontanelles is an important component of the routine neonatal examination. These neonatal anthropometric measurements carried out at birth as routine neonatal evaluation could provide several valuable information regarding the health state of the newborn.5,6 In this regard, the importance of AF size determination at birth is recently gaining attention. In several pieces of literature,7–12 abnormally large fontanels were associated with many clinical conditions including:-hypothyroidism, including intrauterine malnutrition, neonatal meningitis, down syndrome, cardiac disease, osteogenic imperfecta. Moreover, abnormally low AF size is also suggestive of–hyperthyroidism, craniosynostosis, and brain growth retardation.13–16 Therefore, AF size measured may provide sufficient clue to the possibility of risky in the neonate and so call for further diagnosis, special care, and treatment and hence, the diagnosis of an abnormally large or small AF at birth possess an alarming threat to the clinicians for interventional therapies.17
Worldwide, many researches were conducted on determination of AF by different researchers and come up with different results and shown that the size of AF varies by race, gender, geographic location.3,24,29 Most of those researches agreed up on that - maternal (race, geographic location, socio-demography),3,24,27 neonatal-related factors like: gender, birth weight, head circumference28 and, labor and pregnancy-related outcomes3,24,27–29 are associated with AF size.
For meaningful interpretation of fontanelles size, normal reference values are essential.18 However, fontanel size substantially varies between different populations and therefore population-specific normal range is required. According to the study done in Nigeria,19 there is a need for different populations to have their reference values.
Nevertheless, mean AF and its associated factors related to data are extremely scarce in developing countries including Ethiopia. It is the reason that the current study is imperative. Therefore, the present study proposed in an attempt to fill the gap by providing information on the mean AF size and associated factors of neonates born at JUMC.
Methods and Materials
Study Area and Period
The institutional-based cross-sectional study design was implemented in Jimma University Medical Center (JUMC). The study was conducted from April to October 2019.
Study Variables
The dependent variable is AF size
Independent variables: maternal socio-demographic variables: age, place of residence, marital status, educational status, occupation, monthly income; Condition during pregnancy and labor: birth order (number of pregnancy), the onset of labor, duration of labor, mode of delivery, gestational age; newborn related variables: gender of the neonate, birth weight, crown heel length, head circumference, age of neonate.
Sample Size and Sampling Technique/Procedure
A sample size of 417 was calculated using single population proportion formula using the pilot study of 50 respondents before data collection for the main study at the same setup and taking SD=0.63 from a pilot study at 95% level of confidence Zα/2= (95%=1.96) and 5% marginal error (E), and adding 10% non-response rate were included to the study as a sample. Mothers of the neonates were randomly selected to collect data from periods of 24 hours.
Eligibility Criteria
Inclusion Criteria
All term and apparently health neonates on the first day of life with gestational age between 37–42 weeks, birth weight 2500 gm. to 4000 gm. and neonates with written or verbal informed consent from their mothers were included in this study.
Exclusion Criteria
All neonates with cephalohaematoma, caput succedaneum, severe molding, abnormal presentation of labor and birth injury of the head region. Babies with neurological problems (eg, severe birth asphyxia, congenital neurological defects) and babies delivered outside JUMC were excluded in this study. Pregnancies affected by the maternal disease (eg, hypertension, diabetes mellitus, and renal disease) that might have an impact on fetal growth were also excluded in this study. Neonate with congenital anomalies, multiple pregnancies, and maternal TORCH (tetanus, syphilis, rubella, cytomegalovirus, and herpes simplex) infection that normally cause variation in size of AF during pregnancy were excluded. Neonates with any medical and physical disorders (apparent neurological, skeletal or congenital problems) and obvious chromosomal abnormalities were also excluded.
Data Collection Procedure, Tools, and Neonatal Measurement Procedure
The interview questionnaire (S3) was adapted after a review of different pieces of literature. It consisted of socio-demographic, condition during pregnancy and labor, and neonatal characteristics. AF size was measured in term newborn babies using the method proposed by Popich and Smith and measurements were taken within 24 hours after birth. They were first prepared in English, then translated to the local language, then translated back to English to check for consistency of the meaning. Data on the medical condition of the neonates were collected from the attending physician and mother of the newborn while data on other newborn-related variables were collected from measuring the parameters. Data on maternal conditions were collected using a questionnaire with a direct face-to-face interview with the mother. The conditions during delivery, birth order, the onset of labor, duration of labor and mode of delivery (Spontaneous vertex delivery or Cesarean Section) of the neonate were obtained from the mother and attending physician.
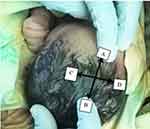
The AF was examined when the neonate was calm and held in the upright position by an assistant20 and the index finger was introduced in turn into each of the four corners of the baby’s AF as proposed by Davies et al 197521 (Figure 1). Then, a small dot was made with washable ink on the scalp immediately distal to the fingertip. Then, a piece of white paper was firmly pressed over the fontanel so that the four dots were transferred onto the paper as traced and marked. The distance between the anterior and posterior points and between the transversal points was measured and recorded with a fresh ruler with an accuracy of the closest millimeter. The size of the fontanel was then calculated as a mean sum of these diameters. The head circumference of each baby was measured with calibrated non-stretchable plastic tape around neonate’s head at the same level from occipital prominence to supra-orbital ridge approximately at glabella of the neonate (Figure 2).
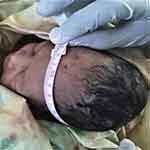 |
Figure 2 Measuring of head circumference among term neonate born at JUMC, Southwest Ethiopia, 2019. |
Birth weight was measured using the infant weight scale in grams, while head circumference and AF size in centimeters. To measure crown heel length, one of the midwives was gently held the crown of the head and positioned the head so that the upper margin of the external auditory meatus and the lower margin of the orbit of the eye was perpendicular. The other midwife was stretch the body and the legs of the neonate with the sole of feet, and then the plastic tape was used to measure the length from head to the sole22 (Figure 3).
 |
Figure 3 Measuring crown heel length among term neonate born at JUMC, Southwest Ethiopia, 2019. |
All measurements were recorded in the questionnaires (S3) designed for the study. Data collection instruments used were a data collection questionary, non-stretchable plastic tape, weight scale, washable ink, fresh ruler, surgical glove, gown, and pen.
Data Quality Control
A well-structured questionnaire prepared in English was used to collect data. Two B.Sc degree nurses were recruited into data collection and the data collection was supervised by experienced health personnel. In the case of measurement variation among two data collectors, average values were taken as the actual AF size. Two days of theoretical and three days of practical training were provided for data collectors before data collection. The collected data was checked for its completeness, accuracy, and clarity at the moments of data collection and every day by the principal investigator. The head circumference was measured with non-stretchable plastic tape with an accuracy of ±1mm and birth weight with an infantile weight scale with an accuracy of ±100gm. AP and transverse diameters were measured with a ruler from paper marked with a dot by washable ink with the accuracy of the closest mm. A pilot test was prior to data collection.
Data Processing and Analysis
The data was coded and entered into Epidata version 3.1 and then transported to SPSS version 20 for analysis after which the variables were checked for Homogeneity, Linearity, normality and homoscedasticity. Descriptive statistics like frequency, percentage, mean, standard deviation and range were used to describe dependent and independent variables. ANOVA (analysis of variance) was used to compare the mean AF size of neonates among groups of marital status, educational status, occupation and monthly income of the family. An independent sample t-test was used to compare the difference in AF size between genders, mode of delivery, the onset of labor, and place of residence. Correlation of AF size with birth weight, crown heel length, duration of labor, and gender of the neonate was also done. The candidate variables (P-value <0.2) under simple linear regression were transferred to Multiple Linear regressions to see the association with mean AF size at 95% Confidence interval. The significance level was declared at <0.05 p-value.
Ethical Consideration
Ethical clearance was obtained from the Institutional Review Board (IRB) of Jimma University (Ref.no: RPSCMF 10/11/2011). This study was conducted in accordance with the Declaration of Helsinki. Data collection was started after the study protocol (participants (mothers of neonates)) under the age of 18 years were reviewed and approved by the IRB of Jimma University to provide informed consent on their own behalf. Permissions were obtained from hospital managers/medical directors and after written (S2) or (verbal for those unable to read and write) informed consent (S1) was obtained from mothers of the baby. The supportive letter was written to JUMC. The purpose of the study was briefly explained to the neonate’s mothers. Data that were collected from the participants were kept in secured and order to maintain confidentiality. Voluntary participation was ensured and participants are free to out.
Results
Socio-Demographic Characteristics of Women Respondents
A total of 417 mothers of the neonates with singleton have participated in the study with a response rate 100%. Out of 417 women respondents, 149 (35.7%) women were within the age group 22–26 from whom 267 (64%) of them were Urban residents, while 150 (36%) were rural. Among total women respondents, 387 (92.8%) were married and more than half 243 (58.3%) were housewives in occupation. 124 (29.7%) of them unable to read and write (Table 1).
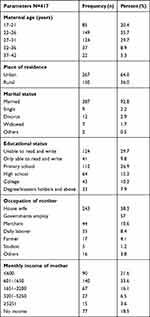 |
Table 1 Showing Frequency Distributions of Socio-Demographic Status of the Mother Respondents That Gave Birth at JUMC, Southwest Ethiopia, 2019 |
General Newborn Characteristics
During the study period, 1061 newborns (1043 live births and 18 stillbirths) were delivered from a total of 1026 women. A total of 417 term and healthy newborns (210 male and 207 females) with (male to female ratio 1.01: 1.0) were included in the study for final analysis. Of 417 newborns, 282 (67.6%) and 135 (32.4%) delivered Spontaneous vertex and cesarean section, respectively. The mean gestational age of the newborn was 39.29 weeks, which ranges from 37–42 weeks (Table 2).
 |
Table 2 General Characteristics of New-Born at JUMC, Southwest, Ethiopia, 2019 |
Anterior Fontanel Size Measurements
The mean AF size for apparently healthy term neonate for the study populations was 3.018cm with a standard deviation (±SD) of 0.909 cm. The mean AP diameter in the study populations was 3.307cm±SD (1.04) whereas the mean TD (transverse diameter) was 2.729cm± SD (0.88) in the total sample. A statistically significant difference between mean AP and TD was found (p<0.001). The 3rd and 97th percentile for mean AP diameter were 0.954cm and 5.00cm, respectively, while for TD (transverse diameter) 0.954cm and 4.3cm in the total sample (Table 3).
 |
Table 3 Distribution of AF Dimension with Respect to Sex and Percentile |
Independent sample T-test analysis between gender of the neonate (t=−5.225 p=0.000) showed a significant difference between groups. However, mean AF size between neonate of mother different in place of residence (t=1.468 p=0.7621), the onset of labor (t=−0.0446 p=0.658), and mode of deliveries (t= −.397 p=0.181) showed hardly significant association (Table 4).
 |
Table 4 T-Test Analysis with Frequency and Percentage Between Neonate of Mother Different in Place of Residence, Gender, Onset of Labour and Mode of Deliveries |
The one-way ANOVA (analysis of variance) analysis results among neonate born from the mother different in educational status (F=3.601; p= 0.003), occupation (F= 3. 232; p= 0.004) and monthly income (F= 4.97; p=0.000) showed significant difference among groups. However, the mean AF size of a newborn delivered from a mother with a different marital status showed a non-significant difference (p=0.352) (Table 5).
 |
Table 5 Showing ANOVA Analysis of Mean AF Size with Frequency and Percentage of Among Women Different in Maternal Marital Status, Educational Status, Occupation and Monthly Income |
The predictor birth weight (r=0.673, p=0.000), crown heel length (r=0.774, p=0.000) were significantly correlated with AF size while the correlation of head circumference is not significant (r=0.023, p=0.069).
Factors Associated with AF Size
After checking for regression assumption A multiple linear regression analysis revealed that an increasing of neonatal birth weight by one gram, increased AF size by 0.001cm (B=0.001, 95% CI: 0.000–0.001, p=0.000), an increasing of crown heel length by one cm, increased AF size by 0.048 (B=0.048, 95% CI, 0.018–0.078, p=0.002), an increasing of labor duration by one hour, decreased AF size by 0.028cm (B= −0.028, p=0.001, 95% CI: −0.45; −0.012), and being female reduced AF size by 0.275cm as compared to being male (B=−0.275, 95% CI: −.441; −.109, p=0.001) (Table 6). The analytical results showed that the model regression coefficient R2=0.561 and R2 adjusted =0.543 indicated that the AF size is explained by independent variables by 54.3% (Table 7).
 |
Table 6 Showing Multiple Linear Regression Analysis of the Variables |
 |
Table 7 Model Summary for Multiple Linear Regression Analysis |
Discussion
The mean AF size for apparently healthy term neonate for the study populations was 3.018cm with a standard deviation (±SD) of 0.909 cm. The finding was approximate with a study done for Black American (3.08 ± 0.8 cm), Gondar Ethiopia (3.00 ± 0.62 cm) and Nigeria (2.97 cm±0.71)4,23,24 respectively. However, the result was founded to be inconsistent with the study done for Caucasian America (2.67 ± 0.7 cm), Popich and Smith (2.1 cm ±1.5), Kanpur India (3.37 ±0.61).20,23,25 The discrepancy might be due to measuring instrument, race and geographical variations. In Popich and Smith study steel tape was used to measure AF dimensions after 48 hours.
In the current study mean the difference between the two genders was established and showed statistically significant difference (p<0.05) in mean AF size between male (3.24 ±.784) and Female (2.79 ±.97). The finding was in agreement with Caucasian and Black American,23 Gondar,4 Hispanic,27 Iranian neonates.28 The discrepancies might be due to female lead in skeletal maturation as evidenced by a previous study in Nigeria.19 However, this finding is in agreement with a study conducted in the USA,20 Iraq neonate,29 Iranian,16 Sri Lankan,18 Nigeria24 show a non-significant difference in mean AF size between male and female. The difference may be due to differences in sample size, measuring instrument and race.
The analytical results among neonates born from the mother difference in educational status occupation and monthly income showed a significant difference (p<0.05) between groups. Our finding is inconsistent with the previous study in Gondar,4 Addis Ababa,26 Nigeria.19 The discrepancies might be due to the difference in their level of knowledge to accomplish their daily activity. Further study was recommended on a previous study in Nigeria19 to establish relationships between AF size and maternal economic status.
A statistically significant difference between AF size and durations of labor (p<0.05) was in agreement with the Hispanic neonate,27 Gondar.4 The possible explanations might be due to the probability of increased molding resulted from the increased duration of labor as evidenced by a Hispanic study.
In our finding, there were significant positive correlations between AF size and birth weight (r=0.673 p<0.05) was matched with a previous study done in Hispanic,27 Gondar4 and Switzerland.22 But, the finding contrary to the previous report from in Iranian,16,28 India25 they report significant negative correlations. The studies in Nigeria reported a non-significant difference.19,24 The difference might be due to differences in race, geographical locations and measuring instruments. A significant positive correlation was also seen in the case of crown heel length (r=0.774 p<0.05) was consistent with the study done on Sri Lankan24 neonate. This finding was contrary to a previous report in South Eastern Nigeria,24 Brazil30 and make reference link reported no significant correlation, Iranian28 reported significant negative correlations. This difference might be due to a difference in the measuring instrument used.
In this study, a multiple linear regression analysis revealed that neonatal birth weight (B=0.001, p=0.000), crown heel length (B=0.048, p=0.002), labor duration (B= −0.028, p=0.001), and gender of the neonates (B=−0.275, p=0.001) were statistically significantly associated with AF size.
Conclusions and Recommendations
This study demonstrated that the mean AF size of the study population was 3.018 cm with a standard deviation (±SD) of 0.909 cm (range 0.4–5.50cm). The study demonstrated that the normal range of AF size was between 0.954 (3rd percentile) and 4.30cm (97th percentiles) among neonates born in JUMC. This normal range can serve as reference values for clinical purposes. A multiple linear regression analysis revealed that birth weight, crown heel length, duration of labor, and neonatal gender had a statistically significant association with AF size. Researchers should generate more information by using more advanced study design. Estimation of AF size by tans-fontanel ultrasound may give more accurate results than manual estimation.
Strength and Limitations of the Study
Strength
The study might provide the valuable information due to the scarcity of well-characterized information on the topic. Besides, unlike other studies, the study used a linear regression model to enhance the AF size measurement quality.
Limitation
The result of the study would be very interesting to conduct the study after the effect of molding resolved. However, the study is limited to newborns delivered on their first day of life because of routine hospital discharge of healthy neonates within 24 hours that is not reliable to get on the next day. Besides, as the study was cross-sectional, it does not provide information on AF size trends over time.
Data Sharing Statement
Any of the data used for analysis in the study is available from the corresponding author and ready in case of reasonable request.
Informed Consent
Informed consent was obtained.
Acknowledgments
We would like to acknowledge Haramaya University for sponsoring this thesis work. Our appreciation goes to all data collectors and study participants. We also acknowledge Jimma University for clearing the paper ethically.
Funding
The thesis was funded by Haramaya University.
Disclosure
The authors declare no conflicts of interest.
References
1. Kiesler J, Ricer R. The abnormal fontanel. Am Fam Physician. 2003;67(12):2547–2552.
2. Paladini D, Vassallo M, Sglavo G, Pastore G, Lapadula C, Nappi C. Normal and abnormal development of the fetal anterior fontanelle: a three-dimensional ultrasound study Naples Italy. Ultrasound Obstet Gynecol. 2008;32(6):755–761. doi:10.1002/uog.5368
3. Standring S. Gray’s Anatomy “The Anatomical Basis of Clinical Practice.
4. Oumer M, Guday E, Abebe Muche AT, Muche A. Anterior fontanelle size among term neonates on the first day of life born at the University of Gondar Hospital, Northwest Ethiopia. PLoS One. 2018;13(10):1–13. doi:10.1371/journal.pone.0202454
5. Haidari MJ, Jahanshahi K, Farahani M. The shapes of head and face in normal male newborns in south- east of Caspian Sea (Iran-Gorgan). J Anat Soc India. 2003;52(1):28–31.
6. Tiansyah RA, Mangunatmadja I, Pulungan AB. Head circumference and anterior fontanel measurements in newborns. Paediatr Indones. 2012;52(3):0–6.
7. Karamizadeh Z, Saneifard H, Amirhakimi G, Karamifar H, Alavi M. Evaluation of congenital hypothyroidism in Fars Province, Iran. Iran J Pediatr. 2012;22(1):107–112.
8. Sharma D, Shastri S, Sharma P. Intrauterine growth restriction: antenatal and postnatal aspects Rajasthan India. Clin Med Insights Pediatr. 2016;10(15):67–83. doi:10.4137/CMPed.S40070
9. Philip AGS. Neonatal meningitis in the new millennium. Neo-Reviews. 2003;4(3).
10. Paladini D, Sglavo G, Penner I, Pastore G, Nappi C. Fetuses with down syndrome have an enlarged anterior fontanelle in the second trimester of pregnancy in Naples Italy. Ultrasound Obstet Gynecol. 2010;30(10):824–829. doi:10.1002/uog.5129
11. Goldenberg L. Bulging anterior fontanelle sign of congestive heart failure infants. Clin Pediatr (Phila). 2016;9(1):42–43.
12. Balasubramanian M, Hurst J, Brown S, et al. Compound heterozygous variants in NBAS as a cause of atypical osteogenesis imperfecta. Health Sci. 2017;94(3):65–74.
13. Chawla R, Alden TD, Bizhanova A, Kadakia R, Brickman W, Kopp PA. Squamosal suture craniosynostosis due to hyperthyroidism caused by an activating thyrotropin receptor mutation (T632I). Thyroid. 2015;25(10):1167–1173. doi:10.1089/thy.2014.0503
14. Uttchin J. Management of craniosynostosis. Plast Reconstr Surg. 2002;111(6):1–17.
15. Lajeunie E, Le MM, Bonaiti-pellie C, Marchac D, Renier D. Genetic study of nonsyndromic coronal craniosynostosis. Am J Med Genet. 1995;504(4):500–504. doi:10.1002/ajmg.1320550422
16. Esmaeili M, Esmaeili M, Ghane Sharbaf F, Bokharaie S. Fontanel size from birth to 24 months of age in Iranian children. Iran J Child Neurol. 2015;9(4):15–23.
17. Periyasamy V, Mamatha H. Morphometric evaluation of anterior fontanelle: a fetal cadaveric study. Int J Health Sci. 2014;4(September):107–113.
18. Perera PJ, Wickramasinghe AR, Ranathunga N, Fernando MP, Warnakulasooriya D. Statistical characteristics of anterior fontanelle size at birth of term Sri Lankan newborns: a descriptive cross-sectional study. Ceylon Med J. 2013;58(3):96–100. doi:10.4038/cmj.v58i3.6102
19. Okorie EM, Opara PI, Alikor EA, Akani NA. Variations in anterior fontanel sizes in Nigerian children in Port Harcourt metropolis. J Pediatr Neonatal Care. 2018;8(1):1–9.
20. Poplch GA, Smith DW. Fontanels: range of normal size. J Pediatr. 1972;80(5):749–752. doi:10.1016/S0022-3476(72)80125-2
21. Davies DP, Ansari BM, Cooke TJ. Anterior fontanelle size in the neonate. Arch Dis Child. 1975;2–4.
22. Duc G, Largo RH. Anterior fontanel: size and closure in term and preterm infants. Pediatrics. 1986;78(5).
23. Faix RG. Fontanelle size in black and white term newborn infants. J Pediatr. 2010;100(2):82–84.
24. Uzukwu-Edeani CV, Ibeziako SN, Ikefuna AN, Uchendu UO. Normal anterior fontanelle sizes in newborn Igbo babies in south-eastern Nigeria. S Afr J Child Health. 2013;7(2):50–53. doi:10.7196/sajch.516
25. Mattur S, Kumar R, Mathur GP. Anterior fontanel size. Indian J Pediatr. 2019;31:1992–1995.
26. Gebremeskel T, Kinfu Y, Worku B. The size of anterior fontanel in neonates and infants in Addis. Ethiop Med J. 2008;46(1):47–53.
27. Jackson GL, Hoyer A, Longenecker L, Engle WD. Anterior fontanel size in term and late preterm hispanic neonates: description of normative values and an alternative measurement method. Am J Perinatol. 2010;27(212):307–312. doi:10.1055/s-0029-1241738
28. Shajari H, Rashidiranjbar N, Ashrafi M. Anterior fontanelle size in healthy Iranian neonates on the first day of life. Acta Med Iran. 2010;49(8):1–4.
29. Algabban N. The normal standards of anterior fontanel size in Iraqi neonates in Iraq neonate. Iraqi J Comm Med. 2008;21(2):153–158.
30. Pedros FS, Rotta N, Quintal A, Giordani G. Evolution of anterior fontanel size in normal infants in the first year of life. J Child Neurol. 2008;23:1419–1423.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.