Back to Journals » Infection and Drug Resistance » Volume 15
Determinants of Multidrug-Resistant Mycobacterium tuberculosis Infection: A Multicenter Study from Southern Ethiopia
Authors Badgeba A , Shimbre MS , Gebremichael MA , Bogale B , Berhanu M, Abdulkadir H
Received 7 March 2022
Accepted for publication 29 June 2022
Published 5 July 2022 Volume 2022:15 Pages 3523—3535
DOI https://doi.org/10.2147/IDR.S363628
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Abdulkerim Badgeba,1 Mulugeta Shegaze Shimbre,2 Mathewos Alemu Gebremichael,3 Biruk Bogale,4 Menur Berhanu,5 Hanan Abdulkadir6
1Department of Public Health, College of Medicine and Health Sciences, Werabe University, Werabe, Ethiopia; 2School of Public Health, Cheeloo College of Medicine, Shandong University, Jinan, Shandong, 250012, China; 3Department of Public Health, College of Health Sciences, Bonga University, Bonga, Ethiopia; 4School of Public Health, College of Medicine and Health Sciences, Mizan Tepi University, Mizan Aman, Ethiopia; 5Ohio State University Global One-Health Initiatives, Arba Minch, Ethiopia; 6Department of Reproductive Health, School of Public Health, College of Medicine and Health Sciences, Arba Minch University, Arba Minch, Ethiopia
Correspondence: Mathewos Alemu Gebremichael; Biruk Bogale, Email [email protected]; [email protected]
Background: Multidrug-resistant tuberculosis (MDR-TB) continues to be a public health problem. Globally in 2019, a total of 465,000 people developed rifampicin-resistant TB (RR-TB), of which 78% had MDR-TB. There is a paucity of evidence on the determinants of MDR-TB in southern Ethiopia. Hence, this study aimed to assess the determinants of MDR-TB in southern Ethiopia.
Methods: A hospital-based case–control study was conducted in southern Ethiopia. The cases were all MDR-TB patients attending TB clinics, and controls were all patients who were declared as cured or treatment completed. The cases were selected by consecutive sampling, and a simple random sampling technique was used for controls. Multivariable logistic regression analysis was done to identify determinants of MDR-TB. Adjusted odds ratios (AORs) with 95% confidence intervals (CIs) were computed, and statistical significance was declared at a P-value less than 5%.
Results: A total of 191 participants, 67 cases, and 124 controls were included. TB patients facing social stigma (AOR = 8.9, 95% CI: 2.3– 34.6), living in a household with one room (AOR = 12.3, 95% CI: 2.3– 63.5), and two rooms (AOR = 9.7, 95% CI: 1.7– 54.8), having the previous history of TB treatment (AOR = 11.8, 95% CI: 2.9– 47), having baseline body mass index (BMI) less than 18.5Kg/m2(AOR = 4.5, 95% CI: 1.2– 16.8), and having pulmonary TB (AOR = 5.1, 95% CI: 1.33– 19.8) were determinants of MDR-TB.
Conclusion: In this study, TB patients facing social stigma, living in one- and two-roomed houses, having a previous history of TB treatment, having low baseline BMI and pulmonary type of TB had higher odds of MDR-TB. Therefore, health workers in TB control programs should include mental health services in the TB care protocol, and priority should be given to malnutrition screening as a first-line diagnosis, nutritional supplements, and health education about proper housing.
Keywords: multidrug-resistant tuberculosis, determinants, case-control, southern Ethiopia
Background
Tuberculosis (TB) remains one of the major causes of morbidity, and the leading cause of death from a single infectious agent and ranking above Human Immunodeficiency Virus/Acquired Immunodeficiency Syndrome (HIV/AIDS).1,2 TB is caused by the Mycobacterium tuberculosis complex and other closely related species like Mycobacterium bovis and Mycobacterium africanum. The bacilli spread like tiny particles (droplet nuclei with 1–5 microns in diameter) expelled by the person with infectious pulmonary or laryngeal TB disease.1,2
Drug-Resistant TB (DR-TB) is resistant to one of the four first-line antibiotic drugs used to treat TB. The most common is isoniazid-resistant TB. Multidrug-resistant TB (MDR-TB) is caused by an organism that is resistant to at least an isoniazid and rifampin, the two most potent TB drugs that are used to treat all persons with TB disease. 2,3 Extensively drug-resistant tuberculosis (XDR-TB) is a subset of MDR-TB with additional resistance to any fluoroquinolone (ie Levofloxacin or Moxifloxacin) and at least one of the three injectable second-line drugs (ie, amikacin, kanamycin, or capreomycin).2,3
TB and DR-TB continue to be public health problems. Globally in 2019, about 10 million people developed TB and 1.4 million died, and around 465,000 people developed rifampicin-resistant TB (RR-TB), of which 78% had MDR-TB. 1 Among these 3.3% of new TB cases and 18% of previously treated cases had MDR/RR-TB. Similarly, there were estimated 182,000 deaths from MDR/RR-TB. About 49% of the global burden sharing MDR-TB cases are estimated to occur in India (27%), China (14%), and the Russian Federation (8%). 1
The 2020 World Health Organization (WHO) global tuberculosis report suggested that 2.6% of new TB cases and 11% of previously treated cases had MDR-TB in Africa. 1 The estimated DR-TB burden in African countries in 2016 was 92,629. About 42% (39,000/92,629) of the total estimated burden is located in Nigeria (20,000) and South Africa (19,000).4
Ethiopia is one of the 30 high MDR-TB burden countries; where 12% of previously treated TB cases had MDR/RR-TB.1 Of the African countries, Ethiopia ranks 5th among the high burden of MDR-TB with estimated 5800 new cases of MDR-TB each year.4 A systematic review and meta-analysis conducted on MDR-TB in Ethiopia revealed that the pooled prevalence of MDR-TB was 7.24% with the majority of them being previously treated patients.5 The social and economic impact of MDR-TB on patients and the health care system is catastrophic.6 The treatment of MDR-TB is more complicated, more toxic, and less effective with a 48% of success rate, and patients experience higher mortality.6 Additionally, it takes more than 20 months of daily therapy to treat MDR-TB, resulting in costly treatment, social isolation, loss of employment, and long-term socioeconomic and psychological effects.6
MDR-TB case finding and management is challenging for patients due to further economic and social costs faced while seeking help and treatment.7 Due to the lack of national capacity to perform drug susceptibility testing for all incident TB cases, it is important to prioritize patients based on the risk factors to ensure that people are identified and started on the most effective treatment regimen as early as possible to prevent transmission of disease.7
Previous studies identified the different risk factors for MDR-TB. Suboptimal adherence, previous TB treatment, and lack of patient’s direct observation of treatment.8 According to the WHO report, people with poor socioeconomic status and other vulnerable groups and having immune compromising conditions such as HIV, undernutrition, smoking, and drug and alcohol abuse, and having poor access to diagnosis and treatment, poor adherence due to social and economic constraints, as well as poverty and socioeconomic inequalities, poor access to quality health services for prisoners, poor living conditions among refugees and other migrant were determinants of MDR TB.6
There are limited numbers of studies conducted to identify the determinants of MDR-TB in Ethiopia, especially in the southern parts of the country.9,10 This study considered variables like nutritional status that have not been addressed by other studies. Therefore, identifying determinants of MDR-TB is crucial to the prevention and control of MDR-TB. Furthermore, it helps to achieve the global targets that have been set within the context of the Sustainable Development Goals (SDGs) and WHO’s End TB Strategy to eliminate TB. Therefore, the purpose of this study was to identify determinants of MDR-TB in southern Ethiopia.
Methods
Study Design, Period, and Setting
An institution-based case–control study design was conducted from March 18 to May 18, 2021, in southern Ethiopia. The Southern Nation Nationalities and People Region (SNNPR) has six MDR-TB treatment initiation centers, namely Arba Minch, Jinka, Butajira, Nigist Eleni Mohammed Memorial, Dilla, and Mizan Tepi University Teaching Hospitals. This study was conducted at Arba Minch, Butajira, Nigist Eleni Mohammed Memorial, and Dilla Hospitals. In these MDR-TB treatment initiation centers, routine care is being provided to patients, including clinical reviews like the assessment of drug side effects, assessment of adherence problems, and drug prescription. In Ethiopia, TB/MDR-TB management (diagnosis, treatment, and follow-up) is being implemented according to the national guidelines for the management of TB, DR-TB, and leprosy.24,26
Population
The source population for the cases was all confirmed MDR-TB cases attending TB clinics and controls were all patients who declared cured or treatment completed at the end of first-line anti-TB treatment at the selected hospitals. The study population for the case was all MDR-TB cases and those who fulfilled inclusion criteria attending TB clinic during the study period, whereas for the control was all patients who were declared as cured or treatment completed at the end first-line anti-TB treatment and who fulfilled inclusion criteria during the study period.
Inclusion criteria for cases were all patients with MDR-TB which was confirmed by culture and/or drug susceptibility testing, and on treatment and for controls were all patients on first-line anti-TB who declared cure or treatment completed at the end of first-line anti-TB treatment. However, patients who were known to be mentally ill and severely ill were excluded.
Sample Size Determination
The sample size was calculated using the Epi Info version 7.0 software sample size calculation formula for Unmatched Case–Control Study. Considering the following assumptions: 95% confidence level, power of 80%, case-to-control ratio of 1:2, and proportion of control with the history of contact with MDR-TB patients 6.17%.9 These gave a total sample size of 183 (61 cases and 122 controls) by adding 10% non-response, the final sample size for the study was 201 (case 67 and control 134).
Sampling Technique
From six MDR-TB treatment centers (Arba Minch, Jinka, Butajira, Nigist Eleni Mohammed Memorial, Dilla, and Mizan Tepi University Teaching hospitals), four hospitals were selected by simple random sampling (SRS) technique. Accordingly, Arba Minch, Butajira, Nigist Eleni Mohammed Memorial, and Dilla Hospitals were selected. The sample size was proportionally allocated to each hospital according to the flow of TB patients. MDR-TB patients on the treatment were selected as the cases by consecutive sampling technique and an SRS technique was used to pick the controls. Unit TB registration book of patients who were registered as treatment complete or cured of March 1, 2020, to February 30, 2021, was used as a baseline source of information to prepare the sampling frame in each study facility.
Study Variables
The dependent variable in the present study was the MDR-TB. Independent variables were socio-demographic factors such as residence, age, sex, educational status, occupation, social stigma, disclosure of TB status, transportation, being in refugee, and imprisonment. Behavioral factors such as alcohol consumption, cigarette smoking, chewing Khat, and sleeping habit. Housing conditions like the number of rooms, number of windows, and family size. Treatment-related factors such as previous TB treatment history, treatment failure, interruption of anti-TB drugs, lack of direct observation by health workers, patient hospitalization for TB treatment, duration of first-line anti-TB treatment, and adverse effects of the anti-TB drug. Clinical factors like nutritional status, contact history, episode of TB illness, type of TB, sputum smear, and comorbidity (HIV/AIDS, DM, and chronic obstructive pulmonary disease).
Operational Definitions
Multidrug-Resistance (MDR)
Resistance to at least Isoniazid and Rifampicin. Extensive drug resistance (XDR): Resistance to Isoniazid and Rifampicin (ie MDR) as well as any fluoroquinolone, and any of the second-line injectable anti-TB drugs (Capreomycin, kanamycin, and amikacin).2,24,26
Contact History
Close contact is defined as living in the same household or in frequent contact with a source case like a caregiver with sputum smear-positive TB. Source cases who are sputum smear-negative but culture-positive are also infectious but to a much lesser degree.11
Adverse Drug Effect
A response to a drug that is noxious and unintended which occurs at doses normally used for prophylaxis, diagnosis, treatment, and physiological modification (like peripheral neuropathy, anemia, hepatitis, hearing impairment, acute kidney injury).8
Social Stigma
In the present study, social stigma was measured by if he/she answered “yes” to the question “Have you ever faced any social stigma (isolation, lack of support, discrimination, and loss of employment) related to your previous or current TB?”
Nutritional Status
BMI was used to measure nutritional status and categorized as severely underweight – BMI < 16.5kg/m2, underweight – BMI under 18.5 kg/m2, normal weight– BMI ≥ 18.5 to 24.9 kg/m2, overweight – BMI ≥25 to 29.9 kg/m2, and obesity – BMI ≥30 kg/m2.25,27
Data Collection Tool and Procedure
The interview was conducted for MDR-TB patients during in-patient ward and follow-up services and randomly selected controls were interviewed by giving appointment day by phone to those who have been declared completed treatment or cured patients. In addition to the primary data, secondary data were retrieved from the document of patients. Data were collected by six BSc nurses recruited from TB clinics of selected hospitals. Additionally, three master’s health officers were involved in supervision activities.
Regarding the TB status of patients, data were taken from the patient documents in the selected MDR-TB treatment centers in Ethiopia. MTB (Mycobacterium tuberculosis) diagnosis and treatment were made according to the National Guidelines of Ethiopia on TB, Drug-resistant TB, and Leprosy.24,26 Smear-positive pulmonary TB (PTB+) was defined when a patient has positive acid-fast bacilli (AFB) results for at least one or two initial sputum specimens by microscopy, and diagnosis for smear-negative pulmonary TB (PTB−) was established when direct microscopy indicated two AFB negative findings, and no response to a course of broad-spectrum antibiotics, and radiological abnormalities in line with pulmonary TB, and decision by a clinician to treat with a full course of anti-TB drugs or a patient whose diagnosis is based on culture positive for MTB.
According to the Ethiopian national guideline, Xpert MTB/RIF test is recommended as the initial diagnostic test for all persons with presumptive TB. However, if Xpert service is not readily available on the same day, sputum microscopy should be used as the primary diagnostic test for tuberculosis in the interim to avoid diagnostic delay.24,26 When there is strong clinical evidence that TB has affected body organs other than the lungs, a patient is diagnosed with extra-pulmonary tuberculosis (EPTB) and a physician decides to treat the patient with a full course of anti-TB treatment. During the first two months, patients received daily rifampicin, pyrazinamide, isoniazid, and ethambutol followed by daily rifampicin and isoniazid for 4 months or more in the continuation phase.
Regarding MDR-TB, all individuals diagnosed with TB undergo the drug resistance screening test at least for Rifampicin at baseline using rapid DST technique by Xpert or FL-LPA. Furthermore, the diagnosis of MDR-TB was made based on the National Diagnostic Algorithm for MDR-TB Patients in Ethiopia.24,26
Data Quality Assurance
To ensure the quality of data, two-day training was given to data collectors and supervisors. A questionnaire was pretested on 5% of the sample size before the data collection. A questionnaire was translated into Amharic for common understanding. To check the consistency of the meaning, the Amharic version was translated back to English. The collected data were reviewed and crosschecked daily by the supervisors and principal investigators to check for accuracy, consistency, and completeness of the questionnaires. Data cleaning was done before analysis. In the MDR-TB treatment centers, instruments and reagents were checked for reliability and reproducibility of the test before any test started. All new lots of reagents were tested with a known positive and negative control.
Data Processing and Statistical Analysis
The collected data were coded and entered into Epi-data version 4.2 and exported to STATA version 14 for further management and analysis. Frequency and cross-tabulations were used to check the missing values. Descriptive statistics like frequencies, proportions, percentages, and different summary measures were computed. Bivariable and multivariable binary logistic regression models were applied to identify determinants of MDR-TB. Independent variables which are biologically plausible and showed significant statistical association in the previous studies and having P-values <0.25 in the bivariable analysis were taken into the multivariable analysis to identify determinants of MDR-TB. The Hosmer and Lemeshow model fitness test was used to check the goodness of fit of the model and the model was a good fit (0.76). Variance inflation factor (VIF) was used to assess multicollinearity and there was no multi-collinearity within variables with a maximum value of 7.63 and a minimum value of 1.22. Adjusted odds ratios (AORs) with corresponding 95% confidence intervals (CIs) were estimated to assess the strength of the associations and statistical significance was declared at a P-value less than 5%.
Ethical Approval and Consent to Participate
Ethical clearance was obtained from the Institutional Review Board (IRB) of Arba Minch University, College of Medicine and Health Sciences with a letter reference number IRB/1083/21. A formal permission and support letter was written by Arba Minch University, School of Public Health to Arba Minch, Butajira, Nigist Eleni Mohammed Memorial, and Dilla Hospitals. Respondents were informed about the objective and purpose of the study and informed written consent was obtained. For minors, assent from each respondent and informed written consent was obtained from the family. Clear information was given to inform respondents about the purpose and procedure of the study, the importance of their participation, and the right to withdraw at any time if they want and about privacy and participant was coded anonymously and confidentiality of information was maintained. COVID-19 precaution mechanisms were applied during the data collection process. The study was also conducted following the declaration of Helsinki.
Results
Socio-Demographic Characteristics
A total of 191 patients (124 controls and 67 cases) participated in the study, which yields a response rate of 92.5% for controls and 100% for the cases. The mean age was 30 (±1.3 SD) years and 29.5 (±1.1 SD) years for the cases and controls, respectively. Regarding patients’ sex, 38 (56.7%) cases and 61 (49%) controls were males. Above half, 41 (61.2%) of cases and 122 (98%) of controls were urban residents. Concerning marital status, among cases 35 (52%) and controls 62 (50%) were single. About 13 (19.4%) of cases and 41 (33%) of controls attended primary school (Table 1).
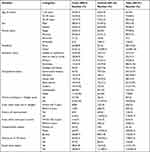 |
Table 1 Socio-Demographic Characteristics of Respondents at Selected Hospitals in Southern Ethiopia, 2021 |
Housing Conditions
Thirty-four (50.75%) cases and 29 (23.39%) of controls had one-roomed houses. Regarding family size, 31 (46.27%) cases and 40 (32.26%) of control had five to six family members living in a house. Fifty-eight (86.6%) of cases and 122 (98.4%) of controls houses had windows (Table 2).
 |
Table 2 Housing Condition of Respondents at Selected Hospitals in Southern Ethiopia, 2021 |
Behavioral Factors
About 23 (34%) of cases and 26 (21%) of controls were alcohol consumers, and 15 (22.4%) cases and 12 (9.68%) of controls were smokers of cigarettes and 15 (22.4%) of cases and 21 (16.9%) of controls were Khat chewers during their lifetimes (Table 3).
 |
Table 3 Behavioral Factors of Respondents at Selected Hospitals in Southern Ethiopia, 2021 |
Treatment-Related Factors
More than half, 35 (52.24%) of cases and 15 (12.1%) controls had a history of previous treatment for TB. Of those patients with a history of previous treatment for TB, 14 (40%) of cases and 12 (80%) of controls were hospitalized for previous TB treatment. Nineteen (54.3%) of cases and five (33.3%) of controls had a history of anti-TB drug interruptions for at least a day and 31 (88.6%) of cases and 8 (53.3%) of control were encountered drug adverse effects during previous TB treatment (Table 4).
 |
Table 4 Treatment-Related Factors of Respondents at Selected Hospitals in Southern Ethiopia, 2021 |
Clinical Related Factors
Twelve (17.9%) of cases and 10 (8.1%) of the controls had a contact history with known MDR-TB patients. Of the study participants, 5 (7.5%) of cases and 11 (8.9%) of controls had known chronic diseases. The majority, 59 (88.06%) of the cases and 74 (59.68%) of controls had pulmonary TB. Among these, 49 (83.03%) of cases and 32 (43.24%) of controls had smear-positive pulmonary TB (Table 5). Regarding the BMI of respondents, a total of 47 (70.15%) was undernourished among cases, whereas a total of 48 (38.71%) were undernourished among controls (Figure 1).
 |
Table 5 Clinical Factors of Respondents at Selected Hospitals in Southern Ethiopia, 2021 |
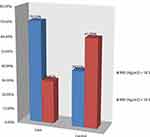 |
Figure 1 The proportion of body mass index (BMI) of respondents at selected hospitals in southern Ethiopia, 2021. |
Determinants of MDR-TB
Bivariable and multivariable binary logistic regression analyses were computed. Variables that had a p-value <0.25 in the bivariable logistic regression analysis were entered into the multivariable logistic regression analysis. In multivariable logistic regression analysis, social stigma, number of living rooms, previous history of TB treatment, baseline BMI, and type of TB were significantly associated factors with MDR-TB (Table 6).
 |
Table 6 Bivariable and Multivariable Binary Logistic Regression Analysis on the Determinants of Multi-Drug Resistant TB Among TB Patients at Selected Hospitals in Southern, Ethiopia, 2021 |
Social stigma related to the current or previous TB illness was statistically associated with MDR-TB. The likelihood of developing MDR-TB was 8.9 times higher among patients who faced social stigma related to current or previous TB illness when compared to their counterparts (AOR = 8.9, 95% CI: 2.3–34.6). Similarly, the odds of MDR-TB were 12.3 times higher among patients living in a house with one room (AOR = 12.3, 95% CI: 2.3–63.5), and 9.7 times higher with two rooms as compared to TB patients living in a house with four and more rooms (AOR=9.7,95% CI: 1.7–54.8) (Table 6).
Exposure to previous TB treatment was another factor associated with MDR-TB. The risk of MDR-TB was 11.8 times higher among patients who had a history of previous treatment as compared to TB patients who had no previous history of TB treatment (AOR = 11.8, 95% CI: 2.9–47). The odds of MDR-TB were 4.5 times higher among TB patients with baseline BMI less than 18.5Kg/m2 as compared to patients with baseline BMI ≥18.5 Kg/m2 (AOR = 4.5, 95% CI: 1.2–16.8). Moreover, pulmonary TB patients had 5.1 times higher chance of having MDR-TB in comparison to patients having extrapulmonary TB (AOR = 5.1, 95% CI: 1.33–19.8) (Table 6).
Discussion
In this study, social stigma related to current or previous TB illness, number of living rooms, previous history of TB treatment, baseline BMI, and type of TB were determinants of MDR-TB.
The likelihood of MDR-TB was 8.9 times higher among patients who faced social stigma related to current or previous TB illness when compared to their counterparts. This finding is consistent with studies conducted in Ethiopia, Serbia, and china.12–14 This might be explained by the fact that stigma results in emotional distress, and lack of social support consequently this contributes to treatment interruptions, treatment failure, recurrence, and resistance.
The number of the living room was an important determinant of MDR-TB in the present study. The odds of MDR-TB was 12.3 times higher among patients living in a house with one room and 9.7 times higher among patients living in a house with two rooms as compared to TB patients living in a house with four or more rooms. Studies conducted in southern Ethiopia, Sudan, and Addis Ababa supported this finding.9,15 One possible reason could be that the concentration of Mycobacterium tuberculosis in the air is determined by the space available in the room, and the presence of adequate ventilation in the house. Therefore, overcrowded conditions increase the transmission of drug-resistant Mycobacterium tuberculosis.6 However, a study conducted in Bhutan showed that having more than 2 rooms in the house also emerged to be marginally associated with having MDR-TB.17 This could be due to differences in the classification of variables and differences in the housing conditions.
The occurrence of MDR-TB was strongly associated with the previous treatment with anti-TB drugs. This study revealed that the odds of MDR-TB was 11.8 times higher among patients who had a history of previous TB treatment as compared to patients who had no previous history of TB treatment. This finding is consistent with studies conducted in China, Bhutan, Sudan, Addis Ababa, Bishoftu, and Adama hospital TB.13,14,15,17–19 The possible explanation for this might be that previous TB treatment may be attributed to inappropriate treatment regimens, inadequate or irregular drug supply, and poor adherence to anti-TB drugs resulting in the emergence of MDR strains on any TB drugs. The resistance could be due to repeated and inappropriate ways of taking the medication that made the bacteria mutate and develop resistance against the drugs.2,20
Nutritional status was another determinant of MDR-TB in the present study. Underweight (<18.5Kg/m2) patients had a 4.5 higher fold of developing MDR-TB when compared to patients having a baseline BMI ≥18.5 Kg/m2. Undernutrition depletes essential nutrients, which would be important for the immunity system for defense against infections and this deficiency consequently leads to the MDR-TB. Furthermore, undernutrition is linked with increased adverse effects like liver toxicity and causes malabsorption of key anti-TB drugs such as rifampicin, increased relapse after cure, and delay sputum conversion.21
The odds of MDR-TB was 5.1 times higher among TB patients with pulmonary TB as compared to patients with extrapulmonary TB. This finding is in line with studies conducted in St Peter’s TB Specialized Hospital, and ALERT Hospitals, Addis Ababa.14,16 Patients with smear-positive pulmonary TB have a higher bacterial load and may not respond to treatment within a short period compared with patients who have a low bacterial load. Nevertheless, this result is inconsistent with a study conducted at Bishoftu, Adama hospital TB, and Shenen Gibe Hospital where pulmonary TB had no significant association with the MDR-TB.22,23
As a limitation, recall bias could be considered as one potential challenge, since some of the information was based on the recall of the study participants’ past events. Data collectors were recruited from the TB clinic so that information bias may be introduced due to knowledge of the disease status that may affect the response of the subjects.
Conclusions and Recommendations
This study showed that TB patients facing any social stigma, living in one- and two-roomed houses, having a previous history of TB treatment, having low baseline body mass index and pulmonary type of TB had higher odds of MDR-TB. Therefore, to alleviate the burden of MDR-TB, patients with these identified risk factors should be given priority in TB control programs. Improving the socio-economic and living conditions of TB patients should be considered in the national TB programs. Health care workers in TB clinics should pay attention to malnourished patients and should provide gene expert MTB/RIF tests for patients with malnutrition. Governmental and non-governmental organizations should work to reduce the stigma related to TB patients, it is necessary to include mental health services in the TB care process. The policymakers should revise the national TB protocol for universal drug sensitivity testing to prioritize putting malnourished TB patient gene expert MTB/RIF test as a first-line diagnosis. Researchers should conduct a qualitative study to explore cultural and belief-related social stigma towards TB patients.
Abbreviations
AIDS, Acquired Immunodeficiency Syndrome; ALERT, All African leprosy and tuberculosis rehabilitation training; BMI, Body Mass Index; COPD, Chronic Obstructive Pulmonary Disease; DM, Diabetes Mellitus; DR-TB, Drug-resistant Tuberculosis; HIV, Human immunodeficiency virus; HSTP, Health Sector Transformation Plan; MDR-TB, Multi-Drug Resistant Tuberculosis; NEMMH, Nigist Eleni Mohammed Memorial Hospital, RR-TB, Rifampicin Resistant Tuberculosis, SDGs, Sustainable Development Goals; SNNPR, South Nation Nationalities and People Region; TB, Tuberculosis; WHO, World health organization; XDR-TB, Extensive drug-resistant Tuberculosis.
Data Sharing Statement
All relevant data are available on the paper and the anonymized STATA data set of study will be obtained from the corresponding author in a reasonable request.
Acknowledgments
The authors acknowledge the Arba Minch University, College of Medicine and Health Sciences for facilitation to do this thesis and all South Nations, Nationalities and people’s region Multidrug-Resistant TB treatment initiation center focal person for their commitment to providing the necessary information. We would also like to acknowledge data collectors and supervisors for accomplishing their tasks and study participants for their honest responses.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
Funding for the data collection was obtained from Arba Minch University, College of Medicine and Health Sciences.
Disclosure
The authors declare that they have no competing interests in this work.
References
1. World Health Organization. Global tuberculosis report 2020. Geneva; 2020. Available from: https://www.who.int/publications-detail.redirect/9789240013131.
2. The Federal Democratic Republic Of Ethiopia, Ministry of Health (MOH). National Guidelines for TB, DR-TB, and Leprosy in Ethiopia sixth edition 2017. Available from: https://www.afro.who.int/publications/national-guidelines-tb-drug-resistant-tb-and-leprosy-ethiopia-sixth-edition.
3. CDC. Multidrug-resistantTuberculosis. National Center for HIV/AIDS, Viral Hepatitis, STD, TB Prev; 2012. Available from: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&cad=rja&uact=8&ved=2ahUKEwi328KBzuXzAhVMxIUKHdq2CvQQFnoECAMQAQ&url=https%3A%2F%2Fwww.cdc.gov%2Fnchhstp%2Fpublications%2Fdocs%2Fnchhstp-annualreport-2012-508.pdf&usg=AOvVaw08miU8aCDrWufGLBWQkapj.
4. Ismail N, Omar SV, Ismail F, et al. Drug-resistant tuberculosis in Africa: current status, gaps, and opportunities. Afr J Lab Med. 2018;7(2):1. doi:10.4102/ajlm.v7i2.781
5. Girum T, Muktar E, Lentiro K, Wondiye H, Shewangizaw M. Epidemiology of multidrug-resistant tuberculosis (MDR-TB) in Ethiopia: a systematic review and meta-analysis of the prevalence, determinants and treatment outcome. Trop Dis Travel Med Vaccines. 2018;4:1–12. doi:10.1186/s40794-018-0061-9
6. World Health Organization. Companion handbook to the WHO guidelines for the programmatic management of drug-resistant tuberculosis; 2014. Available from: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&cad=rja&uact=8&ved=2ahUKEwjqw9fkzuXzAhXEzoUKHctVAXIQFnoECAYQAQ&url=https%3A%2F%2Fapps.who.int%2Firis%2Fbitstream%2Fhandle%2F10665%2F130918%2F9789241548809_eng.pdf&usg=AOvVaw1JfS7pFYotDNwhFtEqmTrF.
7. Belay A, Dagnaw WW, Kumsa A, et al. Guidelines On Programmatic Management Of Drug Resistant.
8. Federal Ministry of Health of Ethiopia. National guidelines for management of tb, dr-tb and leprosy in Ethiopia. Addis Ababa; 2018; Available from: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&cad=rja&uact=8&ved=2ahUKEwivheyPzXzAhVOCxoKHVsZAWIQFnoECAIQAQ&url=https%3A%2F%2Fwww.impaact4t.org%2Fwpcontent%2Fuploads%2F2019%2F08%2FEthiopiaNational-guideline-for-TB-Leprosy-and-DR_TB-6th-ed-Aug.2018.pdf&usg=AOvVaw2AiUI9VZiIjlTn1HxJ_AtY.
9. Biru D, Endrias Markos W. Determinants of drug-resistant tuberculosis in Southern Ethiopia: a Case-Control Study. Infect Drug Resist. 2020;Volume 13:1823–1829. doi:10.2147/IDR.S256536
10. Fikre A, Tewelde T, Shaweno T. Determinants of multi-drug resistant tuberculosis among tuberculosis patients in Southern Ethiopia: a case-control study. J Med Bacteriol. 2019;8(1):1–12.
11. Christof C, Nußbaumer-Streit B, Gartlehner G. WHO-Leitlinie: prävention und Kontrolle von Tuberkulose-Infektionen [WHO Guidelines on Tuberculosis Infection Prevention and Control]. Gesundheitswesen. 2020. 82(11):885–889. PMID: 32977345. doi:10.1055/a-1241-4321
12. Stosic M, Vukovic D, Babic D, et al. Risk factors for multidrug-resistant tuberculosis among tuberculosis patients in Serbia: a case-control study. BMC Public Health. 2018;18(1):1–8. doi:10.1186/s12889-018-6021-5
13. Zhang C, Wang Y, Shi G, et al. Determinants of multidrug-resistant tuberculosis in Henan province in China: a case-control study. BMC Public Health. 2015;16(1):1–8. doi:10.1186/s12889-016-2711-z
14. Assefa D, Seyoum B, Oljira L. Determinants of multidrug-resistant tuberculosis in Addis Ababa, Ethiopia. Infect Drug Resist. 2017;10:209. doi:10.2147/IDR.S134369
15. Workicho A, Kassahun W, Alemseged F. Risk factors for multidrug-resistant tuberculosis among tuberculosis patients: a case-control study. Infect Drug Resist. 2017;10:91. doi:10.2147/IDR.S126274
16. Hirpa S, Medhin G, Girma B, et al. Determinants of multidrug-resistant tuberculosis in patients who underwent first-line treatment in Addis Ababa: a case-control study. BMC Public Health. 2013;13(1):1–9. doi:10.1186/1471-2458-13-782
17. Tenzin C, Chansatitporn N, Dendup T, et al. Factors associated with multidrug-resistant tuberculosis (MDR-TB) in Bhutan: a nationwide case-control study. PLoS One. 2020;15(7):e0236250. doi:10.1371/journal.pone.0236250
18. Desissa F, Workineh T, Beyene T. Risk factors for the occurrence of multidrug-resistant tuberculosis among patients undergoing multidrug-resistant tuberculosis treatment in East Shoa, Ethiopia. BMC Public Health. 2018;18(1):1–6. doi:10.1186/s12889-018-5371-3
19. Elduma AH, Mansournia MA, Foroushani AR, et al. Assessment of the Risk Factors Associated with Multidrug-Resistant Tuberculosis in Sudan: A Case-Control Study. Epidemiology and health; 2019:41.
20. US WASHINGTON. National action plan for combating multidrug-resistant tuberculosis; 2015. Availablefrom:https://obamawhitehouse.archives.gov/sites/default/files/microsites/ostp/national_action_plan_for_tuberculosis_20151204_final.pdf.
21. TB FACTS. ORG. Nutrition & TB - Malnutrition, under nutrition; 2021. Available from: https://tbfacts.org/nutrition-tb/.
22. Gobena D, Ameya G, Haile K, Abreha G, Worku Y, Debela T. Predictor of multidrug-resistant tuberculosis in the southwestern part of Ethiopia: a case-control study. Ann Clin Microbiol Antimicrob. 2018;17(1):1–7. doi:10.1186/s12941-018-0283-8
23. Demile B, Zenebu A, Shewaye H, Xia S, Guadie A. Risk factors associated with multidrug-resistant tuberculosis (MDR-TB) in a tertiary armed force referral and teaching hospital, Ethiopia. BMC Infect Dis. 2018;18(1):1. doi:10.1186/s12879-018-3167-9
24. FMOH. Guidelines for management of tb, dr-tb and leprosy in Ethiopia; 2022. Available from: https://www.afro.who.int/sites/default/files/2019-04/Ethiopia-National%20guidelines%20for%20TB%2C%20DR.TB%20and%20Leprosy%20in%20Ethiopia%20-%20Sixth%20Edition.pdf.
25. Mengesha MM, Gebremichael MA, Watumo D, Hallström IK, Jerene D. Poor adult tuberculosis treatment outcome and associated factors in Gibe Woreda, Southern Ethiopia: an institution-based cross-sectional study. PLOS Global Public Health. 2022;2(3):e0000161. doi:10.1371/journal.pgph.0000161
26. FMOH. Guidelines for Clinical and Programmatic Management of TB, TB/ HIV, DR-TB and Leprosy.
27. Weir CB, Jan A. BMI classification percentile and cut off points. Treasure Island (FL): StatPearls Publishing; 2021. Available from: https://pubmed.ncbi.nlm.nih.gov/31082114/.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
