Back to Journals » International Medical Case Reports Journal » Volume 13
Detection of Rickettsia Endosymbiont Bemisia Tabaci in the Amputated Limbs of Three Buerger’s Disease Patients
Authors Fazeli B , Mirhosseini A, Hashemi Z, Taheri H
Received 2 August 2019
Accepted for publication 4 February 2020
Published 18 February 2020 Volume 2020:13 Pages 33—40
DOI https://doi.org/10.2147/IMCRJ.S225839
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ronald Prineas
Bahare Fazeli, 1, 2 Ali Mirhosseini, 1 Zahra Hashemi, 1 Hossein Taheri 3
1Immunology Research Center, Inflammation and Inflammatory Diseases Division, School of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran; 2Vascular Independent Research and Education, European Foundation, Milan, Italy; 3Surgery Department, Farabi Hospital, Mashhad, Iran
Correspondence: Bahare Fazeli
Immunology Research Center, Inflammation and Inflammatory Diseases Division, School of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran
Tel +98 51 38002379
Fax +985138414499
Email [email protected]
Abstract: Until recently, the aetiology of Buerger’s disease (BD) has been unknown. Although there is a close relationship between BD and smoking, it cannot explain the low prevalence of BD among smokers or the disease’s geographical distribution. Infectious pathogens, such as Rickettsial infection, have also been suggested as the trigger of BD development, but this theory has neither been proven nor ruled out. The aim of this study was to evaluate the footprint of Rickettsial infection in tissue specimens obtained from amputees with Buerger’s disease. Forty-nine tissue biopsies were obtained from three below-the-knee amputees who also had a diagnosis of BD according to Olin’s criteria (between 14– 21 biopsies for each patient). After extraction of DNA from the tissue samples, the existence of 16srRNA was evaluated using a PCR test. The sequence of PCR products was evaluated using Geneious 11.1.2 software and NCBI blast. The 16srRNA was found in 3 to 7 samples from each patient. The sequence of the PCR products had a 98% homology with Rickettsia Tabaci. The sequences of the three patients were aligned, and no difference was found in the sequence of 16srRNA amongst the patients. Rickettsia Tabaci is a pathogen that infects tobacco leaves. Thus, BD might be an infectious disease for which smoking could be the route of pathogen entry into the bloodstreams of the sufferers. However, further studies are highly recommended to confirm this hypothesis.
Keywords: thromboangiitis obliterans, Buerger’s disease, rickettsia, tobacco, smoking
Introduction
Buerger’s disease (BD) is a non-atherosclerotic, inflammatory and thrombotic occlusive peripheral vascular disease, usually seen in male smokers and it can result in gangrene and limb loss. BD is more common in the Middle East, the Far East and southeast Asia.1
To date, the aetiology of BD remains unknown. Although there is a close relationship between BD and smoking, it cannot explain the low prevalence of BD amongst smokers or the disease’s geographical distribution.2 Infectious pathogens, such as Rickettsial infection, have been suggested as the trigger of BD development,3–5 but this theory has neither been proven nor ruled out.
We present three cases of Buerger’s disease in which the patients underwent some below-knee amputations and we evaluated the footprint of Rickettsial infection in tissue specimens obtained from amputees. Under sterile operating room conditions, tissue biopsies were obtained from the anterior and posterior tibial arteries, the small and great saphenous veins, the microvasculature close and far from the site of gangrene, after which DNA was extracted from the tissue samples.
The existence of 16srRNA specific for Rickettsiales were evaluated using a polymerase chain reaction (PCR) test following Giulieri et al primers sequences.6 The sequence of the primers and the PCR programme is summarised in Table 1. The purified DNA of Rickettsia conorii (AMPLIRUN®, Spain) was considered the positive control. Two negative controls were considered for this study. One was the DNA extracted from the blood of a healthy young man to verify whether the primers amplified any homo sapiens gene. The next negative control was nuclease free water to control any contamination during the process of PCR testing.
 |
Table 1 Primers and Program Used for PCR Amplification for 16srRNA Specific for Rickettsiales. |
The PCR products were sequenced by Macrogen’s sequencing service. The sequence of PCR products was analysed using Geneious 11.1.2 software and the basic local alignment search tool of the national center for biotechnology information (NCBI BLAST). Written informed consent was provided by the patients for molecular investigations on the tissue samples and to have the case details and any accompanying images published and got an ethical code from Mashhad University of Medical Sciences (Ethical code: MUMS-951595).
Case Presentation
Case 1
A 42-year-old Caucasian man with a ten-year-old diagnosis of Buerger’s disease reported in September 2013 complaining of gangrene and severe pain at rest in his left forefoot. His past medical history included a history of cold sensitivity, foot claudication, thrombophlebitis migrans, night burning pain of toes, and amputations of the left fourth toe and below the right knee in the five years prior to consulting with us. The patient was and had been a heavy smoker (three packs of cigarettes per day) for 28 years.
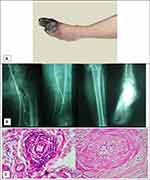
Upon physical examination, the first, second and third toes of the left foot were completely gangrenous, with cyanosis of the fifth toe (Figure 1A). The dorsalis pedis (DP) and tibialis posterior (TP) pulses of the left foot were absent. The popliteal pulses of both sides were also absent. The femoral pulses were intact.
The laboratory tests revealed that he had normal fasting blood sugar and a normal lipid profile, and the angiography of his left limb was characteristic of Buerger’s disease (Figure 1B).
The patient underwent a below-knee amputation of the left leg. In the pathology study of the amputated leg, infiltration of inflammatory cells within all layers of the vessel walls, preserved vascular architecture and intima thickening were observed (Figure 1C). The PCR 150bp band for 16srRNA was found in eight samples from 21 tissue biopsies. Next, the PCR bands were separated from the electrophoresis gel, after which the sequence of PCR products was evaluated using Geneious 11.1.2 software and NCBI BLAST.
Case 2
A 45-year-old Caucasian man with a 14-year-old diagnosis of Buerger’s disease reported in August 2011 complaining of gangrene and severe pain at rest in the first and third toes of his left foot. His past medical history included a history of Raynaud’s phenomenon in the hands, calf claudication, thrombophlebitis migrans and night burning pain of left toes. In addition, he had undergone an amputation of the second left toe and left lumbar sympathectomy in May 2009. Approximately five months prior to reporting to us, he had undergone autologous stem cell therapy. He had also been treated with aspirin and amlodipine for the five years prior to reporting to us.
The patient was and had been a heavy smoker (two to three packs of cigarettes per day) for 30 years.
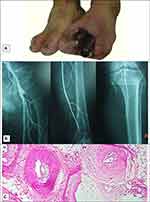
Upon physical examination, the third toe of the left foot was completely gangrenous, and the gangrene had extended to the first toe, along with forefoot discoloration (Figure 2A). The DP and TP pulses of both feet were absent. The popliteal pulses were also absent on the left side. The femoral pulses were intact. The radial pulse of the right hand was absent, and the Allen’s test of both hands was positive.
The laboratory tests revealed that he had normal fasting blood sugar and a normal lipid profile, and the angiography of his left limb was characteristic of Buerger’s disease (Figure 2B).
Due to the progression of the gangrene to the forefoot, the patient underwent a left below-knee amputation. In the pathology study of the amputated leg, organised thrombus, infiltration of inflammatory cells within all the layers of the vessel walls, preserved vascular architecture and intima thickening were observed (Figure 2C). The PCR 150bp band for 16srRNA was found in five samples from 14 tissue biopsies. Next, the PCR bands were separated from the electrophoresis gel, after which the sequence of PCR products was evaluated using Geneious 11.1.2 software and NCBI BLAST.
Case 3
A 40-year-old Caucasian man with a one-year-old diagnosis of Buerger’s disease reported in July 2015 complaining of gangrene and severe pain at rest in his right forefoot. His past medical history included a history of Raynaud’s phenomenon of the right foot, foot claudication that manifested as burning rather than cramping pain, and night paraesthesia. The patient was and had been a heavy smoker (one pack of cigarettes per day) for 20 years.
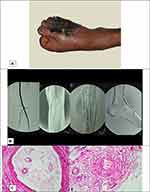
Upon physical examination, the second through fifth toes of the right foot were completely gangrenous, and the gangrene had also extended to the forefoot (Figure 3A). The DP of both feet and TP of the right foot were absent. The popliteal and femoral pulses of both sides were intact.
The laboratory tests revealed that he had normal fasting blood sugar and a normal lipid profile, and the angiography of his left limb was characteristic of Buerger’s disease (Figure 3B).
The patient underwent a below-knee amputation. In the pathology study of the amputated leg, infiltration of inflammatory cells within all the layers of the vessel walls, preserved vascular architecture and intima thickening were observed (Figure 3C). The PCR 150bp band for 16srRNA was found in three samples from seven tissue biopsies, close to the margin of gangrene. Next, the PCR bands were separated from the electrophoresis gel, and the sequence of PCR products was evaluated using Geneious 11.1.2 software and NCBI BLAST. The PCR 150bp band for 16srRNA was found in five samples from 14 tissue biopsies, after which the PCR bands were separated from the electrophoresis gel. The sequence of PCR products was evaluated using Geneious 11.1.2 software and NCBI BLAST.
Sequencing of 16srRNA and Data Analysis
The sequence of the 16srRNA PCR products from the amputees was TCGCAACCCTCATTCTTATTTGCCAGCACTTCGGGTGGGAACTTTAAGGATACTGCCAGTGACAAACTGGAGGAAGGCGGGGACGACGTCAAGTCATCATGGCCCTTACGACCAGGGCTACACACGTGCTACAATGGTGTTTACAGAGGA. The sequence had a 98% homology, with the Rickettsia endosymbiont of Bemisia Tabaci (R. Tabaci) with E value 2e-39. The sequences of the 16sRNA PCR products of these three amputees were aligned using Geneious 11.1.2 software. No differences were found in the sequence of 16srRNA amongst the patients. As the homology was 98%, we decided to design another pair of primers specific to R. Tabaci to confirm our results. However, no reference gene for 16SrRNA of R. Tabaci has been introduced by NCBI. Therefore, we aligned the five submitted sequences of R. Tabaci that were found according to the previous BLAST and then designed the primers. The sequence of the primers and the PCR programme is summarised in Table 2.
 |
Table 2 Primers and Program Used for PCR Amplification for 16srRNA Specific for Rickettsia Endosymbiont Besimia Tabaci |
The sequence of the R.Tabaci 16SrRNA PCR products from the same samples was AGATTTATCCCCAACCACGGGAAAATTAGCGACACACTGCCCGTCACGCCATGGGAGTTGGTTTTACCTGAAGGTGGTGAGCTAACGCAAGAGGCAGCCAACCACGGGAAAATTAGCGACAATAGCCATCAGCTCTTCGATTCGATGGGTTCTTAGAGGACAGGACCCTGGCTTGCTCTATTTCTCCCCTGCTCTGGCACTGCCTCACACTCAGGACTTGCTAGGGCCATTTAGAGTGGGAGGGTCCTGT
After alignment with the sequence of the 16srRNA of Rickettsia Endosymbiont of Besimia Tabaci, 110 nucleotides had 98.67–100% homology with different species of R. Tabaci with an E value of 1-e41 – 9-e34 (Figure 4). Notably, 23 base pairs (bp) were repetitive and are bolded in the sequence. The remaining 130 bp had 100% homology with homo sapiens oestrogen-related receptor beta (ERRB). The PCR test for R. Tabaci was conducted three times at a minimum of two-week intervals and sequenced three times to evaluate the accuracy of the results.
 |
Figure 4 Alignment of the PCR product sequence of 16srRNA of Rickettsia Endosymbiont Besimia Tabaci with submitted sequences for the same pathogen in NCBI. |
Discussion
To date, the aetiology of BD remains unknown. Infectious pathogens, including oral bacteria and Rickettsia, have been suggested as the trigger of BD development,3–5,7 but this theory has neither been proven nor ruled out. Even in this theory, it is still unknown if the clinical manifestation of BD is due to the pathogenesis of the infectious pathogen or due to an autoimmunity that is induced by the pathogen.
Amongst the suggested infectious pathogens, Rickettsia infection drew our attention because it could explain the ratio of male to female involvement with BD, the pain characteristics, the involvement of small- and medium-sized vessels and also the pathological characteristics of BD.8
Following evaluation of the IgG class antibody against Rickettsia rickettsii, which has cross-reactions with the spotted fever group, and Rickettsia typhi, which has cross-reactions with the typhus fever group, 92% of the patients were positive for Rickettsia rickettsii antibodies.5
In this study, we aimed to evaluate the presence of any Rickettsia pathogen inside the vascular tissue of the amputees using PCR for 16sRNA, specifically for Rickettsials. Notably, the specific PCR band was detected only in the vessels with thrombosis. Each band was sequenced and then BLASTed to detect the type of Rickettsia.
Homology of about 98% between the sequence of our detected band and the sequence of Rickettsia Endosymbiont of Bemisia Tabaci emerged as a hypothesis if the infected tobacco leaf could serve as a reservoir for horizontal transmission of Rickettsia to humans (i.e. botanosis). To evaluate our hypothesis, we designed specific primers for R. Tabaci. Through the sequencing of the specific PCR band, approximately half of the PCR product had 98.63–100% homology with R. Tabaci, and the remainder of the sequence belonged to the ERRB homo sapiens gene. According to the negative controls and owing to the fact that the specific primers had been BLASTed and could not detect any homo sapiens gene, our findings may indicate that the Rickettsia integrated its genome inside its host genome. It is not known whether integration within the ERRB gene is significant or merely a coincidence. The function of ERRB is not well understood in humans, but it is known that it plays a role in the regulation of cell cycles.9 Rickettsia may integrate its genome inside the ERRB gene to break the control of ERRB on endothelial cell proliferation as its cellular host. However, this hypothesis requires further evaluation.
All in all, because R. Tabaci can influence the response of its insect host (whitefly) to heat stress and because infected whiteflies are most commonly found in areas with high temperatures,10,11 this hypothesis could explain the geographical distribution of BD. Also, it may explain the prevalence of BD amongst people from the low and middle classes of society due to their tendency to smoke cheap brands of cigarettes containing infected tobacco leaves.
However, the root of entrance of R. Tabaci to the body is the main challenge to this hypothesis. It is not known whether the pathogen survives the high temperature associated with burning tobacco or whether its gene is overtaken by another pathogen, such as oral bacteria, and then passes through the body.
Although more evidence and case studies are needed to reach the conclusion that R. Tabaci plays a role in the development of BD, our findings might provide a clue for understanding the disease’s pathophysiology.
Conclusion
All in all, further studies about the possible transmission of an infectious pathogen from the tobacco leaf to humans are highly recommended. Because, inhibition of tobacco leaf disease, may reduce the risk of smokers developing vascular disease and further studies from different geographical areas could prove or disprove this hypothesis.
Acknowledgment
The authors would like to thank Dr SA Rahim Rezaee and Hiva Sharebiani for their constructive comments on this study.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Olin JW, Shih A. Thromboangiitis obliterans (Buerger’s disease). Curr Opin Rheumatol. 2006;18(1):18–24. doi:10.1097/01.bor.0000198000.58073.aa
2. Fazeli B. Buerger’s disease as an indicator of socioeconomic development in different societies, a cross-sectional descriptive study in the North-East of Iran. Arch Med Sci. 2010;6(3):343–347. doi:10.5114/aoms.2010.14253
3. Bartolo M, Antignani PL, Todini AR, Ricci G. Buerger’s disease: etiologic role of the rickettsiae? J Mal Vasc. 1987;12(1):82–84.
4. Fazeli B, Ravari H, Ghazvini K. Rickettsia infection could be the missing piece of the Buerger’s disease puzzle. Int Angiol. 2017;36(5):410–416. doi:10.23736/S0392-9590.17.03420-4
5. Fazeli B, Ravari H, Farzadnia M. Does a species of Rickettsia play a role in the pathophysiology of Buerger’s disease? Vascular. 2012;20(6):334–336. doi:10.1258/vasc.2011.cr0271
6. Giulieri S, Jaton K, Cometta A, Trellu LT, Greub G. Development of a duplex real-time PCR for the detection of Rickettsia spp. and typhus group rickettsia in clinical samples. FEMS Immunol Med Microbiol. 2012;64(1):92–97. doi:10.1111/j.1574-695X.2011.00910.x
7. Iwai T, Inoue Y, Umeda M, et al. Oral bacteria in the occluded arteries of patients with Buerger disease. J Vasc Surg. 2005;42(1):107–115. doi:10.1016/j.jvs.2005.03.016
8. Fazeli B. Is Rickettsia the key to solving the puzzle of Buerger’s disease? Vascular. 2014;22(5):393–394. doi:10.1177/1708538113491256
9. Madhu Krishna B, Chaudhary S, Mishra DR, et al. Estrogen receptor α dependent regulation of estrogen related receptor β and its role in cell cycle in breast cancer. BMC Cancer. 2018;18(1):607. doi:10.1186/s12885-018-4528-x
10. Brumin M, Levy M, Ghanim M. Transovarial transmission of rickettsia spp. and organ-specific infection of the whitefly Bemisia tabaci. Appl Environ Microbiol. 2012;78(16):5565–5574.
11. Brumin M, Kontsedalov S, Ghanim M. Rickettsia influences thermotolerance in the whitefly Bemisia tabaci B biotype. Insect Sci. 2011;18(1):57–66. doi:10.1111/j.1744-7917.2010.01396.x
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.