Back to Journals » Nature and Science of Sleep » Volume 14
Detection of Common Arrhythmias by the Watch-PAT: Expression of Electrical Arrhythmias by Pulse Recording
Authors Pillar G , Berall M , Berry RB , Etzioni T, Henkin Y, Hwang D, Marai I, Shehadeh F, Manthena P, Rama A, Spiegel R , Penzel T , Tauman R
Received 25 January 2022
Accepted for publication 11 April 2022
Published 21 April 2022 Volume 2022:14 Pages 751—763
DOI https://doi.org/10.2147/NSS.S359468
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ahmed BaHammam
Giora Pillar,1 Murray Berall,2 Richard B Berry,3 Tamar Etzioni,1 Yaakov Henkin,4 Dennis Hwang,5 Ibrahim Marai,6,7 Faheem Shehadeh,6 Prasanth Manthena,8 Anil Rama,9 Rebecca Spiegel,10 Thomas Penzel,11 Riva Tauman12
1Sleep Laboratory, Carmel Medical Center and Technion Faculty of Medicine, Haifa, Israel; 2Center of Sleep and Chronobiology, University of Toronto, Toronto, ON, Canada; 3UF Health Sleep Center, University of Florida, Gainesville, FL, USA; 4Cardiology Department, Soroka Medical Center, Be’er Sheva, Israel; 5Kaiser Permanente San Bernardino County Medical Center, Fontana, CA, USA; 6Cardiology Department, Rambam Medical Center, Haifa, Israel; 7Baruch Padeh Medical Center and the Azrieli Faculty of Medicine in the Galilee, Poriya, Israel; 8Sleep clinic, Kaiser Permanente Los Angeles Medical Center, Los Angeles, CA, USA; 9Sleep Clinic, Kaiser Permanente San Jose Medical Center, San Jose, CA, USA; 10Department of Neurology and Sleep Center, Stony Brook University Hospital, Stony Brook, NY, USA; 11Charite Universitätsmedizin Berlin, Sleep Medicine Center, Berlin, Germany; 12Sleep Disorders Center, Tel Aviv Sourasky Medical Center and Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel
Correspondence: Giora Pillar, Sleep Laboratory, Carmel Medical Center and Technion Faculty of Medicine, Haifa, 31096, Israel, Tel +972 4 8250258, Fax +972 48250699, Email [email protected]
Background: The WatchPAT (WP) device was shown to be accurate for the diagnosis of sleep apnea and is widely used worldwide as an ambulatory diagnostic tool. While it records peripheral arterial tone (PAT) and not electrocardiogram (ECG), the ability of it to detect arrhythmias is unknown and was not studied previously. Common arrhythmias such as atrial fibrillation (AF) or premature beats may be uniquely presented while recording PAT/pulse wave.
Purpose: To examine the potential detection of common arrhythmias by analyzing the PAT amplitude and pulse rate/volume changes.
Patients and Methods: Patients with suspected sleep disordered breathing (SDB) were recruited with preference for patients with previously diagnosed AF or congestive heart failure (CHF). They underwent simultaneous WP and PSG studies in 11 sleep centers. A novel algorithm was developed to detect arrhythmias while measuring PAT and was tested on these patients. Manual scoring of ECG channel (recorded as part of the PSG) was blinded to the automatically analyzed WP data.
Results: A total of 84 patients aged 57± 16 (54 males) participated in this study. Their BMI was 30± 5.7Kg/m2. Of them, 41 had heart failure (49%) and 17 (20%) had AF. The sensitivity and specificity of the WP to detect AF segments (of at least 60 seconds) were 0.77 and 0.99, respectively. The correlation between the WP derived detection of premature beats (events/min) to that of the PSG one was 0.98 (p< 0.001).
Conclusion: The novel automatic algorithm of the WP can reasonably detect AF and premature beats. We suggest that when the algorithm raises a flag for arrhythmia, the patients should shortly undergo ECG and/or Holter ECG study.
Keywords: home sleep apnea test, WatchPAT, obstructive sleep apnea, arrhythmia, atrial fibrillation
Introduction
Sleep apnea is a very common sleep disorder, associated with arrhythmias. Premature beats and atrial fibrillation (AF), which are the most common cardiac arrhythmias, are increasingly observed in patients with sleep apnea.1,2
While the gold standard for the diagnosis of sleep apnea is still considered polysomnography (PSG), Home Sleep Apnea Testing (HSAT) in general, and the WatchPAT (WP) in particular, are gaining more and more popularity.3–6 This has even further increased during the last 2 years with the COVID19 epidemic.7 The WP is based on peripheral arterial tone (PAT) signal’s amplitude and rate, oxygen saturation and actigraphy. It has been previously shown to be accurate in detecting both respiratory indices and sleep staging.5,6,8–11 Although we have recently shown the WP may be used in patients with AF for detecting sleep apnea,12 it has never been shown to be able to detect AF or other arrhythmias.
Detecting arrhythmias in patients with sleep apnea is of great importance, specifically AF, as it is known to be associated with both morbidity and mortality.13 The prevalence of AF in patients with sleep apnea is substantially larger than in the general population1,14,15 and the prevalence of sleep apnea among patients with AF is even much higher, estimated between 49% and 62%.16,17 Moreover, studies have demonstrated that patients with sleep apnea who undergo electrical cardioversion are at greater risk for recurrence of AF.18–20 Additionally, treatment of sleep apnea may reduce the risk of recurrence of AF by 40%.21,22 Furthermore, the presence of sleep apnea in patients with arrhythmias diminishes the efficacy of antiarrhythmic therapy.23–25 Thus, detecting sleep apnea in patients with AF is of great clinical importance, it is considered as a modifiable risk factor for AF, and the treatment of sleep apnea in such patients may reduce morbidity and mortality.22,26 Likewise, the ability of flagging patients suspected of having arrhythmias, especially AF, a phenomenon which could be paroxysmal, could provide valuable information to the patient and to the physician who may use this additional information for further investigation. Hence, it is of great importance and clinical implication to detect AF events in WP studies, especially in patients with high pre-test probability of arrhythmias.
Therefore, the question whether the WP may detect AF or other arrhythmias is relevant and important. While arrhythmias are electrical phenomena detected by ECG, they may have a mechanical reflection observed in the monitoring of pulse. Therefore, the purpose of the current study was to quantify the accuracy of the WP in detecting AF and/or premature beats in patients with sleep apnea. We hypothesized that in patients with undisturbed electromechanical transmission, the WP should be able to accurately detect these arrhythmias.
Materials and Methods
This was a multicenter study. Participants were recruited as part of a larger study at 11 Medical Centers. The following centers participated:
- Carmel Medical Center, Haifa, Israel
- Centre for Sleep and Chronobiology, Toronto, Canada
- UF Health Sleep Center, University of Florida, USA
- Soroka Medical Center, Beer Sheva, Israel
- Kaiser Permanente Fontana Medical Center, USA
- Rambam Medical Center, Israel
- Kaiser Permanente LA Medical Center, USA
- Kaiser Permanente San Jose Medical Center, USA
- Stony Brook University Hospital, USA
- Charite Universitätsmedizin, Berlin, Germany
- Sourasky Medical Center, Tel Aviv, Israel
In all centers an IRB approval was obtained, and all participants have signed an informed consent prior to participation. The list of sites:
- Carmel Medical Center, Haifa, Israel
- Center of Sleep and Chronobiology, Toronto, ON, Canada
- UF Health Sleep Center, University of Florida, Gainesville, FL, USA
- Soroka Medical Center, Be’er Sheva, Israel
- Kaiser Permanente Fontana Medical Center, Fontana, CA, USA
- Rambam Medical Center, Haifa, Israel
- Kaiser Permanente Los Angeles Medical Center, Los Angeles, CA, USA
- Kaiser Permanente San Jose Medical Center, San Jose, CA, USA
- Stony Brook University Hospital, Stony Brook, NY, USA
- Charite Universitätsmedizin Berlin, Sleep Medicine Center, Berlin, Germany.
- Sourasky Medical Center, Tel Aviv, Israel
Sample (Population) Selection
In order to increase the likelihood of arrhythmias in the recordings, we used the same cohort we previously reported with selectively recruiting higher percentage of patients with potential central sleep apnea.27 This cohort consisted of many patients with a previous diagnosis of heart failure and/or AF. Thus, this is an a-priori deliberate decision to have a unique population and not a typical sleep lab population. Of a total of 84 participants in the current study (see below), 50 had a previous diagnosis of CHF and/or AFib (33 had CHF without known AF, 9 had a previous diagnosis of AF without known CHF, and 8 patients had both conditions), comprising 59.5% of the study population. Since this population was also studied for the potential ability of the WP to detect central sleep apnea,27 the local staff at each participating center attempted recruiting participants in whom they estimated that there was a relatively high risk of having central apneas. Since there is an overlap between central apneas, CHF and arrhythmias, in the current study we have utilized the same population, albeit for different purposes and with a different algorithm. The contribution of the various centers was not equally distributed, and the various sites had recruited the following number of participants: 1, 2, 3, 5, 5, 8, 8, 8, 11, 12, 21 (total of 84). Since the study population was detailed elsewhere,27 this population is briefly described in Table 1. As can be seen, there were no significant differences in medical history by gender.
 |
Table 1 Anthropometric Characteristics and Medical History Parameters |
All participants underwent a full in lab PSG (which is in use in each center) and simultaneous recording of WP200U (Itamar-Medical, Caesarea, Israel). The WP200U signals were analyzed for the presence of arrhythmia (AF or premature beats) by a novel automatic algorithm which is described below. These results were compared to the ECG’s manual scoring for arrhythmias which is recorded as part of the PSG. The manual scoring was performed centrally by an authorized independent cardiologist scorer according to European Society of Cardiology (ESC) guidelines and textbook of Cardiovascular medicine. This cardiologist was blinded to the WP200U analysis. Furthermore, the cardiologist who scored the recordings was not aware of the participants’ prior diagnoses. The only prescoring information that the cardiologist was provided with was the presence or absence of pacemaker in any specific record.
Polysomnography
The reference used for comparison in this study was FDA approved in lab PSG from multiple manufacturers used in the eleven study sites. Multi-channel PSG configurations compliant with accepted standards and manual scoring according to the American Academy of Sleep Medicine (AASM) directives28 were used. Enrolled patients underwent a simultaneous recording of standard PSG and WP in the clinical sleep lab. Manual scoring of ECG channel for AF, premature beats and other arrhythmias was performed by an independent cardiologist scorer who was blinded to the automatic scoring of the WP (see below). The rules to score these arrhythmias was corresponded to the ESC guidelines and textbook of Cardiovascular medicine rules.29
WatchPAT System
The WP system used in this study is a Home Sleep Apnea Testing (HSAT) system based on a wrist-worn device and a finger probe which acquires Peripheral Arterial Tone (PAT) signals and arterial oxygen saturation levels, together with actigraphy data from an accelerometer that is embedded in the wrist unit, and a Snoring and Body Position (SBP) sensor that is positioned under the sternal notch. The WP algorithm detects offline apnea/hypopnea events, respiratory effort–related arousals, sleep/wake status, and determines sleep stages.5,6,8–11 The automatic algorithm for arrhythmia detection is described below.
The WP assesses changes in vascular tone at the fingertip, with transient vaso-constrictory events being associated with sympathetic activation that typically terminates sleep disordered breathing events. While this characteristic was used to detect SDB, in the current study the focus was on the pulse shape and pulse to pulse timing and variability as described hereby:
Algorithm of the WP to Detect Arrhythmias
The PAT is related to the electromechanical coupling arises in the electrical rhythm generated in the heart. Only the timing of QRS-complexes (ventricular contraction) is directly observable on the PAT signal. Therefore, the arrhythmia detection algorithm in WatchPAT mostly relies on this property. The detection of AF in the WP depends on the irregularly irregular nature of the QRS-complexes that accompanies this condition and is a consequence of the abnormal depolarization events of the atria.
Algorithm to Detect Atrial Fibrillation
The algorithm begins with a preprocessing phase to identify the individual pulses in the PAT signal (see Figure 1 for normal sinus rhythm). A sequence of pulse-to-pulse durations is generated. We refer to these values as RR periods, although they are extracted from the PAT signal, since they accurately estimate the RR period in the ECG signal, allowing for small differences in pulse propagation to the periphery.
The irregularly irregular trait typical to AF is evident in the distribution of RR periods within time frames of a few tens of seconds. The typical distribution of AF is unimodal, wide, and flat. While this pattern is not unique to AF, the chaotic nature of this condition suggests that similar distributions would also be apparent in the derivatives of the sequence (see Figure 2), which is not the case with other types of irregular heart rate or sleep apnea (see Figure 3). This characteristic leads to the following algorithm for detection of AF:
- Get the sequence of RR-periods
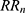 within the timeframe of interest. Calculate the first and second derivatives sequences
within the timeframe of interest. Calculate the first and second derivatives sequences 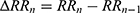 and
and 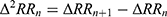 .
. - Estimate the distribution of each of the three sequences by calculating the histograms
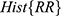 ,
, 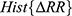 ,
, 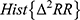 .
. - Test each of the three histograms for three characteristics: unimodality, wide distribution, flat distribution.
- Only if each of the three histograms features all the three characteristics (ie, all nine tests produce positive results), mark the time frame as AFib.
- Get the sequence of RR-periods
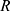 .
.
Three time frames were selected for the comparison of AF detection between the WP and the ECG of the PSG: First, a direct comparison of AF duration (second by second, see Table 2). Second, a six minutes threshold for AF performance was decided a-priori to analyses and was chosen since it has been shown that a single episode lasting > 6 minutes might be sufficient to increase risk for stroke in patients without overt clinical AF.30 60-seconds threshold was included to demonstrate the performance of the device in shorter AF episodes.
 |
Table 2 Sensitivity Specificity and Predictive Values for Detecting Time in AF by the WP Compared to ECG |
Algorithm for Detection of Premature Beats
Premature beats are detected based on the identification of specific patterns in the sequence of RR-periods and amplitudes of the respective pulses in the PAT signal.
The primary attribute of premature beats is, obviously, an RR period shorter than the typical at the contiguous heart rate. In addition, premature beats often demonstrate reduced stroke volume, which leads to lower amplitude in the PAT signal, and, in some cases, to complete elimination of the pulse (see Figure 4 for example). The algorithm, hence, considers two possible patterns:
- A beat with an RR period shorter than its surrounding beats, with normal or reduced amplitude, and followed by a normal or prolonged beat, is identified as a premature beat (case I).
- When the RR period found for a single beat, compared to its surrounding beats, is long enough to be the accumulated duration of a premature and a normal beat, an undetected premature beat is assumed (case II).
In both cases, additional shape analysis of the pulse is performed to detect artifacts in the PAT signal and remove the marking of premature beats if they are triggered by artifacts.
In cases of premature beats, there would usually be an interest in the origin of the event, whether it is atrial or ventricular. However, the properties in an ECG signal that help in identifying the origin of the premature beat, are the existence of the P-wave and the formation of the QRS-complex, which are not directly observable in the PAT signal, and hence an ECG test is required to distinguish between atrial and ventricular premature heartbeats.
Statistical Analysis
The ECG marked AF/premature beat by the certified cardiologist (gold standard) and the automatic WP analysis’s arrhythmia during sleep were compared as descriptive statistics. Scatter graphs with linear regressions coefficients and Pearson correlation were generated. Sensitivity, specificity, positive and negative predictive values of the WP-derived AF detection compared to AF based on the ECG channel of the PSG were calculated based on either second by second, event by event or maximal event length. Premature beats detection was compared between the two methods based on the number of premature beats detected per minute of sleep. Whenever applicable, in all cases p<0.05 was considered statistically significant.
Results
A total of 84 patients were included in the analysis, 54 males and 30 females. The mean age was 57±16 (range: 22–83) with no differences in age and BMI between genders (Table 1). Patient’s medical history presented high percentages of hyperlipidemia, hypertension, heart failure and diabetes. There were no significant differences in comorbidities between genders (Table 1).
Atrial Fibrillation (AF) Analysis
AF duration analysis (second by second) as measured by the WP algorithm compared to ECG derived from the PSG is presented in Table 2. It shows the sensitivity, specificity and predictive values for the detection of AF by the WP, with the gold standard being the ECG. As can be seen, the specificity was very high, indicating very low false positive results. On the other hand, sensitivity was only moderately high indicating some degree of false negative events.
If looking on these times not based on second by second but rather based on event by event, as recommended in an ECG analysis performance standard,29,30 then the results for episodes of at least 60 seconds improve with a sensitivity of 81%, but a reduction in the positive predictive value to 80%. In short AF episodes (episode less than 60 seconds) the ability of the WP to detect the AF episode is limited.
As for detecting of patients with long episodes of AF, ie longer than 6 min, the Sensitivity, Specificity, PPV and NPV of WP200U were 91.7%, 98.6%, 91.7% and 98.6% respectively, as can be seen in Table 3. The area under the receiver operating curve (sensitivity vs 1-specificity) for this figure was AUC=0.9919.
 |
Table 3 Sensitivity Specificity and Predictive Values for Detecting Patients with AF Episodes Longer Than 6 min, by the WP Compared to ECG |
Premature Ectopic Beats Analyses
For premature beats analysis, time which was marked as AF or pacemaker rhythm by the ECG scorer was excluded. Files left with valid sleep time ≥ 600 seconds were included in this analysis (N=71).
Correlation Analysis
The relationship between the WP200U’s average number of premature beats detected per minute of sleep and ECG ones are presented using scatterplot and linear regressions coefficients in Figure 5. As can be seen, there is a very high and significant correlation between the two methods in detecting premature contractions (R=0.98, p<0.001). Since it is argued that the average premature contractions rate is less important and less predictive of a real pathology than the maximal premature contractions per minute, we provide a separate analysis for the maximal rate of premature contractions (Figure 6). We defined the maximum per patient rate of events detected by each system by examining each 600 seconds’ window. As can be seen, the correlation is still very high (R=0.95, p<0.001), probably somewhat less than with the average due to 1 outlier in whom the ECG detected a maximal rate of 24 premature beats per min compared to 13/min with the WP.
Table 4 shows the Sensitivity, Specificity, and predictive values for detecting at least 5 premature beats per min (in some places considered threshold for pathological premature beats). The Area Under the Curve (AUC) of the ROC curve for premature Ev/min in 600-sec window versus ECG when utilizing a threshold of 5 Ev/min for ECG was 0.990.
 |
Table 4 Sensitivity, Specificity, PPV and NPV of WP200U Rate of Premature Beats in 600-Sec Window versus ECG Using Threshold = 5 Ev/min |
Discussion
The major findings of the current study are that in patients undergoing overnight PAT study, without directly measuring ECG, the PAT may provide a reasonably good ability to detect major arrhythmias such as AF and premature beats. While this detection is not diagnostic, it should serve as a “flag raising” indicating further clinical investigation, referring the patient for a standard ECG and/or 24h Holter ECG recording.
The question of the capability of detecting cardiac arrhythmia by indirect measurement of oximetry pulse wave (photoplethysmographic (PPG) waveform) has been previously raised,31 but a specific algorithm was not reported. A combined measure of both ECG signal and PPG waveform had been previously reported as a good system to alarm for potentially life-threatening arrhythmias such as asystole, extreme bradycardia, extreme tachycardia, ventricular tachycardia, and ventricular fibrillation.32 By coupling ECG signal with PPG signal the suggested algorithm could also detect electro-mechanical transmission failure, which is extremely important especially in an intensive care setting.32 However, that study did not indicate arrhythmia based on the PPG signal alone. Paradkar and Chowdhury, on the other hand, did show that based on measuring PPG signal alone life-threatening arrhythmias may be detected.33 They showed that tachycardia, bradycardia, asystole, ventricular tachycardia and ventricular fibrillation may be diagnosed by analyzing pulse-wave with an overall true positive rate of 93% and a true negative rate of 54% (sensitivity of 67% and specificity of 88.5%). By analyzing 219 2-minute pulse recordings from 121 participants with AF, McManus et al showed that a smartphone app can accurately discriminate pulse recordings during AF episodes from other rhythms including sinus rhythm and premature contractions.34 While that was a relatively pioneer study, it was tested only for brief time periods, in awake subjects. In addition, they looked specifically to discriminate only on AF from all other rhythms, rather than attempting to discriminate sinus rhythm from arrhythmias. Yet, their results are in concert with the current results, indicating the ability to identify AF by measuring pulse-wave. Several additional studies have shown positive and encouraging results for recognizing AF by assessing pulse only, with high sensitivity and specificity of 95%.35 A recent systematic review of the literature regarding the performance of mobile health devices, usually measuring pulse, in diagnosing AF concluded that although the evidence for clinical effectiveness is limited, smart watches and health devices may be useful in detecting AF demonstrating sensitivities between 66–100%, and specificities between 93–99%.36 Thus, our findings are not surprising, yet adding the dimension of all night recording in relatively vulnerable patients. Our results for detection of AF with 77–91% sensitivity (based on time in AF or episodes longer than 6 min), and specificities between 98–99%) indicate a relatively strong algorithm within the reported range in the literature. Yet, as can be seen especially in the relatively wide confidence intervals of the sensitivity and PPV (Tables 2–4), the implication is that not negligible number of arrhythmias may be missed by analysis of pulse.
In short AF episodes (episode less than 60 seconds) the ability of the WP to detect the AF episode is limited. Thus, in such cases where the longest episode is shorter than 60 seconds a manual review of the pulse trace is recommended.
While the necessity and technical plausibility for identifying AF from measuring pulse is clear cut and obvious, for detecting premature beats it is more complex, both theoretically and practically. Previous studies have shown that analysis of PPG waveform may accurately detect premature beats, although PPGs composed of pulses with a prominent dicrotic notch tend to increase the rate of false classifications.37 Studying 40 patients by simultaneous recording of PPG and ECG during 24-hour monitoring indicated high accuracy for detecting AF, and somewhat lower for premature beats or other arrhythmias.38 McManus et al showed that a smartphone app can accurately classify premature contractions with sensitivity of 67–73% and specificity of 97–98%.34 Hence, our results of identifying premature contractions with a sensitivity of 74.3% and a specificity of 99.7% are encouraging, and in agreement with previous similar publications.
The importance of detecting arrhythmias during sleep studies, especially in patients with sleep apnea cannot be underestimated. This has a huge importance and implications for AF, although somewhat less clear for premature contractions. The presence of AF is a prognostic marker for increased both morbidity and mortality,13 and this is further emphasized in patients with sleep disordered breathing (SDB). Patients with OSA have a significantly elevated risk of heart failure, arrhythmias in general, and AF in particular.1,13–22,39 Although the direct correlation between heart failure, SDB, and cardiac arrhythmias is yet to be fully understood, it has been shown that the presence of one may promote the existence of the others.1,13–22,39 SDB may diminish the successful treatment of AF,18–20,23–25 while successful treatment of OSA may reduce the recurrence and the complications associated with AF.21,22,26 Hence, detecting AF in patients with sleep apnea is of great clinical importance. In this light, the results of the current study indicating the ability of flagging patients suspected of having arrhythmias during WP studies, is of great value, and may have substantial clinical implications. Early identification of both conditions may lead to better and more successful treatment, with improved prognosis.13–26,39
In some circumstances, premature beats may be related to scar formation in the heart, to the presence of chronic ischemic heart disease, to active structural and coronary heart disease, lethal arrhythmias, stroke and to both all-cause and cardiac mortality.40–42 Therefore, the current results of flagging for their potential existence, sending the patients who underwent a WP study to undergo an ECG test, may promote the diagnosis of unfavorable premature beats and eventually improve prognosis.
Our study has several limitations. First, the sample size for such a study is only small to moderate. Although the representation of arrhythmias in these 84 participants is very high due to the selection of the patients, 84 participants are still not very large sample size. Second, by nature this is a retrospective study. Although the algorithm tested is novel, and so is the topic, it was tested on patients who were recruited for another study. We deliberately re-analyzed the studies of this population since there was a high prevalence of CHF and arrhythmias in this population. Yet, some more prospective studies would be needed to re-test this algorithm, specifically in general population or the common sleep clinic populations. Finally, this is a cross sectional study and not longitudinal, and we do not have information of follow up on the patients with vs without arrhythmias.
Conclusion
In conclusion, we believe this study indicates that a “flag” for the existence of arrhythmias as AF and premature beats may be raised by this algorithm in WP studies (ie patients undergoing sleep studies using the PAT system), which may be of substantial clinical importance. It should be also kept in mind that some arrhythmias may be missed by pulse recording and this is not a replacement for ECG recordings. Larger scale studies are required to strengthen and establish the results of the current study.
Compliance with Ethical Standards
This study was supported by an unrestricted grant from Itamar-Medical LTD.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
The authors do not intend to share individual deidentified participant data.
Disclosure
Giora Pillar is a consultant to Itamar-Medical, and has current research funding from DayZz, KeepMed and InnoBev and has received speaker fees from TEVA, Pfizer and Sanofi. Richard Berry has current research funding from Philips Respironics and Res Med. Thomas Penzel has received unrestricted grants from &gesund, Itamar, Löwenstein Medical, Philips/Respironics, Resmed and speaker fees from Inspire, Heel Pharma, Philips, and UCB; reports grants from Cidelec, personal fees from Bayer Healthcare, personal fees from Jazz Pharma, personal fees from Cerebra, personal fees from National Sleep Foundation, grants, personal fees from Löwenstein Medical, grants from Novartis, Shareholder from Advanced Sleep Research, Shareholder from The Siestagroup GmbH, Shareholder from Nukute, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Almeneessier AS, Alasousi N, Sharif MM, Pandi-Perumal SR, Hersi AS, BaHammam AS. Prevalence and predictors of arrhythmia in patients with obstructive sleep apnea. Sleep Sci. 2017;10(4):142–146. doi:10.5935/1984-0063.20170025
2. Wang S, Cui H, Ji K, et al. Relationship between obstructive sleep apnea and late gadolinium enhancement and their effect on cardiac arrhythmias in patients with hypertrophic obstructive cardiomyopathy. Nat Sci Sleep. 2021;13:447–456. PMID: 33790677; PMCID: PMC8006971. doi:10.2147/NSS.S270684
3. Krishnaswamy U, Aneja A, Kumar RM, Kumar TP. Utility of portable monitoring in the diagnosis of obstructive sleep apnea. J Postgrad Med. 2015;61(4):223–229. doi:10.4103/0022-3859.166509
4. Safadi A, Etzioni T, Fliss D, Pillar G, Shapira C. The effect of the transition to home monitoring for the diagnosis of OSAS on test availability, waiting time, patients’ satisfaction, and outcome in a large health provider system. Sleep Disord. 2014;2014:418246. PMID: 24876974; PMCID: PMC4020217. doi:10.1155/2014/418246
5. Hedner J, White DP, Malhotra A, et al. Sleep staging based on autonomic signals: a multi-center validation study. J Clin Sleep Med. 2011;7(3):301–306. doi:10.5664/JCSM.1078
6. Pillar G, Bar A, Betito M, et al. An automatic ambulatory device for detection of AASM defined arousals from sleep: the WP100. Sleep Med. 2003;4(3):207–212. doi:10.1016/S1389-9457(02)00254-X
7. Kole A. Home sleep apnea testing in the era of COVID-19: a community perspective. J Clin Sleep Med. 2020;16(9):1633. doi:10.5664/jcsm.8614 PMID: 32501209; PMCID: PMC7970616.
8. Pillar G, Bar A, Shlitner A, Schnall R, Shefy J, Lavie P. Autonomic arousal index: an automated detection based on peripheral arterial tonometry. Sleep. 2002;25(5):543–549. [PMID: 12150321]. doi:10.1093/sleep/25.5.541
9. Bar A, Pillar G, Dvir I, Sheffy J, Schnall RP, Lavie P. Evaluation of a portable device based on peripheral arterial tone for unattended home sleep studies. Chest. 2003;123(3):695–703. doi:10.1378/chest.123.3.695
10. Bresler M, Sheffy K, Pillar G, Preiszler M, Herscovici S. Differentiating between light and deep sleep stages using an ambulatory device based on peripheral arterial tonometry. Physiol Meas. 2008;29(5):571–584. PMID: 18460762. doi:10.1088/0967-3334/29/5/004
11. Yalamanchali S, Farajian V, Hamilton C, Pott TR, Samuelson CG, Friedman M. Diagnosis of obstructive sleep apnea by peripheral arterial tonometry: meta-analysis. JAMA Otolaryngol Head Neck Surg. 2013;139(12):1343–1350. doi:10.1001/jamaoto.2013.5338
12. Tauman R, Berall M, Berry R, et al. Watch-PAT is useful in the diagnosis of sleep apnea in patients with atrial fibrillation. Nat Sci Sleep. 2020;12:1115–1121. PMID: 33299372; PMCID: PMC7721305. doi:10.2147/NSS.S278752
13. Miyasaka Y, Barnes ME, Gersh BJ, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114(2):119–125. doi:10.1161/CIRCULATIONAHA.105.595140
14. Cintra FD, Leite RP, Storti LJ, et al. Sleep apnea and nocturnal cardiac arrhythmia: a populational study. Arq Bras Cardiol. 2014;103(5):368–374. PMID: 25252161; PMCID: PMC4262096. doi:10.5935/abc.20140142
15. Hohl M, Linz B, Böhm M, Linz D. Obstructive sleep apnea and atrial arrhythmogenesis. Curr Cardiol Rev. 2014;10(4):362–368. PMID: 25004989; PMCID: PMC4101201. doi:10.2174/1573403x1004140707125137
16. Gami AS, Pressman G, Caples SM, et al. Association of atrial fibrillation and obstructive sleep apnea. Circulation. 2004;110(4):364–367. doi:10.1161/01.CIR.0000136587.68725.8E
17. Stevenson IH, Teichtahl H, Cunnington D, Ciavarella S, Gordon I, Kalman JM. Prevalence of sleep disordered breathing in paroxysmal and persistent atrial fibrillation patients with normal left ventricular function. Eur Heart J. 2008;29(13):1662–1669. doi:10.1093/eurheartj/ehn214
18. Mazza A, Bendini MG, Cristofori M, et al. Baseline apnoea/hypopnoea index and high-sensitivity C-reactive protein for the risk of recurrence of atrial fibrillation after successful electrical cardioversion: a predictive model based upon the multiple effects of significant variables. Europace. 2009;11(7):902–909. doi:10.1093/europace/eup107
19. Linz D, Hohl M, Ukena C, et al. Obstructive respiratory events and premature atrial contractions after cardioversion. Eur Respir J. 2015;45(5):1332–1340. doi:10.1183/09031936.00175714
20. Linz D, McEvoy RD, Cowie MR, et al. Associations of obstructive sleep apnea with atrial fibrillation and continuous positive airway pressure treatment: a review. JAMA Cardiol. 2018;3(6):532–540. doi:10.1001/jamacardio.2018.0095
21. Qureshi WT, Nasir UB, Alqalyoobi S, et al. Meta-analysis of continuous positive airway pressure as a therapy of atrial fibrillation in obstructive sleep apnea. Am J Cardiol. 2015;116(11):1767–1773. doi:10.1016/j.amjcard.2015.08.046
22. Shukla A, Aizer A, Holmes D, et al. Effect of obstructive sleep apnea treatment on atrial fibrillation recurrence: a meta-analysis. JACC Clin Electrophysiol. 2015;1(1–2):41–51. doi:10.1016/j.jacep.2015.02.014
23. Szymanski FM, Filipiak KJ, Platek AE, et al. Presence and severity of obstructive sleep apnea and remote outcomes of atrial fibrillation ablations - a long-term prospective, cross-sectional cohort study. Sleep Breath. 2015;19(3):849–856. doi:10.1007/s11325-014-1102-x
24. Neilan TG, Farhad H, Dodson JA, et al. Effect of sleep apnea and continuous positive airway pressure on cardiac structure and recurrence of atrial fibrillation. J Am Heart Assoc. 2013;2(6):e000421. doi:10.1161/JAHA.113.000421
25. Monahan K, Brewster J, Wang L, et al. Relation of the severity of obstructive sleep apnea in response to anti-arrhythmic drugs in patients with atrial fibrillation or atrial flutter. Am J Cardiol. 2012;110(3):369–372. doi:10.1016/j.amjcard.2012.03.037
26. Lau DH, Nattel S, Kalman JM, Sanders P. Modifiable risk factors and atrial fibrillation. Circulation. 2017;136(6):583–596. doi:10.1161/CIRCULATIONAHA.116.023163
27. Pillar G, Berall M, Berry R, et al. Detecting central sleep apnea in adult patients using WatchPAT-a multicenter validation study. Sleep Breath. 2020;24(1):387–398. PMID: 31402439; PMCID: PMC7127995. doi:10.1007/s11325-019-01904-5
28. Berry RB, Brooks R, Gamaldo CE, et al. The AASM Manual for the Scoring of Sleep and Associated Events. Rules, Terminology and Technical Specifications. Scoring Manual Version 2.2. Darien, Illinois: American Academy of Sleep Medicine; 2015.
29. Camm AJ, Kirchhof P, Lip GYH; European Heart Rhythm Association, European Association for Cardio-Thoracic Surgery. Guidelines for the management of atrial fibrillation: the task force for the management of atrial fibrillation of the European Society of Cardiology (ESC). Eur Heart J. 2010;31(19):2369–2429. doi:10.1093/eurheartj/ehq278
30. Chen LY, Chung MK, Allen LA. Atrial fibrillation burden: moving beyond atrial fibrillation as a binary entity A scientific statement from the American heart association. Circulation. 2018;137(20):e623–e644. doi:10.1161/CIR.0000000000000568
31. Alian AA, Shelley KH. Photoplethysmography. Best Pract Res Clin Anaesthesiol. 2014;28(4):395–406. PMID: 25480769. doi:10.1016/j.bpa.2014.08.006
32. Krasteva V, Jekova I, Leber R, Schmid R, Abächerli R. Real-time arrhythmia detection with supplementary ECG quality and pulse wave monitoring for the reduction of false alarms in ICUs. Physiol Meas. 2016;37(8):1273–1297. PMID: 27454550. doi:10.1088/0967-3334/37/8/1273
33. Paradkar N, Chowdhury SR. Cardiac arrhythmia detection using photoplethysmography. Annu Int Conf IEEE Eng Med Biol Soc. 2017;2017:113–116. PMID: 29059823. doi:10.1109/EMBC.2017.8036775
34. McManus DD, Chong JW, Soni A, et al. PULSE-SMART: pulse-based arrhythmia discrimination using a novel smartphone application. J Cardiovasc Electrophysiol. 2016;27(1):51–57. doi:10.1111/jce.12842
35. Krivoshei L, Weber S, Burkard T, et al. Smart detection of atrial fibrillation†. Europace. 2017;19(5):753–757. doi:10.1093/europace/euw125
36. Lopez Perales CR, Van Spall HGC, Maeda S, et al. Mobile health applications for the detection of atrial fibrillation: a systematic review. Europace. 2021;23(1):11–28. doi:10.1093/europace/euaa139
37. Sološenko A, Petrėnas A, Marozas V, Sörnmo L. Modeling of the photoplethysmogram during atrial fibrillation. Comput Biol Med PMID: 28061368. 2017;81:130–138. doi:10.1016/j.compbiomed.2016.12.016
38. Eerikainen LM, Bonomi AG, Schipper F, et al. Detecting atrial fibrillation and atrial flutter in daily life using photoplethysmography data. IEEE J Biomed Health Inform. 2020;24(6):1610–1618. PMID: 31689222. doi:10.1109/JBHI.2019.2950574
39. Bandi PS, Panigrahy PK, Hajeebu S, Ngembus NJ, Heindl SE. Pathophysiological mechanisms to review association of atrial fibrillation in heart failure with obstructive sleep apnea. Cureus. 2021;13(7):e16086. doi:10.7759/cureus.16086 PMID: 34345562; PMCID: PMC8325395.
40. Madsen JK, Sørensen JN, Kromann-Andersen B, et al. Ventricular premature beats on Holter monitoring in patients admitted with chest pain, in whom acute myocardial infarction is not confirmed. The prognostic value and relationship to scars or ischemia on thallium-201 scintigraphy. Clin Cardiol. 1987;10(5):305–310. doi:10.1002/clc.4960100503 PMID: 2439244.
41. Ozawa K, Funabashi N, Takaoka H, Ueda M, Kobayashi Y. Risk stratification using a combination of left ventricular fibrosis and number of morphological types of ventricular premature beats in cardiomyopathy subjects without obstructed coronary arteries. Int J Cardiol. 2014;176(1):236–239. PMID: 25037689. doi:10.1016/j.ijcard.2014.06.070
42. Suba S, Fleischmann KE, Schell-Chaple H, et al. Diagnostic and prognostic significance of premature ventricular complexes in community and hospital-based participants: a scoping review. PLoS One. 2021;16(12):e0261712. doi:10.1371/journal.pone.0261712
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.






