Back to Journals » Infection and Drug Resistance » Volume 13
Detection and Control of Biofilm Formation by Staphylococcus aureus from Febrile Neutropenic Patient
Authors El-Nagdy AH , Abdel-Fattah GM, Emarah Z
Received 2 May 2020
Accepted for publication 28 July 2020
Published 7 September 2020 Volume 2020:13 Pages 3091—3101
DOI https://doi.org/10.2147/IDR.S259914
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Suresh Antony
Ahmed Hazem El-Nagdy,1 Gamal M Abdel-Fattah,2 Ziad Emarah3
1Microbiology Department, Faculty of Dentistry, Horus University, Damietta el gadeeda, Egypt; 2Botany Department, Faculty of Science, Mansoura University, Mansoura, Egypt; 3Medical Oncology Unit Internal Medicine Department Faculty of Medicine, Mansoura University, Mansoura, Egypt
Correspondence: Ahmed Hazem El-Nagdy
Microbiology Department, Faculty of Dentistry, Horus University, Damietta el gadeeda, Egypt
Tel +20 1000953030
Email [email protected]
Introduction: Febrile neutropenia (FN) is the evolution of fever in a patient with neutropenia over 38.0°C. Neutropenia is diagnosed when absolute neutrophil count (ANC) < 1500 cells/μL. FN represents a common complication of cancer treatment. Hence, it is featured to be a major cause of morbidity and mortality in cancer patients. Staphylococcus aureus is one of the most important microorganisms isolated from the blood of febrile neutropenic patients. Infections caused by S. aureus range from mild to life-threatening diseases. Biofilm production by S. aureus is one of the most significant virulence factors of the bacterium as it prevents the penetration of antibiotics. Recently, it has been shown that S. aureus carries the ica operon responsible for biofilm production. The aim of the work is to determine a genotypic characterization that includes not only the detection of icaA and icaD genes in S. aureus but also the determination of their relation to clinical and microbiological features. Empiric antibacterial treatment was recommended for cancer patients receiving chemotherapy.
Materials and Methods: The relation between the presence of icaA and icaD and biofilm production was determined in a collection of 66 S. aureus samples from febrile neutropenic patients. Biofilm-forming ability was tested on Congo Red agar plates. Also, the effect of the most sensitive antibiotics on the bacterial cells was determined by an electron microscope.
Results: Of the bacterial samples, 48 were biofilm-productive and 18 were non-biofilm productive. For the biofilm productive bacteria, 37.5% were positive for icaA, 22.9% were positive for icaD and 10.4% were positive for both. Linezolid was the most effective antibiotic and it is highly recommended for the treatment of febrile neutropenia caused by biofilm-productive S. aureus. Severe changes were found on the bacterial cell after being treated with Linezolid. The icaA and icaD genes were present in only 50% of biofilm-productive bacteria.
Conclusion: The ica operon is present in only 50% of biofilm-productive S. aureus and Linezolid is the best antibiotic against these bacteria.
Keywords: febrile neutropenia, Staphylococcus aureus, icaA, icaD, biofilm production
Introduction
FN is defined as the rising of body temperature with other symptoms of infection in a neutropenic patient. It is also defined as an oral fever measurement higher than 38.3°C in a neutropenic patient without any environmental factors, where the fever persists for at least an hour.1 Neutropenia is characterized by abnormally low numbers of neutrophil granulocytes in patient’s blood; less than 1500 cells/mm3 and less than 500 cells/mm3 in severe cases.1 The most common cause can be detected with a chemotherapy receiving complication especially in case of being myelosuppressive.2
The Multinational Association for Supportive Care in Cancer (MASCC) has divided the neutropenic patients into low-risk and high-risk according to the risk index. This is very important to determine the most suitable therapeutic strategies for treatment.3 Treatment of febrile neutropenic patients starts with empirical antibiotics until the neutrophil count becomes normal (more than 500 cells/mm3). The body temperature also becomes normal. Antifungal agent must be added in cases of recurrence or continuous fever.4
Staphylococci are the most common organism associated with febrile neutropenia. S. aureus is considered to be the most dangerous of the numerous types of staphylococci. S. aureus is transmitted directly from infected persons by using contaminated objects or by droplet infections when sneezing or coughing.5
Infections caused by S. aureus range from mild to life-threatening diseases. A skin infection is the most common staphylococcal infection and it often causes abscesses. Bacteremia can also occur if the bacteria enter the bloodstream, and this can affect almost all the body parts.6
Staphylococcal infections occur by two mechanisms including direct invasion to the tissue, e.g. endocarditis and septic arthritis, and through the production of exotoxins. Some of these exotoxins have local effects and others have severe systemic effects as they induce release of cytokine from T cells, causing disease to all parts of the host body.7
The laboratory diagnostics of infections caused by S. aureus depend on the detection of the pathogen while species diagnostics depend on the delimitation of coagulase-negative Staphylococcus spp. either by the phenotypic method or by the genotypic method.8
S. aureus has the ability to form a biofilm of settled microbes. Those microbes are characterized by their ability to attach to a substrate or surfaces and to any available moist solid surface, including host tissue, forming a matrix using extracellular polymer substances. These microbes exhibit a special phenotype depending on the growth phase and gene expression and play a serious role in providing resistance to both host immune response and antibiotics.9
On one level, the most common genes known to play an important role in biofilm production are icaA and icaD. Extracellular matrix excreted by biofilm-producing bacteria consists mainly of polysaccharides, especially intercellular adhesion polysaccharide, i.e. poly-β (1-6)-N-acetyl-glucosamine which is coded by the ica operon.10
On the other level, the biofilm matrix is built passively or actively. Passive matrix rearrangement occurs due to the sorption of substances that strengthen it or as a result of damaging factors,11 while active modification is carried out by biofilm microbes. Micro-dependent matrix reconstruction is realized due to anti-directional processes include increasing production of matrix biopolymers, synthesizing matrix enzymes, enhancing cell reproduction, preventing autolysis, and rejecting of biofilm constituents into the environment. Moreover, these ways are the evolution of the skeleton of the biofilm takes place.12 The matrix not only mechanically links the biofilm into a single structure, but also fills the intercellular spaces, forming a three-dimensional filtering system.13
Identification of the biofilm matrix depends on a number of methods based on its color. These methods are traditionally used and include the utilization of definite stains or sets of fluorescent dyes marketed by manufacturers as biofilm matrix detection agents or by the detection of specific antigens.14
Controlling the formation of biofilm occurs by direct or indirect pathways. Direct pathways include using substances that directly react with biofilm matrix molecules. These substances may be pharmaceutical preparations like Dispersin B or polysaccharides oxidative substances like sodium periodate.15 Indirect pathways involve controlling the reactions of microbial metabolism through suppression of the synthesis of biofilm matrix polymers and the production of enzymes that destabilize it.16
Materials and Methods
The study was carried out at the Microbiology Diagnostics and Infection Control Unit at the Medical Microbiology and Immunology Department, Oncology Center, Mansoura University. The study includes 414 blood samples collected from inpatients with fever (>38°C) receiving chemotherapy attending Mansoura University Hospitals. The patients were 165 males and 249 females. A written approval was taken before sample withdrawal. The study was performed in accordance with the ethical standards laid down in Mansoura Oncology Center.
Collection of Blood Samples and Selection of Target Organism
Blood samples were collected from these patients – 1 mL in medical disposable blood collection EDTA tube under septic conditions – and a Complete Blood Picture (CBC) test was done to determine the ANC for each sample so that we would be able to select febrile neutropenic patients. The CBC test was performed automatically using Sysmex KX 21 which is best known for its high accuracy in determining white blood cells count with differential. A blood culture test was performed using two systems. The first system was BacT/Alert 3D 60 as an automated microbial detection system based on the colorimetric detection of CO2 produced by growing microorganisms. The second one was Vitek 2 compact that has the capacity to improve therapeutic success and patient outcomes through reliable microbial identification.
Confirmatory Tests to Ensure the Isolated Bacteria
Isolated bacteria were subjected to several tests including microscopic examination after Gram-stain, catalase test, coagulase test, Api staph test (A strip utilizes a battery of miniaturized biochemical tests), and mannitol salt agar. Each of these tests has a standard result for S. aureus so that we were able to confirm our samples.
Congo Red Test
Congo Red test was performed to detect biofilm production by S. aureus. Although the Congo Red test is not the most sensitive test to determine biofilm production, it can be reliably used because it is acceptably sensitive and specific.17 Tested bacterial isolates were inoculated then incubated at 35°C for 24 h. Black and brown colonies with a dry crystalline consistency indicated biofilm production while red and dark red colonies are non-biofilm producers.
Antibiotic Sensitivity Testing
Bacterial isolates were cultivated on Blood Agar as an enriched medium to encourage their growth. The standard disc diffusion microbial sensitivity test based on the Kirby–Bauer method on Mueller–Hinton agar was done for all S. aureus isolates to assess the antibiotic resistance as per Clinical and Laboratory Standard Institute guidelines.18 Cells of 3 to 5 colonies were inoculated into 5 mL nutrient broth and incubated 2 to 8 hours until turbidity of the suspension matched 0.5 McFarland turbidity standards. This resulted in a suspension containing approximately 1–2 x108 CFU/mL. Mueller–Hinton agar plates were inoculated by the inoculum using a sterile cotton swab. The antibiotic-impregnated discs were placed on the agar plates within 15 minutes of inoculation using sterilized forceps. The plates were then inverted and incubated for 16–18 hours at 37°C. The diameters of inhibition zones were measured in millimeters using a ruler on the undersurface of the plate. Interpretation was done by referring to the zone diameter interpretive standards for S. aureus and the isolates were reported as sensitive, intermediate, or resistant. The antibiotics used were Tetracycline 30 mg, Ciprofloxacin 5 mg, Vancomycin 30 mg, Erythromycin 15 mg, Ceftriaxone 30 mg, Levofloxacin 5 mg, Ofloxacin 5 mg, Ampicillin 10 mg, Cefoxitin 30 mg, and Linezolid 30 mg.
Specimen Preparation for Scanning Electron Microscope (SEM) and Transmission Electron Microscope (TEM)
Two samples were prepared to be examined with an electron microscope (EM). Each sample was examined two times using TEM and SEM. The first sample was normal bacterial isolates and the second one was treated with linezolid 30 mg. Both samples were examined to determine the recent changes of the bacterial cell with the effect of the antibiotic and to ensure the presence of biofilm so as to determine its thickness. Ultrathin sections were examined at 80 kV using a JEOL 2100 EM in the Faculty of Agriculture, Mansoura University, Egypt.
Specimen Preparation for Electron Microscope
All samples were fixed in 4F1G fixative for electron microscopy studies, samples processed and examined as follows:19
Fixation
- Fix 1 mm tissue blocks in 4% formaldehyde and 1% glutaraldehyde in 0.1 M PB (pH 7.4) for at least 2 hours to overnight.
- Immerse in 8% (0.2 M) sucrose in 0.1 M PB 3×15 min or overnight.
- Post-fix in 1% osmium tetroxide in 0.1 M PB for 1 hour.
Dehydration
The dehydration was in 50% ethanol for 15 min, 70% ethanol for 15 min, 95% ethanol for 15 min, 100% ethanol for 2×15 min, 100% acetone for 1 hour, 1:1 Embed 812: propylene oxide for 1–2 hour, and 2:1 Embed 812: propylene oxide overnight in a desiccator with the top off.
Embedding
Samples were embedded in beam capsules then were baked in a 60°C oven for 48 h.
Sectioning
Firstly, thick sections (0.5–1.0 µm) were cut on a slide, and sections were dried on a slide warmer, and stained with Toluidine blue for 2–5 min. Secondly, sections were observed under a microscope for the precise location to cut ultrathin sections at 60–90 nm thick, and sections were collected onto grids. Finally, sections were examined under a transmission electron microscope (JEOL JEM-2100).
Coating
This step was used for SEM only; samples after dehydration were coated with gold by a Sputter Coating Evaporator instrument. Examination was done by scanning electron microscope (JEOL JSM 6510 lv).
Molecular Characterization
Molecular characterization was performed to determine if icaA and icaD genes are present in S. aureus isolates that was defined to be able to produce biofilm around them. The relation between the presence of these genes and biofilm production was determined. The process consists of three steps: bacterial DNA extraction from a colony on blood agar with a genomic BYF DNA extraction mini kit according to manufacturer instructions. The second step was centered on polymerase chain reaction (PCR) for detection of icaA 5-TCTCTTGCAGGAGCAATCAA which was used as the forward primer (corresponding to nucleotides 4796–4815) and 5-TCAGGCACTAACATCCAGCA which was also used as the reverse primer (corresponding to nucleotides 4964–4983) and detection of icaD ATGGTCAAGCCCAGACAGAG was used as the forward primer (corresponding to nucleotides 5422–5441), and 5-CGTGTTTTCAACATTTAATGCAA was used as the reverse primer (corresponding to nucleotides 5616–5597). PCR was performed using the method described previously20 using a thermal cycler. PCR cycling conditions used were 30 cycles of 1 min of denaturation at 94°C, annealing for 1 min at 58°C followed by 1 min extension at 72°C with final extension for 5 min at 72°C. PCR was performed with Taq polymerase (Helini). The third step was centered on analyzing the reaction mixtures by 1% agarose gel electrophoresis to resolve PCR products. The amplified product sizes were estimated by comparison with the 50 bp DNA ladder. Amplicons for icaA and icaD produced fragments of 189 and 195 bp, respectively.
Results
Bacterial Isolation
This study was carried out on 414 febrile patients. CBC tests allowed us to select neutropenic patients. The distribution of these patients was 180 non-neutropenic and 234 neutropenic. The distribution of the neutropenic patients according to blood culture was 28.2% S. aureus, 14.5% Gram-positive other than S. aureus, and 57.3% were Gram-negative isolates as shown in Table 1.
 |
Table 1 Distribution of 234 Samples Collected from Febrile Neutropenic Patients According to Causative Organism |
Confirmatory Test for Isolated Bacteria
Isolated bacteria were purple in color and cocci in shape by microscopic examination, catalase and coagulase positive (showed colt appearance in the test tube), the Api-Staph test strip after inoculation with bacterial isolates and occurrence of chemical reactions matched standard results for S. aureus, and mannitol salt agar changed to yellow as an indication of fermentation occurrence.
Isolation of Biofilm-Productive S. aureus
Congo Red test results were black and brown colony color for 72.7% of the isolates, indicating biofilm-productive bacteria (positive result) and red or dark red colonies were 24.4% of the isolates, representing non-biofilm-productive bacteria (negative result), as shown in Table 2.
 |
Table 2 Type of Isolates According to Congo Red Test |
Antibiotic Sensitivity Test
Table 3 summarizes the number and percentage of interpretation for each antibiotic. The table shows that Linezolid and Vancomycin are the most suitable antibiotics recommended to be used against S. aureus in febrile neutropenia.
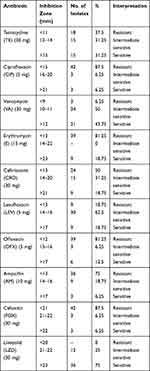 |
Table 3 Antibiotic Susceptibility of the Isolated Bacteria Against Ten Antibiotics (Interpretation by Inhibition Zone) |
Electron Microscope Examination
Untreated S. aureus examination displayed condensed growth with rod-shaped undamaged cells. Also, it showed a smooth and intact surface without significant variation in length that seems to be several microns in thickness as shown in Figure 1. Moreover, S. aureus showed normal cell morphology and undamaged ultra-structure intact inner membrane, slightly waved outer membrane and each bacterial cell seems to be divided into two parts surrounded with cell membrane as shown in Figure 2.
 |
Figure 1 Examination of normal bacterial cells under scanning electron microscope. |
 |
Figure 2 Examination of normal bacterial cells under transmission electron microscope. |
After anti-microbial treatment with antibiotic, treated S. aureus was prepared for examination in the border line between growth and clear zone that showed enlarged malformed and uncharacterized cell shape. Some bacterial cells had burst with deep craters in their cell wall. Also, many lysed cells were observed. The cells condensed in the shape of a cluster and no growth was observed after the border line. Furthermore, non-uniform electron density cytoplasm and malformed enlarged cells with slightly ruptured as shown in Figures 3 and 4.
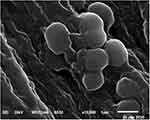 |
Figure 3 Examination of treated bacterial cells under scanning electron microscope. |
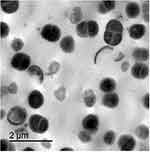 |
Figure 4 Examination of treated bacterial cells under transmission electron microscope. |
Molecular Detection for icaA and icaD in Biofilm-Productive S. aureus
Table 4 shows that 37.5% of the isolates carry the icaA gene, 22.9% of the isolates carry icaD genes, and 10.4% of the isolates carry both genes at the same time. The percentage of the samples carrying at least one gene was 50%. Table 5 shows the mean age of febrile neutropenic patients who carry at least one gene, and those who do not carry any genes, and determines the significance of age in these patients. The mean age for patients who carry genes was 51.58 ± 17.90 and the P -value was 0.019, so it is significant.
 |
Table 4 Distribution of icaA and icaD Genes in Staphylococcal Isolates |
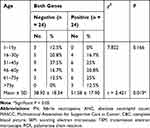 |
Table 5 Significance of Ages in Cases Carrying at Least One Gene |
Discussion
FN is the development of fever in a patient with neutropenia. Fever is an oral temperature measure of ≥38.3°C or a temperature of ≥38.0°C for a period of an hour. Neutropenia is characterized by abnormally low numbers of neutrophil granulocytes in the patient’s blood.21
FN is caused by different microorganisms, including Gram-positive and Gram-negative bacteria. From blood cultures of FN cancer patients, staphylococci were some of the most common isolated organisms. S. aureus is considered to be the most dangerous of the numerous types of staphylococci. It causes skin infections, bone infections, pneumonia, and heart valve infections.22
S. aureus is the most common causative organism of FN in our results, representing 28.2%. This result confirms the results of Boyce23 and Hakim et al24 that S. aureus is the most common isolated organism from blood cultures of FN cancer patients.
The distribution of microorganisms isolated from samples collected from febrile neutropenic patients in our research was 57.3% Gram-negative bacteria and 42.7% were Gram-positive. This result matches the previous results that confirmed the predominance of Gram-negative bacteria as the causative organisms of febrile neutropenia reported by Bodey et al25 and Glauser et al,26 and is in contrast to the result of Durnaa et al27 that Gram-positive bacteria are the most common causative organism.
Congo Red test result showed that 72.7% of S. aureus were positive (biofilm producer) and 24.4% were negative (non-biofilm producer). This result is very similar to the result of Darwish et al28 that 70.4% of staphylococcal isolates were positive to Congo Red test (biofilm producers) and completely in contrast to the result of El-Hendi et al,29 which includes 23.6% biofilm productive staphylococcal isolates only.
These results explained the fact that biofilm formed by S. aureus is connected into a single system using the extracellular matrix. This helps the bacteria to mechanically link the biofilm into a single structure and fill the intercellular spaces, forming a three-dimensional filtering system. This is considered as virulence factors of S. aureus that enable bacteria to resist the effect of antibiotics.30
Antibiotic culture and sensitivity tests were performed to determine the most effective antibiotic recommended for the treatment of FN caused by S. aureus. The results showed that Linezolid and Vancomycin were the most effective antibiotics against isolated bacteria, while Ciprofloxacin and Cefoxitin were the antibiotics that have least effect on isolated bacteria.
As a comparison between the recent results and previous results, antimicrobial susceptibility patterns for S. aureus isolates exhibited 92.8% susceptibility to ciprofloxacin, Levofloxacin and Ofloxacin, 85.7% to tetracycline and a high resistance pattern of 71.4% was recorded in ceftriaxone and 42.8% in Vancomycin31 Meanwhile, in our research, percentages of ciprofloxacin, Levofloxacin, Ofloxacin, and tetracycline sensitivity were 12%, 81.25%, 18.75%, and 62.5%, respectively, and the percentages of Ceftriaxone and Vancomycin resistance were 50% and 6.25%, respectively. So, our results match these results for Levofloxacin and approach results for Tetracycline and Ceftriaxone.
EM has long been used in the discovery and description of microorganisms like bacteria. Use of EM has been known to exist since the late 19th century. One of the main advantages of using EM for diagnosis is that it does not require organism-specific reagents for recognizing the pathogenic agent.
Shalamanou32 reported that there was formation and/or degradation of the cell wall as well as pleomorphism, giant cell formation, and clustering of the damaged bacterial cell wall of three Gram-negative bacteria, i.e. Enterobacter cloacae, Pseudomonas aeruginosa, and Serratia marcescens after treatment with a minimal inhibitory concentration of chlorhexidine gluconate and examination by SEM.
Bhaskar et al33 treated Klebsiella pneumoniae with Cefotaxime (30 µg). He found wrinkled with rough-surfaced bacterial cells when examining with SEM. Previous researchers noticed loose attachment causing loss of permeability of bacterial cell membrane in addition to crinkling and malformation of bacterial cell wall after being examined with TEM.
In this research, non-treated control bacterial isolates were examined to show normal cell morphology with undamaged ultra-structure intact inner membrane and slightly waved outer membrane. Each bacterial cell seems to be divided into two parts surrounded by cell membrane.
The effect of Linezolid (30 mg) was tested by SEM and TEM in the border line between growth and clear zone. Differences between the two samples were reported. Observations showed enlarged malformation in the cell shape so that the shape became uncharacterized.
On one level, some bacterial cells had burst with deep craters in their cell wall. Also, many lysed cells were observed. The cells were condensed in the shape of a cluster.
On the other level, some other bacteria have an apparent hole in the cell surface, others are abounded, varying markedly in size and shape. Many of them were smaller than usual while others were abnormally large. No growth was observed after the border line. These changes in the bacterial cell are similar to those reported by Hartmann et al34 and similar to the result of usage silver nanoparticles reported by Mirzajani et al.35
A PCR detection test was performed to detect the prevalence of icaA and icaD genes for S. aureus isolates isolated from febrile neutropenic patients. The results were 37.5% of these patients carry icaA gene, 22.9% carry icaD gene, and 10.4% of the isolates carried both genes. These results indicate that biofilm production by S. aureus was associated with the presence of icaA or icaD genes with a percentage of 50%. This percentage is less than the percentage found by El-Mahallawy et al,36 who reported that 83.3% of biofilm productive S. aureus carry ica genes.
On the other hand, the results were proportionally equal to the result of Arciola et al21 which determined a relation between the presence of the gene and slime production with a percentage of 60%.
Febrile neutropenic patients carrying ica genes showed a significant indication to the age of those patients. The death rate in older patients receiving chemotherapy was very high due to immune deficiency, cytokine storm, inappropriate diet, mutations, etc. This explains that the numbers in the last two groups were lower than expected. These results match the result of Aapro et al37 in the sense that older age is considered an important patient-related risk factor that should be considered in the overall assessment of FN risk before administering chemotherapy.
Conclusion
S. aureus is a Gram-positive bacterium, facultative anaerobic mostly aerobic, catalase, coagulase and hemolysis positive, mannitol, sucrose. It is the most common organism associated with febrile neutropenia. S. aureus is considered to be the most dangerous of the numerous types of staphylococci. Infections caused by S. aureus range from mild to life-threatening diseases. One of the most dangerous infections caused by S. aureus is bacteremia. Bacteremia occurs when the bacteria enter the bloodstream of the patient and affect almost all the body parts.
Biofilm production is one of the most important virulence factors of S. aureus as it enables them to resist or inhibit the effect of different antibiotics. The icaA and icaD genes are considered to be responsible for the production of biofilm. In our research, we found that they were present in 50% of biofilm-productive S. aureus.
The most suitable antibiotic recommended to be used against S. aureus in febrile neutropenic patients is Linezolid. It has a destructive effect on the biofilm. In addition to its effect, it has the ability to make enlarged malformations in the cell shape and cytometry as it was clear in the electron microscopic examination.
Ethics Approval and Consent to Participate
This study was approved by the official ethics committee of the Oncology center – Mansoura University Institutional Review Board (IRB), reference number; R/17.1.39. Written approval was signed by a parent or a legal guardian of pediatric patients less than 18 years old. The study procedures were done in accordance with the Declaration of Helsinki. Patients above 18 years old have provided written approval.
Acknowledgments
All the due thanks go to the Head of the Department of Microbiology and Immunology, Oncology Center, Mansoura University for facilitating laboratory use procedures. Thanks also to the director of the electronic microscope unit at the Faculty of Agriculture, Mansoura University.
Disclosure
The authors report no conflicts of interest for this work.
References
1. Zimmer AJ, Freifeld AG. Optimal management of neutropenic fever in patients with cancer. J Oncol Pract. 2019;15(1):19–24. doi:10.1200/JOP.18.00269
2. Ko KI, Root CM, Lindsay SA, et al. Starvation promotes concerted modulation of appetitive olfactory behavior via parallel neuromodulatory circuits. Elife. 2015;4:e08298. doi:10.7554/eLife.08298
3. Van der Velden WJ, Herbers AH, Netea MG, Blijlevens NM. Mucosal barrier injury, fever and infection in neutropenic patients with cancer: introducing the paradigm febrile mucositis. Br J Haematol. 2014;167(4):441–452. doi:10.1111/bjh.13113
4. de Souza Viana L, Serufo JC, da Costa Rocha MO, Costa RN, Duarte RC. Performance of a modified MASCC index score for identifying low-risk febrile neutropenic cancer patients. Support Care Cancer. 2008;16(7):841–846. doi:10.1007/s00520-007-0347-3
5. Flowers CR, Seidenfeld J, Bow EJ, et al. Antimicrobial prophylaxis and outpatient management of fever and neutropenia in adults treated for malignancy: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2013;31(6):794–810. doi:10.1200/JCO.2012.45.8661
6. Yadegarynia D, Fatemi A, Mahdizadeh M, Movahhed RK, Alizadeh MA. Current spectrum of bacterial infections in patients with nosocomial fever and neutropenia. Caspian J Intern Med. 2013;4(3):698.
7. Coates R, Moran J, Horsburgh MJ. Staphylococci: colonizers and pathogens of human skin. Future Microbiol. 2014;9(1):75–91. doi:10.2217/fmb.13.145
8. Kanoksil M, Jatapai A, Peacock SJ, Limmathurotsakul D. Epidemiology, microbiology and mortality associated with community-acquired bacteremia in northeast Thailand: a multicenter surveillance study. PLoS One. 2013;8(1). doi:10.1371/annotation/e199ebcc-0bc1-4be1-ad91-ad2a8c0c9382
9. Clarisse T, Michèle S, Olivier T, et al. Detection and quantification of staphylococcal enterotoxin A in foods with specific and sensitive polyclonal antibodies. Food Control. 2013;32(1):255–261. doi:10.1016/j.foodcont.2012.11.021
10. Dunsing V, Irmscher T, Barbirz S, Chiantia S. Purely polysaccharide-based biofilm matrix provides size-selective diffusion barriers for nanoparticles and bacteriophages. Biomacromolecules. 2019;20(10):3842–3854. doi:10.1021/acs.biomac.9b00938
11. Diemond-Hernández B, Solórzano-Santos F, Leaños-Miranda B, Peregrino-Bejarano L, Miranda-Novales G. Production of icaADBC-encoded polysaccharide intercellular adhesin and therapeutic failure in pediatric patients with staphylococcal device-related infections. BMC Infect Dis. 2010;10(1):68. doi:10.1186/1471-2334-10-68
12. O’Neill E, Pozzi C, Houston P, et al. A novel Staphylococcus aureus biofilm phenotype mediated by the fibronectin-binding proteins, FnBPA and FnBPB. J Bacteriol. 2008;190(11):3835–3850. doi:10.1128/JB.00167-08
13. Boles BR, Horswill AR. Agr-mediated dispersal of Staphylococcus aureus biofilms. PLoS Pathog. 2008;4:4. doi:10.1371/journal.ppat.1000052
14. Tribedi P, Sil AK. Cell surface hydrophobicity: a key component in the degradation of polyethylene succinate by P seudomonas sp. AKS 2. J Appl Microbiol. 2014;116(2):295–303. doi:10.1111/jam.12375
15. Quinn L, Barros C, Vitale S, Casey E. Extraction and identification of components of the biofilm matrix in Pseudomonas species biofilms. Access Microbiol. 2019;1(1A):386. doi:10.1099/acmi.ac2019.po0219
16. Rendueles O, Kaplan JB, Ghigo JM. Antibiofilm polysaccharides. Environ Microbiol. 2013;15(2):334–346. doi:10.1111/j.1462-2920.2012.02810.x
17. Hentzer M, Riedel K, Rasmussen TB, et al. Inhibition of quorum sensing in Pseudomonas aeruginosa biofilm bacteria by a halogenated furanone compound. Microbiology. 2002;148(1):87–102. doi:10.1099/00221287-148-1-87
18. Melo PDC, Ferreira LM, Nader Filho A, Zafalon LF, Vicente HIG, Souza VD. Comparison of methods for the detection of biofilm formation by Staphylococcus aureus isolated from bovine subclinical mastitis. Braz J Microbiol. 2013;44(1):119–124. doi:10.1590/S1517-83822013005000031
19. Wayne PA. Clinical and laboratory standards institute. Performance standards for antimicrobial susceptibility testing; 2011.
20. Cheville NF, Stasko J. Techniques in electron microscopy of animal tissue. Vet Pathol. 2014;51(1):28–41. doi:10.1177/0300985813505114
21. Arciola CR, Baldassarri L, Montanaro L. Presence of icaA and icaD Genes and slime production in a collection of Staphylococcal strains from catheter-associated infections. J Clin Microbiol. 2001;39(6):2151–2156. doi:10.1128/JCM.39.6.2151-2156.2001
22. Freifeld AG, Bow EJ, Sepkowitz KA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis. 2011;52(4):e56–e93. doi:10.1093/cid/cir073
23. Boyce JM, Havill NL, Kohan C, Dumigan DG, Ligi CE. Do infection control measures work for methicillin-resistant Staphylococcus aureus? Infect Control Hosp Epidemiol. 2004;25(5):395–401. doi:10.1086/502412
24. Hakim H, Flynn PM, Knapp KM, Srivastava DK, Gaur A. Etiology and clinical course of febrile neutropenia in children with cancer. J Pediatr Hematol Oncol. 2009;31(9):623. doi:10.1097/MPH.0b013e3181b1edc6
25. Bodey GP, Buckley M, Sathe Y, Freireich EJ. Quantitative relationships between circulating leukocytes and infection in patients with acute leukemia. Ann Intern Med. 1966;64(2):328–340. doi:10.7326/0003-4819-64-2-328
26. Glauser M, Boogaerts M, Cordonnier C, Palmblad J, Martino P. Empiric therapy of bacterial infections in severe neutropenia. Clin Microbiol Infect. 1997;3:s77–s86. doi:10.1111/j.1469-0691.1997.tb00648.x
27. Durnaa B, Dzierzanowska D. Infection in neutropenic cancer patients–etiology, microbiological diagnostics, treatment. Wiadomoscilekarskie. 2006;59(7–8):506–511.
28. Darwish SF, Asfour HA. Investigation of biofilm forming ability in Staphylococci causing bovine mastitis using phenotypic and genotypic assays. Sci World J. 2013;2013:1–9. doi:10.1155/2013/378492
29. El-Hendi AA, El-Fotouh MAA, El-Shalakany AH, Shalaby HM. Biofilm formation by blood stream staphylococcal isolates from febrile neutropenic cancer patients. Menoufia Med J. 2016;29(2):349. doi:10.4103/1110-2098.192435
30. Seif-El-Din SS, El-Rehewy MS, Ghazaly MM, Abd-Elhamid MH. Biofilm formation by blood stream Staphylococcal isolates from febrile pediatric cancer patients at South Egypt Cancer Institute. The Journal of American Science. 2011;7(1):674–686
31. Percival SL, Malic S, Cruz H, Williams DW. Introduction to biofilms. In: Percival SL, Knottenbelt DC, Christine A, editors Biofilms and Veterinary Medicine. Berlin, Heidelberg: Springer; 2011:41–68.
32. Shalamanov DS. Chlorhexidine gluconate-induced morphological changes in gram negative microorganisms. Biotechnol Biotechnol Equip. 2005;19(1):121–124. doi:10.1080/13102818.2005.10817165
33. Bhaskar BH, Mulki SS, Joshi S, Adhikary R, Venkatesh BM. Molecular Characterization of Extended Spectrum β-lactamase and Carbapenemase Producing Klebsiella pneumoniae from a Tertiary Care Hospital. Indian J Crit Care Med. 2019;23(2):61–66. doi:10.5005/jp-journals-10071-23118
34. Hartmann M, Berditsch M, Hawecker J, Ardakani MF, Gerthsen D, Ulrich AS. Damage of the bacterial cell envelope by antimicrobial peptides gramicidin S and PGLa as revealed by transmission and scanning electron microscopy. Antimicrob Agents Chemother. 2010;54(8):3132–3142. doi:10.1128/AAC.00124-10
35. Mirzajani F, Ghassempour A, Aliahmadi A, Esmaeili MA. Antibacterial effect of silver nanoparticles on Staphylococcus aureus. Res Microbiol. 2011;162(5):542–549. doi:10.1016/j.resmic.2011.04.009
36. El-Mahallawy HA, Loutfy SA, El-Wakil M, El-Al AKA, Morcos H. Clinical implications of icaA and icaD genes in coagulase negative staphylococci and Staphylococcus aureus bacteremia in febrile neutropenic pediatric cancer patients. Pediatr Blood Cancer. 2009;52(7):824–828. doi:10.1002/pbc.21964
37. Aapro MS, Bohlius J, Cameron DA, et al. 2010 update of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumors. Eur J Cancer. 2011;47(1):8–32. doi:10.1016/j.ejca.2010.10.013
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
