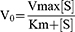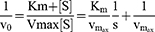Back to Journals » Drug Design, Development and Therapy » Volume 16
Design, Synthesis and Pharmacological Evaluation of 2-(3-BenzoyI-4-Hydroxy-1,1-Dioxido-2H-Benzo[e][1,2]thiazin-2-yI)-N-(2-Bromophenyl) Acetamide as Antidiabetic Agent
Authors Rashid F, Ahmad M , Ashfaq UA , Al-Mutairi AA , Al-Hussain SA
Received 20 July 2022
Accepted for publication 2 November 2022
Published 22 November 2022 Volume 2022:16 Pages 4043—4060
DOI https://doi.org/10.2147/DDDT.S379205
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tuo Deng
Fatima Rashid,1 Matloob Ahmad,2 Usman Ali Ashfaq,1 Aamal A Al-Mutairi,3 Sami A Al-Hussain3
1Department of Bioinformatics and Biotechnology, Government College University, Faisalabad, Pakistan; 2Department of Chemistry, Government College University, Faisalabad, Pakistan; 3Department of Chemistry, Faculty of Science, Imam Mohammad Ibn Saud Islamic University (IMSIU), Riyadh, 11623, Saudi Arabia
Correspondence: Usman Ali Ashfaq, Department of Bioinformatics and Biotechnology, Government College University, Faisalabad, Pakistan, Email [email protected] Sami A Al-Hussain, Department of Chemistry, Faculty of Science, Imam Mohammad Ibn Saud Islamic University (IMSIU), Riyadh, 11623, Saudi Arabia, Email [email protected]
Purpose: The present study is based on screening new and potent synthetic heterocyclic compounds as anti-diabetic drugs using various computational tools, lab experiments, and animal models.
Methods: A potent synthetic compound 2-(3-benzoyl-4-hydroxy-1,1-dioxido-2H-benzo[e][1,2]thiazin-2-yl)-1-(2-bromophenyl) acetamide (FA2) was checked against diabetes and screened via enzyme inhibition assays, enzyme kinetics against alpha-glucosidase and alpha-amylase. Protein–ligand interaction was analyzed via molecular docking and toxicological analysis via ADMET. Experimental animals were used to examine the compound FA2 safety, delivery, and check various biochemical tests related to diabetes like fasting glucose sugar, cholesterol, triglyceride, HbAc1, creatinine, and insulin level. Histography of liver, kidney, and pancreas was also performed.
Results: Results showed that FA2 had binding energy of ‐7.02 Kcal/mol and ‐6.6 kcal/mol against α‐glucosidase (PDB ID: 2ZE0) and α‐amylase (PDB ID: 1B2Y), respectively. Moreover, in vitro enzyme inhibition assays and enzyme kinetics against α‐glucosidase and α‐amylase were performed, and FA2 showed IC50 at 5.17 ± 0.28 μM and 18.82 ± 0.89 μM concentrations against α‐glucosidase and α‐amylase, respectively. Kinetics studies showed that the FA2 compound impeded α‐glucosidase and α-amylase as a non-competitive mode of inhibition with Ki’ values − 0.320 ± 0.001 and 0.141 ± 0.01, respectively. FA2 was further analyzed on alloxan-induced mice for 21 days. Biochemical tests (fasting glucose sugar, cholesterol, triglyceride, HbAc1, creatinine, and insulin levels) and histological examination of liver and kidney showed that the FA2 compound showed better results than acarbose. Histology of pancreas found to show the maintenance of normal pancreatic acini and Langerhans islets in FA2 treated mice compared to acarbose and nontreated diabetic controls.
Conclusion: Investigating anti-diabetic potential of FA2 compound showed that the selected benzothiazine derivative has tremendous importance in reducing dose concentration and side effects.
Keywords: diabetes, benzothiazine, enzyme kinetics, mice model, drug discovery
Graphical Abstract:
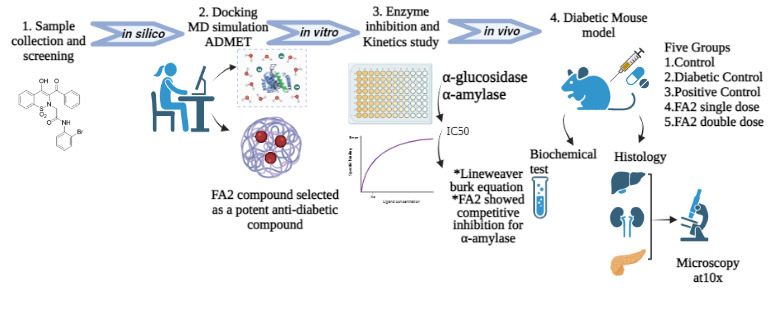
Introduction
Diabetes is a well-known chronic condition characterized by hyperglycemia due to inadequate insulin synthesis. Diabetes affects an estimated 220 million individuals globally. The death toll with diabetes will be more than double between 2005 and 2030, according to ADA.1 As an international diabetes federation (IDF), three common types of diabetes are observed in the human population: T1DM, T2DM, and gestational diabetes. Type 1 and 2 are most common in the human population: type 1 is defined by inadequate insulin level, and type 2 is marked by insulin futility. About 90% of diabetic patients globally have Type 2 diabetes due to an imbalanced diet and physical activities. Thus, the rising prevalence of type 2 DM has become a major clinical issue worldwide.2 T2DM is a metabolic syndrome caused by a deficiency in insulin production and activity.3 T2DM is also related to carbohydrate metabolism abnormalities and is defined by hyperglycemia, a health-threatening concurrent disease worldwide.4 Hyperglycemia also induces oxidative stress (OS) via numerous metabolic ways, such as the overdose of superoxide negative ion (O−2) produced via mitochondrial electron transport chain (ETC).5 OS is also thought to be the key reason for various long-term metabolic diseases, such as heart diseases, diabetes, and the onset of multiple cancers.6 The high glucose level in the blood may disturb redox status, which damages several organs and cause various health issues, such as inducing hyperglycemia and hyperlipidemia. Therefore, maintaining ROS status could be a promising way to prevent diabetes-related difficulties, such as myocardiopathy, renal disorder, retinopathy, skin diseases, alveolar bone loss, and cardiac problems.6–8 The regulation of blood glucose levels and a balanced lifestyle is considered an ideal therapeutic mode for diabetic patients,9 and this therapy is also used to recover diabetic patients with COVID-19.10
The hyperglycemic effect can be reduced with limited intake and digestion of nutritional carbohydrates (by inhibiting the α-glucosidase and α-amylase enzyme activities) in the gastrointestinal track.3,11 The α-glucosidase enzyme is essential for its ordinary physical role. The α-glucosidase contributes to glycolysis by hydrolyzing the α-D-glucopyranosyl bond to generate α-D-glucose (reducing sugar) from sucrose (non-reducing sugar).12 The pancreas secretes amylase to hydrolyze starch into maltose, isomaltose, and oligo-maltose monomers that the colon cannot absorb. Gastrointestinal α-glucosidases, such as maltase, iso-maltase, and sucrase are membrane-bounded proteins found in the small intestine epithelium. Gastrointestinal α-glucosidases are engaged in completing sugar breakdown to produce absorbable sugar monosaccharides.11,13 The anti-postprandial hyperglycemia effect of acarbose, miglitol, and voglibose were verified by suppressing α-glucosidase function and doctors prescribed these medicines for treatment of T2DM. They are frequently claimed to produce diarrhea, flatulence, abdominal pain, and liver issues, the most prevalent reason for noncompliance. Thus, synthesizing new α-glucosidase antagonists with negligible side effects is a critical approach.14 Researchers are now focusing on finding new heterocyclic compounds as promising candidates for glycosidase inhibitors to counteract these adverse effects. The majority of pharmacists are tempted to perform research in heterocyclic systems. Because of the diverse and adaptable biological features of heterocyclic systems, many heterocyclic scaffolds are used as vital components in many therapeutic molecules. Heterocyclic-derived compounds have the potential to be anti-diabetic medicines.15
Heterocyclic chemistry research accounts for over half of all organic chemistry research worldwide. Many bioactive organic molecules with heterocyclic frameworks serve an important role in medicine.16 Researchers have paid great attention to synthesizing small molecular weight compounds to increase their solubility and excretion rate; for instance, benzoimidazol, benzothiophen, and benzothiazol are reported as good α-glucosidase inhibitors.17 Noori et al18 have reported benzoimidazol with acetamide derivatives as an α-glucosidase inhibitor. Benzothiazine acetamide derivatives are well-known anti-diabetic,16 anti-inflammatory,19 anti-leukemic,20,21 anti-microbial,22 monoamine oxidase (MAO) inhibitors,23 endothelin receptor antagonists (ERA),24 antiallergic and antioxidant compounds.20,25 Benzothiazine derivative FA2 was selected because of their great role in the pharmaceutical field; for instance, benzothiazine carboxamides, sudooxicam, piroxicam, and many other potent benzothiazine oxides have clinical importance. The present study is based on screening FA2 as a potent anti-diabetic compound via computational tools and experimental approaches.20,21,26–28 T2DM can be managed by lowering postprandial hyperglycemia (PPHG) by postponing the activity of α-amylase and α-glucosidase, which are managed the carbohydrate ingestion and glucose absorption in the gastrointestinal tract.29 Thus, this study is planned to explore the potential of benzothiazine acetamide derivative against diabetes.
Materials and Methods
Chemistry
The selected compound (Figure 1), 2-(3-benzoyl-4-hydroxy-1,1-dioxido-2H-benzo[e][1,2]thiazin-2-yl)-1-(2-bromophenyl) acetamide (FA2) was synthesized by reported methodology (Scheme 1).30 The 3-benzoyl-4-hydroxy-1,2-benzothiazine 1,1-dioxide (0.5 g, 1.66 mmoles), 2-bromo-N-(2-bromophenyl) acetamide (0.49 g, 1.67 mmoles) and K2CO3 (0.46 g, 2.33 mmoles) were mixed in dimethyl formamide (10 mL). The reaction mixture was vortex for 10 minutes at 28–30 °C, then heated at 100 °C for 120 minutes. The contents of the flask were poured into ice-cold distilled water to obtain the precipitates of the product (0.64g, 75% yield). Finally, FA2 was recrystallized from ethanol for purification.
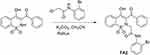 |
Scheme 1 Synthesis of targeted 2-(3-benzoyI-4-hydroxy-1,1-dioxido-2H-benzo[e][1,2jthiazin-2-yI)-N-(2-bromophenyl) acetamide. |
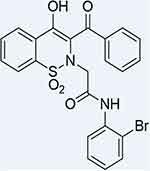 |
Figure 1 Structure of 2-(3-benzoyl-4-hydroxy-1,1-dioxido-2H-benzo[e][1,2]thiazin-2-yl)-1-(2-bromophenyl) acetamide (FA2). |
In silico Study
Target Protein and Benzothiazine Ligands Preparation
The 3D crystalline structures of α-glucosidase (PDB ID: 2ZE0) and α-amylase (PDB ID: 1B2Y) were fetched from the PDB (https://www.rcsb.org/). The 2D construction of FA2 was drawn out via ChemDraw Ultra (Figure 1), minimized its energy by Chimera 1.16, and saved as a .pdb format.
Energy Optimization
The UCSF Chimera tool was utilized to optimize the PDB coordinates of the targeted enzymes and the FA2 molecule for better docking results. These coordinates have the least amount of energy and the most stable configuration.
Molecular Docking Analysis
The FA2 molecule was docked with 2ZE0 and 1B2Y. The ligand and protein structures were saved in .pdbqt format. PyRx-python software was used to calculate the binding scores of the molecules and target enzymes, and discovery studio (DS) visualizer was used to display the protein/ligand interactions. The results were compared with acarbose (PubChem CID: 41774) with the targeted enzymes.
Drug-Likeness Evaluation
Examining FA2 drug-likeness properties is an important step in drug design to check the compound’s properties. Physicochemical parameters such as molecular mass, log P water (miLogP), the number of OH−, and H+ were analyzed via swissADME software (http://www.swissadme.ch/) to evaluate the remedy ability of the best-docked ligand models.31,32 The pharmaceutical drug properties of the synthetic compound FA2 were evaluated according to the Lipinski’s rule of five (RO5).33
ADMET Analysis
Chemical absorption, distribution, metabolism, excretion, and toxicity (ADMET) all show essential shares in the pharmaceutical industry. A potent medicinal compound should be effective without any lethal side effects and have suitable ADMET characteristics at an acceptable dose quantity. Additionally, the pharmacokinetic features of the successful compounds were investigated to forecast their ADMET in the animal body.
The ADMET properties of the FA2 compound were predicted via computational tools, eg, SwissADME (http://www.swissadme.ch/)34 and PreADMET (https://preadmet.bmdrc.kr/).31 Cardiotoxicity was also predicted via predherg software (http://predherg.labmol.com.br).
In vitro Anti-Diabetic Activity
Sample Preparation
FA2 compound was synthesized at the chemistry department, Government College University Faisalabad (GCUF).25 The compound was weighed at 1.903 mg, prepared the stock solution (10 mM) into 500 µL DMSO, and kept at room temperature.
α-Glucosidase Inhibition Assay
The α-glucosidase inhibition assay was conducted to evaluate the conversion of PNPG into p-nitrophenol by α-glucosidase and yellow color was measured at 405 nm via spectrophotometer.35 For this assay, acarbose was used as a reference compound and the reactions were conducted on 96 well plates. A mixture of 10 µL of 10 mM test sample (FA2) and 40 µL of 0.5 U α-glucosidase from S. cerevisiae (sigma Aldrich, Merck) was prepared in 100 µL of 1x PBS (pH 7.4) and reaction plate was incubated at 37 °C for 5–10 minutes. After incubation, 40 µL of 5 mM ρ-nitrophenyl-α-D-glucopyranoside (PNPG, sigma Aldrich, Merck) was added as a substrate to start the reaction and kept at 37 °C for 20–30 minutes. The reaction was ended by using 40 µL of 1 M sodium carbonate. The quantity of p-nitrophenol released from PNPG was observed at 405 nm via spectrophotometer (Bio-Tek with GEN5 software). The percentage inhibition was calculated with the following formula:
In the control reaction, DMSO was used instead of the sample. The 50% inhibition concentration (IC50) of FA2 and acarbose were calculated with the microdilution method and analyzed results through Microsoft Excel software 2013.
α-Amylase Inhibition Assay
The spectrophotometric method was used to perform α-amylase inhibition assay in 96 well plates35 with slight modifications. A mixture of 40 μL of 2 U α-amylase from porcine pancreas (Sigma Aldrich, Merck) and 1 μL of 10 mM FA2 solution (or 10 µL of PBS as a control) was incubated at 37 °C for 5–10 min. After incubation, 40 μL of 1% (w/v) soluble potato starch (Sigma Aldrich, Merck) solution (prepared in 20 mM PBS, pH 6.9) was added as a substrate. The mixture was kept at 37 °C for 20–30 minutes and stopped by adding 40 μL of 3.5-dinitrosalicylic acid (DNS). The reaction mixture was kept at 95 °C for 5 min followed by rapid cooling. Acarbose was used as a reference, and reading was taken at 540 nm wavelength.
The following formula was used to calculate the percentage inhibition:
In the control reaction, DMSO was used instead of the test sample. The 50% inhibition concentration (IC50) of FA2 and acarbose were calculated with the microdilution method and analyzed using Microsoft Excel software 2013.
DPPH Activity
The free radical donating activity of the FA2 compound was measured using DPPH (1,1-diphenyl-2-picrylhydrazyl) radical scavenging method as described by Okawa, Kinjo36 with some modifications and carried out in 96 well plates. A reaction mixture contained 10 µL of 500 µM FA2 (prepared in DMSO) and 190 µL of 300 µM DPPH (Sigma, Merck) was placed in an incubator at 37 °C for 30 minutes. After that plate was read at 517 nm wavelength via a microtiter plate reader (Molecular Devices, USA). Ascorbic acid was used as a positive control.
The following formula was used to calculate the percentage inhibition:
In control, DMSO was added instead of the test sample. The experiment was conducted in the dark and in triplets (n=3). The 50% inhibition concentration (IC50) of FA2 and ascorbic acid were calculated with the microdilution method and analyzed through Microsoft Excel software 2013.
MTT Assay
Cell Culture
The human hepatocarcinoma cell line (HepG2) cells (ATCC, Manassas, USA) were cultured in Dulbecco’s modified Eagle medium (DMEM) containing 10% fetal bovine serum (FBS) and 1% streptomycin−penicillin (100 units/mL and 1% 100 μg/mL) at 37 °C with 5% CO2 incubator. Cell lines were treated with compounds in DMSO (< 1%).
Determination of Cell Viability
The cytotoxicity was checked via MTT assay. HepG2 cells were grown in 96-well plates with 100µM and 200 µM dilutions of FA1. The plate was incubated at 37 °C for overnight. The next day, the treated plate with 80–90% confluency was used for treatment with 20 µL of MTT (5 mg/mL) and kept at 37 °C for 240 min. After incubation, formazan crystals were dissolved in 100 μL of control (DMSO). The absorbance was quantified in a microplate reader at the test wavelength of 570 nm, and a reference wavelength 630 nm. The percentage cell viability was calculated using the following formula:
Inhibitory Kinetics Study
Lineweaver Burk equation was used to describe the inhibition mechanism of the compound FA2 against α-glucosidase and α-amylase. For this, inhibitor [V] and substrate [S] were used as variables, and enzyme concentration was constant. In case of α-glucosidase, the concentration of substrate (PNPG) was used as 1, 3, 5, 7 and 9 mM and the concentration of potato starch as substrate was used as 0.25%, 0.5%, 1%, 1.5% and 2% for α-amylase. The concentration of inhibitor (FA2) was used as 0.1 mM, 0.25 mM, 0.5 mM, 0.75 mM, 1 mM. The inhibition mechanism and Km value were calculated through Lineweaver-Burk and Michaelis Menten equations.
Vo =initial reaction rate,
Km =Michaelis Menten constant,
Km/Vmax =slope,
1/Vmax =-intercept, and
S = substrate
The secondary graph was plotted to evaluate the inhibition constant and intersecting line between Y-intercept and [V] was represented the Ki and Ki’ values. GraphPad Prism software was used to study the enzyme kinetics.
In vivo Anti-Diabetic Activity
Animals
This study used albino laboratory-bred mice (strain balb/c) as an anti-diabetic model. For this purpose, mice were categorized into five groups, and each group contained five mice (25–40 g). Group 1 was designated for untreated mice (positive control). Group 2 comprised alloxan-induced mice without treatment (diabetic control). Group 3 contained diabetic mice treated with acarbose (reference control). Group 4 represented diabetic mice treated with FA2 compound (single dose). Group 5 had diabetic mice treated with FA2 (double dose). The mice were subjected to overnight hunger conditions and the initial fasting blood glucose was measured from the mice’s tail vein. Alloxan was dissolved in normal saline, and intraperitoneal dose of alloxan (120 mg/kg) was induced in overnight starved mice. The mice were fed in hygienic polypropylene cells and kept in a temperature-controlled animal house, faculty of pharmacy, GCUF (approved from ethical review committee) that was well-ventilated and had a steady 12 hours light/dark schedule. A higher level of blood glucose (hyperglycemia) was detected after 72 hours and only those mice with > 250 mg/dl blood glucose concentration were selected for the further ant-diabetic study. During the experiment, acarbose and test compound (FA2) were dissolved in sterile normal saline and injected into mice. Fasting blood glucose level in fasting mice was measured from the mice’s tail vein on the first, seventh, fourteenth-, and twenty-first days during treatment.
Assessment of Blood Glucose Level
The blood sample was taken from the mouse tail vein, and blood sugar level was measured via glucose oxidase-peroxidase reactive strips and a glucometer (on call-ACON LABS INC.).
Biochemical and Histological Examinations
On day 21, mice were anesthetized with chloroform and whole blood from the anesthetic mouse’s submandibular was collected in BD vacutainer plus blood collection tubes (K2EDTA) and serum separator tubes (SST). HbAc1, insulin and cholesterol were measured via VITRO SCIENT kit, and triglycerides and creatinine were checked through Clinical Chemistry Reagents SRL (CCR) kit. These parameters were studied in controls and treated diabetic mice under Anim diagnostic and collection center, Pakistan.
About 2 mice from each group were dissected via a dorsal midline incision, removed organs (pancreas, kidneys, and liver), and fixed in 10% formalin. After fixation, selected organs were embedded with paraffin and sliced to obtain 6µm thick paraffin sections. These slices were tainted with Harris, hematoxylin, and eosin (H&E). The stained tissue samples were examined under the compound microscope at 100X resolution.
Statistical Analysis
Comparison of the control group with the treated group was assessed through two-way ANOVA through GraphPad prism v 8.0.2. The P-value < 0.05 indicates that the data is more significant than the control group. All data were analyzed in triplicate (n=3).
Results
Chemistry
1H NMR (600 MHz, DMSO-d6), δ: 4.04 (s, 2H), 7.06 (t, 1H, J = 7.80 Hz, Ar-H), 7.18–7.24 (m, 3H, Ar-H), 7.55 (d, 1H, J=7.9 Hz, Ar-H), 7.63–7.72 (m, 3H, Ar-H), 7.87–7.89 (m, 2H, Ar-H), 8.03–8.14 (m, 3H, Ar-H), 9.49 (br-s, 1H, NH). 13C NMR (150 MHz, DMSO-d6) δ: 53.9 (1C), 113.0 (1C), 116.2 (1C), 118.0 (1C), 122.0 (1C), 125.0 (1C), 127.1 (1C), 128.4 (1C), 128.70 (2C), 128.74 (2C), 130.1 (1C), 132.9 (1C), 133.0 (1C), 134.0 (1C), 135.2 (1C), 138.4 (1C), 139.1 (1C), 147.8 (1C), 165.6 (1C), 168.0 (1C), 189.2 (1C). LC-MS (ESI) m/z calcd. for C23H17BrN2O5S [M+H]+, 513.00; found, 513.08 (see Figures S1–S3).
Molecular Docking
In silico analysis revealed that the FA2 has the best RMSD value and binding energy against 2ZE0 and 1B2Y proteins. The relative molecular docking analysis has been performed for acarbose as a control and FA2 as a potent antidiabetic inhibitor. The top three complexes were selected based on the highest binding residue interactions, drug properties, and highest binding energy. The docking results against FA2 showed significant binding energies as −7.2 kcal mol−1 and −6.6 kcal mol−1 for 2ZE0 and 1B2Y, respectively, compared with acarbose (Table 1). Almost all the docked compounds are bound on a similar binding pocket as the control (Figure 2 and Table 1).
 |
Table 1 Docking Results of α-Glucosidase and α-Amylase (2ZE0 and 1B2Y) |
 |
Figure 2 3D docking model of acarbose and FA2 against α-glucosidase (2ZE0: A and B) and α-amylase (1B2Y; C and D); 2D structure representing interaction of molecules with enzyme pocket residues. |
Druglikeness and ADMET Analysis
The drug-like properties of the FA2 compound were evaluated in silico based on RO5 and FA2 compound showed < 500 molecular weight, < 10 nON, < 5 nOHNH, and ~5 miLogP, as mentioned in Table 2. The FA2 compound fulfilled the RO5 and showed excellent drug-likeness characteristics compared to acarbose. Acarbose showed some variations of the RO5 (Table 2).
 |
Table 2 Druglikeness Properties of FA2 Compared with Acarbose |
The pharmacokinetics parameters were evaluated via PreADMET and SwissADME online tools to predict the ADME and toxicity (T) of the FA2 compound (Table 3). The data mentioned in Table 3 were collected from both online tools. These parameters were evaluated based on the solubility, human gastrointestinal absorption, blood–brain barrier interactions, P-glycoprotein, and cytochrome P450 (CYP450) inhibitors. The FA2 compound did not cross the blood–brain barrier and showed high gastrointestinal absorption and inhibitor of enzyme CYP450 as compared to the reference compound (acarbose). Toxicity also showed that FA2 compound fulfilled the safety criteria like Ames toxicity, lethal dose limit (LD50) (Table 3), and cardiotoxicity, as shown in Figure 3. Pred-hERG results showed that FA2 compound was non-cardiotoxic with 60% confidence.
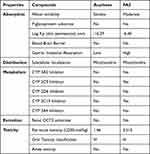 |
Table 3 In silico Pharmacokinetic Analysis: Absorption, Distribution, Metabolism, Excretion and Toxicity of FA2 and Acarbose |
α-Glucosidase and α-Amylase Inhibition Activities
The synthetic compound FA2 was screened and checked for its inhibitory effect on α-glucosidase, α-amylase and DPPH activity at 500 µM concentration (Table 4). The FA2 compound was a potent inhibitor of α-glucosidase and α-amylase with 69.4% and 70.8% inhibition.
 |
Table 4 In vitro Anti-Diabetic and Anti-Oxidant Effect of FA2. The Data is Represented in Triplicate (n=3) and Values are Mean ± SD. All Data Was Repeated in Triplicate (n=3) |
It was found that FA2 inhibited α-glucosidase and α-amylase activities in a dose–response manner. Both enzymatic activities were significantly (p < 0.05) decreased with the increment of FA2 concentration. In the case of α-glucosidase activity, compound FA2 and acarbose (reference control) showed 50% inhibition at 18.82 ± 0.89 µM and 58.8 ± 2.69 µM concentrations, respectively. Similar results were also observed with the α-amylase inhibition assay. Synthetic compound FA2 was inhibited at 5.17 ± 0.28 µM concentration with compared to acarbose. The results showed that synthetic compound FA2 has significant inhibition against α-glucosidase and α-amylase compared with acarbose.
The antioxidant capacity of the FA2 compound was evaluated via DPPH assay. It was found that FA2 showed non-significant results compared with ascorbic acid (control).
Cytotoxicity Analysis
The cytotoxicity of tested compound FA2 was checked on HepG2 cell line. FA2 compound showed no toxicity when compared with control (DMSO) as shown in Figure 3. The percentage cell viability of different concentrations (100 µM, 200 µM, 400 µM and 500 µM) of FA2 was checked and maximum cell viability (98–80%) was observed at different concentrations of FA2 compound.
Kinetics Study
The type of inhibition against α-glucosidase and α-amylase by synthetic compound FA2 was examined via the Lineweaver-Burk equation. According to the Lineweaver-Burk equation, the graph was plotted between the reciprocal of inhibitor velocities [1/V] on the y-axis and the reciprocal of substrate concentrations [1/S]. The results showed that FA2 compound inhibited α-glucosidase and α-amylase in a non-competitive manner (Table 5). Secondary graphs were plotted to calculate the inhibitory constant (Ki) and dissociated constant (Ki’). FA2 showed Ki 0.168 ± 0.02 and Ki’ −0.32 ± 0.001 against α-glucosidase and Ki 0.287 ± 0.07 and Ki’ 0.141 ± 0.01 against α-amylase. Ki value is the indication of affinity for the enzyme inhibitor site, as shown in Figure 4. This type of inhibition shows that FA2 binds at the allosteric site instead of the active site. Thus, FA2 compound has the potential to bind α-glucosidase and α-amylase and inhibit the enzyme activity, whether the substrate is present or not. Ki and Ki’ values of acarbose against α-glucosidase and α-amylase were published earlier and used in this study.37
 |
Figure 4 Lineweaver–Burk plots of inhibition kinetics of α-glucosidase (A) and α-amylase (B) by FA2 compound. Each point represents the average of triplicate (n=3). |
 |
Table 5 Type of Inhibition Against α-Glucosidase and α-Amylase |
In vivo Study
Effect of FA2 Compound Within a Living Body
Effect on Fasting Blood Sugar Level (mg/dL)
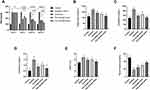
In the current study, the effect of synthesized compound (FA2) on fasting blood sugar (FBS) levels in alloxan-induced diabetic mice was monitored from Day 1 to day 21 as shown in Figure 5A. Graphic presentation of FBS has demonstrated a comparable decline rate in FBS with the positive control (acarbose) and diabetic control. The maximum decline rate was observed on the 21st day of treatment. As shown in Figure 5, the FA2 compound showed a significant (p < 0.05) decline rate in FBS level at high concentration (double dose 2x) when compared with controls.
Biochemical Analysis
To demonstrate the effect of synthesized compounds within a living body, FA2 was injected in alloxan-induced mice from day 1 to day 21 under fasting conditions. Cholesterol, serum creatinine, triglyceride, HbA1C level, and Serum insulin were checked in control, diabetic control, reference control, and treated diabetic animals as shown in Figure 5.
Effect of FA2 Compound on the Liver (Cholesterol, Triglycerides)
Cholesterol and Triglycerides are Two Forms of Lipids Found in the Blood
Triglycerides are fats that store unused calories and supply energy to your body. Cholesterol is required for the formation of cells and the production of some hormones. Normal cholesterol and triglyceride ranges are 200 mg/dL and 40–160 mg/dL, respectively. In this study, cholesterol and triglyceride content in blood were significantly (p < 0.05) low in treated animals compared to diabetic control. Among treated groups, FA2 with a high dose showed a significant (p < 0.05) decline rate in cholesterol and triglyceride compared with the reference compound acarbose as in Figure 5B and C).
Effect of FA2 Compound on Kidneys
Creatinine is a byproduct of creatine, a chemical component that helps muscles obtain the required energy. Creatinine is removed from the blood by the renal as a waste product and excreted in the urine. A creatinine test determines the level of this substance in the blood or urine. Normal creatinine range is from 0.8 to 1.4 mg/dL. In the current study, treated animals with acarbose and FA2 compound showed a significant (p < 0.05) decrease in creatinine content compared with diabetic control. Furthermore, when treated groups (FA2) were compared with the acarbose, a significant (p<0.05) decrease in creatinine level was also observed in high doses of FA2 (Figure 5D).
HbA1c is a measure of erythrocyte hemoglobin in glycation, as erythrocytes have a life span of around 120 days, and it represents total blood glucose levels for 2–3 months. It is also used to monitor diabetic treatment. It has been established that HbA1c is an important complement to regular self-blood glucose testing in achieving the best possible glycemic management. Renal failure can have a variety of effects on HbA1c formation and measurement. When employing some HbA1c assays, nitrogenous waste isocyanate can form carboxylated Hb, which can be indistinguishable from HbA1c. The normal range of HbA1c is up to 6.5%. HbA1c levels in treated animals with FA2 showed a significant (p < 0.05) low percentage compared with diabetic control. Treated group FA2 with low dose showed non-significant results, but FA2 with double dose showed significantly (p < 0.05) lower HbA1c level when compared with acarbose, as shown in Figure 5E.
Effect of FA2 Compound on the Pancreas
Insulin is a peptide hormone produced by pancreatic β-cells, and it is the body’s principal anabolic hormone. It modulates carbohydrate, fat, and protein metabolism by boosting glucose absorption from the bloodstream into hepatic, fat, and skeletal muscle cells. The normal range of serum insulin is up to 150 рmol/L. In the present study, treated animals showed significant (p < 0.05) high serum insulin concentration compared with diabetic control. Synthetic compounds FA2 with high dose showed significant (p < 0.05) high level of insulin in serum (38 ± 2 pmol/L) compared with acarbose (25 ± 3.1 pmol/L) as shown in Figure 5F.
Histological Analysis
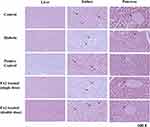
Histology of Liver
Normal hepatic cells with well-preserved cytoplasm, nucleus, nucleolus, and central vein were observed on micrographs of the liver. All treated groups showed normal lobular structure, prominent central vein and properly congested compared with diabetic control as shown in Figure 6.
Histology of Kidneys
Histology of kidney (Figure 6) in normal animals showed a normal number of renal capsules, bowman’s space, glomerulus capillary, and the number of mesangium intraglomerular cells compared with diabetic control. A mild increase in glomerulus Bowman’s space, decrease in mesangium intraglomerular cells were observed in positive control compared with FA2 compounds. No other significant changes were seen in control and treated animals. However, the treatment groups of FA2 (double dose) showed better glomerulus structure improvements than other groups.
Histology of Pancreas
Pancreas histology revealed normal pancreatic acinus and islets of Langerhans in normal control. In the diabetic group, extensive damage of pancreatic islets of Langerhans and a reduced number of zymogen granules were observed as shown in Figure 6. A significant improvement in pancreatic islets of Langerhans was observed in FA2 treated mice compared with acarbose treated mice.
Post-Experiment Procedures
After treatment, all research animals were anesthetized and disposed of under euthanized procedure approved by NIH and the institutional ethical committee (Ref. No. GCUF/ERC/22 dated 03-12-2021).
Discussion
Diabetes is a common chronic metabolic disorder characterized by a rise in plasma sugar due to a lack and/or inadequate use of insulin in the body. Treatment for type 2 diabetes requires lowering glycogen synthesis, improving insulin signaling, increasing insulin production from β-pancreatic cells, or inhibiting glucose digesting enzymes such as α-glucosidase and α-amylase.15,38 Thiazines are a class of heterocyclic chemical compounds with many biological activities (anti-diabetic, antifungal, anti-cancer, anti-inflammatory, anti-parasitic, anticonvulsant, and antiviral activities) and play an important role in the treatment of many autoimmune diseases.16,25 Inhibiting digestive enzymes such as α-amylase and α-glucosidase has been suggested to be useful for glycemic control. The present study was designed to screen the potent benzothiazine derivative FA2 via computational tools in vitro screening and in vivo testing. Taj et al,39 also used molecular docking and ADMET software to screen out the best potent pyrazolo benzothiazine derivative as α-glucosidase inhibitor for treating diabetes. The synthetic compounds exhibited significant potential to control the hyperglycemic conditions in diabetic patients as previously reported synthetic derivatives for instance, benzothiazole40 and pyrrolidines.41 Taj et al,37 reported pyrazolo benzothiazine-based derivative as an α-glucosidase inhibitor at a concentration (IC50) of 5.8 µM. Rahim et al,42 also found the potent synthetic benzimidazole derivatives as α-glucosidase inhibitors. α-glucosidase and α-amylase are considered important enzymes in glycemic control and diabetic complications. Selvarasu et al,43 also found the same results and reported a new synthetic 1-aryl-N-tosyl-1H-tetrazole-5-carboxamide derivative. FA2 showed higher antioxidant activity compared with ascorbic acid. Júnior et al,44 also reported gallic acid as a good antioxidant and diabetic phenolic compound. Several findings have shown the correlation between the production of free radicals and the pathogenesis of diabetes in the treatment of diabetes.45–47 Benzothiazine derivatives are considered to have anti-diabetic properties and are used as a therapeutic agents. The enzyme kinetics results also showed that the FA2 compound had non-competitive inhibition with α-glucosidase/α-amylase and tended to inhibit the function of the α-glucosidase.11 Peng et al,9 also found mixed-type inhibition to investigate kaempferol for the treatment of diabetes. Rouzbehan et al,48 also showed the same kinetics results by using medicinal plants and indicated that the non-competitive mode of inhibition might not be affected the activity of the inhibitor in the presence of high substrate dose. Because there is no competition between substrate and inhibitor, a little concentration of inhibitor is enough to maintain the glucose level.49,50 The role of FA2 was investigated in regulating critical biological pathways involved in type 2 diabetes and the potential of selective benzothiazine agonists as a new therapeutic approach to treat this illness in this work. FA2 compound significantly lowered the blood cholesterol and triglyceride level compared to acarbose in the alloxan-induced diabetic mice model (balb/c strain). Documenting high levels of cholesterol and triglyceride in the blood is considered a direct driver of the thickening of arteries in T2DM patients.51 During diabetes, the liver absorbs spare fatty acids, esterification with glycerol phosphate, and deposits them as triglycerides. This causes a decline in insulin-mediated metabolic activity and may cause diabetes and other metabolic disorders.52 Studies on acarbose, sulfonylureas, and metformin showed that these anti-diabetic agents significantly affect lipid profile at high doses53 and do not rescue the pancreatic β-cells for continual distortion.54 The FA2 compound showed better results in maintaining the cholesterol, triglyceride, creatinine, and HbAc1 level than acarbose at the same concentration in the present study. Histopathology of the liver also showed that the FA2 compound has no side effects on hepatic cells. Histology of kidneys also showed that renal capsules and glomerulus structure in FA2 case had the better condition than acarbose treated mice. It has been reported that renal nephropathy is strongly associated with DM.55 Diabetes-related nephropathy may cause renovascular lesions, renal medulla hypoxia, and cell death due to insufficient oxygen supply to the glomerulus and renal medulla. The decreased oxygen supply alters the stability of the hypoxia-inducible factor (HIF), which may induce kidney fibrosis in diabetic patients.56,57 Insulin regulates blood glucose by directing the liver, muscle tissue, and adipocytes to absorb glucose from the blood. Hyperglycemia condition is linked with insulin resistance and ketoacidosis, causing abnormal absorption of glucose and the development of advanced glycation end products. This process eventually results in pancreatic beta-cell production dysfunction and cell death.54 Present study investigated that benzothiazine derivative showed significant effects in maintaining the level of insulin compared with acarbose. The histography also showed that pancreatic β-cells are more prominent in FA2 compound treated mice compared to acarbose treated mice.
Conclusions
The targeted FA2 compound was synthesized with maximum yield. The anti-diabetic activity of FA2 compound was screened against α-glucosidase and α-amylase, and FA2 showed the best antidiabetic activity. Moreover, it also fulfilled drug safety criteria via cytotoxicity, ADMET, and cardiotoxicity. This compound has the potential to bind with the allostatic site of enzymes to inhibit the enzyme’s activity. In vivo diabetic mouse model study showed that FA2 could be safe for body tissues and histographics showed no significant side effects to pancreatic beta cells. Current research suggests that the FA2 compound is potentially safe for managing the hyperglycemic impact on diabetic patients and other diabetic-related complications. However, more studies and experiments are required to narrow down the effects of FA2 at the gene level, explore related metabolic pathways, and develop advanced biochemical testing to fulfill the pharmacological checklist.
Institutional Review Board Statement
The animal study protocol was approved by the Ethical Review Committee of Government College Faisalabad (Ref. No. GCUF/ERC/22 dated 03-12-2021) for studies involving animals. NIH guidelines were followed for the welfare of the laboratory animals.
Author Contributions
All authors made a significant contribution to the work reported and took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(Suppl 1):S62–9. doi:10.2337/dc10-S062
2. Deshpande AD, Harris-Hayes M. Schootman M %J P therapy. Epidemiol Diabetes Diabetes-Related Compl. 2008;88(11):1254–1264.
3. Wang Y, Yang Z, Wei X. Sugar compositions, α-glucosidase inhibitory and amylase inhibitory activities of polysaccharides from leaves and flowers of Camellia sinensis obtained by different extraction methods. Int J Biol Macromol. 2010;47(4):534–539. doi:10.1016/j.ijbiomac.2010.07.007
4. Lim EL, Hollingsworth KG, Aribisala BS, Chen MJ, Mathers JC, Taylor R. Reversal of type 2 diabetes: normalisation of beta cell function in association with decreased pancreas and liver triacylglycerol. Diabetologia. 2011;54(10):2506–2514. doi:10.1007/s00125-011-2204-7
5. Benhalima K. Contents/contributors/preface. In: Cardiovascular Diabetology: Clinical, Metabolic and Inflammatory Facets. Basel: KARGER; 2008:I–XIV. doi:10.1159/000115119
6. Sharifi-Rad M, Anil Kumar NV, Zucca P, et al. Lifestyle, oxidative stress, and antioxidants: back and forth in the pathophysiology of chronic diseases. Front Physiol. 2020;11. doi:10.3389/fphys.2020.00694
7. Cai L, Kang YJ. Oxidative stress and diabetic cardiomyopathy: a brief review. Cardiovasc Toxicol. 2001;1(3):181–194. doi:10.1385/CT:1:3:
8. Wang Y, Cai L. Diabetes/obesity-related inflammation, cardiac cell death and cardiomyopathy. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2006;31(6):814–818.
9. Peng X, Zhang G, Liao Y, Gong D. Inhibitory kinetics and mechanism of kaempferol on α-glucosidase. Food Chem. 2016;190:207–215. doi:10.1016/j.foodchem.2015.05.088
10. Xiao F, Zhou Y, Zhang M, et al. Hyperglycemia and blood glucose deterioration are risk factors for severe COVID‐19 with diabetes: a two‐center cohort study. J Med Virol. 2022;94(5):1967–1975. doi:10.1002/jmv.27556
11. Phan MAT, Wang J, Tang J, Lee YZ, Ng K. Evaluation of α-glucosidase inhibition potential of some flavonoids from Epimedium brevicornum. LWT Food Sci Technol. 2013;53(2):492–498. doi:10.1016/j.lwt.2013.04.002
12. Lordan S, Smyth TJ, Soler-Vila A, Stanton C, Ross RP. The α-amylase and α-glucosidase inhibitory effects of Irish seaweed extracts. Food Chem. 2013;141(3):2170–2176. doi:10.1016/j.foodchem.2013.04.123
13. Bischoff H. The mechanism of alpha-glucosidase inhibition in the management of diabetes. Clin Invest Med. 1995;18(4):303–311.
14. Feng J, Yang X-W, Wang R-F. Bio-assay guided isolation and identification of α-glucosidase inhibitors from the leaves of Aquilaria sinensis. Phytochemistry. 2011;72(2–3):242–247. doi:10.1016/j.phytochem.2010.11.025
15. Dhameja M, Gupta P. Synthetic heterocyclic candidates as promising α-glucosidase inhibitors: an overview. Eur J Med Chem. 2019;176:343–377. doi:10.1016/j.ejmech.2019.04.025
16. Badshah S, Naeem A. Bioactive thiazine and benzothiazine derivatives: green synthesis methods and their medicinal importance. Molecules. 2016;21(8):1054. doi:10.3390/molecules21081054
17. Huneif MA, Alshehri DB, Alshaibari KS, et al. Design, synthesis and bioevaluation of new vanillin hybrid as multitarget inhibitor of α-glucosidase, α-amylase, PTP-1B and DPP4 for the treatment of type-II diabetes. Biomed Pharmacother. 2022;150:113038. doi:10.1016/j.biopha.2022.113038
18. Noori M, Davoodi A, Iraji A, et al. Design, synthesis, and in silico studies of quinoline-based-benzo[d]imidazole bearing different acetamide derivatives as potent α-glucosidase inhibitors. Sci Rep. 2022;12(1):14019. doi:10.1038/s41598-022-18455-7
19. Ikeda T, Kakegawa H, Miyataka H, Matsumoto H, Satoh T. Anti-allergic and anti-inflammatory actions of 2′-(tetrazole-5-yl)-4-hydroxy-2-methyl-2H-1,2-benzothiazine-3-carboxanilide 1,1-dioxide. Bioorg Med Chem Lett. 1992;2(7):709–714. doi:10.1016/S0960-894X(00)80397-2
20. Lombardino JG, Wiseman EH, Chiaini J. Potent antiinflammatory N-heterocyclic 3-carboxamides of 4-hydroxy-2-methyl-2H-1,2-benzothiazine 1,1-dioxide. J Med Chem. 1973;16(5):493–496. doi:10.1021/jm00263a017
21. Lombardino JG, Wiseman EH, McLamore WM. Synthesis and antiinflammatory activity of some 3-carboxamides of 2-alkyl-4-hydroxy-2H-1,2-benzothiazine 1,1-dioxide. J Med Chem. 1971;14(12):1171–1175. doi:10.1021/jm00294a008
22. Jantová S, Greif G, Špirková K, Stankovský Š, Oravcová M. Antibacterial effects of trisubstituted quinazoline derivatives. Folia Microbiol. 2000;45(2):133–137. doi:10.1007/BF02817411
23. Saddique FA, Zaib S, Jalil S, et al. Synthesis, monoamine oxidase inhibition activity and molecular docking studies of novel 4-hydroxy-N′-[benzylidene or 1-phenylethylidene]-2-H/methyl/benzyl-1,2-benzothiazine-3-carbohydrazide 1,1-dioxides. Eur J Med Chem. 2018;143:1373–1386. doi:10.1016/j.ejmech.2017.10.036
24. Berryman KA, Edmunds JJ, Bunker AM, et al. Endothelin receptor antagonists: synthesis and structure–activity relationships of substituted benzothiazine-1,1-dioxides. Bioorg Med Chem. 1998;6(9):1447–1456. doi:10.1016/S0968-0896(98)00080-7
25. Saddique FA, Aslam S, Ahmad M, et al. Synthesis and α-glucosidase inhibition activity of 2-[3-(Benzoyl/4-bromobenzoyl)-4-hydroxy-1,1-dioxido-2H-benzo[e][1,2]thiazin-2-yl]-N-arylacetamides: an in silico and biochemical approach. Molecules. 2021;26(10):3043. doi:10.3390/molecules26103043
26. Inagaki M, Tsuri T, Jyoyama H, et al. Novel antiarthritic agents with 1,2-Isothiazolidine-1,1-dioxide (γ-Sultam) skeleton: cytokine suppressive dual inhibitors of cyclooxygenase-2 and 5-lipoxygenase. J Med Chem. 2000;43(10):2040–2048. doi:10.1021/jm9906015
27. Lebegue N, Gallet S, Flouquet N, et al. Novel Benzopyridothiadiazepines as Potential Active Antitumor Agents. J Med Chem. 2005;48(23):7363–7373. doi:10.1021/jm0503897
28. Shobana S, Sreerama YN, Malleshi NG. Composition and enzyme inhibitory properties of finger millet (Eleusine coracana L.) seed coat phenolics: mode of inhibition of α-glucosidase and pancreatic amylase. Food Chem. 2009;115(4):1268–1273. doi:10.1016/j.foodchem.2009.01.042
29. Gulati V, Harding IH, Palombo EA. Enzyme inhibitory and antioxidant activities of traditional medicinal plants: potential application in the management of hyperglycemia. BMC Complement Altern Med. 2012;12(1):77. doi:10.1186/1472-6882-12-77
30. Saddique FA, Ahmad M, Ashfaq UA, Muddassar M, Sultan S, Zaki MEA. Identification of cyclic sulfonamides with an N-arylacetamide group as α-glucosidase and α-amylase inhibitors: biological evaluation and molecular modeling. Pharmaceuticals. 2022;15(1):106. doi:10.3390/ph15010106
31. Taidi L, Maurady A, Britel MR. Molecular docking study and molecular dynamic simulation of human cyclooxygenase-2 (COX-2) with selected eutypoids. J Biomol Struct Dyn. 2022;40(3):1189–1204. doi:10.1080/07391102.2020.1823884
32. Taj S, Ahmad M, Alshammari A, Alghamdi A, Ali Ashfaq U. Exploring the therapeutic potential of benzothiazine-pyrazole hybrid molecules against alpha-glucosidase: pharmacological and molecular modelling based approach. Saudi J Biol Sci. 2022;29(3):1416–1421. doi:10.1016/j.sjbs.2021.11.033
33. Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 1997;23(1–3):3–25. doi:10.1016/S0169-409X(96)00423-1
34. Coimbra JRM, Baptista SJ, Dinis TCP, et al. Combining virtual screening protocol and in vitro evaluation towards the discovery of BACE1 inhibitors. Biomolecules. 2020;10(4):535.
35. Daub CD, Mabate B, Malgas S, Pletschke BI. Fucoidan from Ecklonia maxima is a powerful inhibitor of the diabetes-related enzyme, α-glucosidase. Int J Biol Macromol. 2020;151:412–420. doi:10.1016/j.ijbiomac.2020.02.161
36. Okawa M, Kinjo J, Nohara T, Ono M. DPPH (1,1-Diphenyl-2-Picrylhydrazyl) radical scavenging activity of flavonoids obtained from some medicinal plants. Biol Pharm Bull. 2001;24(10):1202–1205. doi:10.1248/bpb.24.1202
37. Taj S, Ahmad M, Ashfaq UA. Exploring of novel 4-hydroxy-2H-benzo[e][1,2]thiazine-3-carbohydrazide 1,1-dioxide derivative as a dual inhibitor of α-glucosidase and α-amylase: molecular docking, biochemical, enzyme kinetic and in-vivo mouse model study. Int J Biol Macromol. 2022;207:507–521. doi:10.1016/j.ijbiomac.2022.03.023
38. He Z, Zhou Z-W, Yang Y, et al. Overview of clinically approved oral antidiabetic agents for the treatment of type 2 diabetes mellitus. Clin Exp Pharmacol Physiol. 2015;42(2):125–138. doi:10.1111/1440-1681.12332
39. Taj S, Ashfaq UA, Aslam S, Ahmad M, Bhatti SH. Alpha-glucosidase activity of novel pyrazolobenzothiazine 5,5-dioxide derivatives for the treatment of diabetes mellitus. In vitro combined with molecular docking approach. Biologia. 2019;74(11):1523–1530. doi:10.2478/s11756-019-00294-z
40. Shah S, Arshia Javaid K, Javaid K, et al. Synthesis, and in vitro and in silico α-glucosidase inhibitory studies of 5-Chloro-2-Aryl Benzo[d]thiazoles. Bioorg Chem. 2018;78:269–279. doi:10.1016/j.bioorg.2018.02.013
41. Kasturi S, Surarapu S, Uppalanchi S, et al. Synthesis and α-glucosidase inhibition activity of dihydroxy pyrrolidines. Bioorg Med Chem Lett. 2017;27(12):2818–2823. doi:10.1016/j.bmcl.2017.04.078
42. Rahim F, Zaman K, Taha M, et al. Synthesis, in vitro alpha-glucosidase inhibitory potential of benzimidazole bearing bis-Schiff bases and their molecular docking study. Bioorg Chem. 2020;94:103394. doi:10.1016/j.bioorg.2019.103394
43. Selvarasu S, Srinivasan P, Mannathusamy G, Maria Susai B. Synthesis, characterization, in silico molecular modeling, anti-diabetic and antimicrobial screening of novel 1-aryl-N-tosyl-1H-tetrazole-5-carboxamide derivatives. Chem Data Collect. 2021;32:100648. doi:10.1016/j.cdc.2021.100648
44. de Lima Júnior JP, Franco RR, Saraiva AL, Moraes IB, Espindola FS. Anacardium humile St. Hil as a novel source of antioxidant, antiglycation and α-amylase inhibitors molecules with potential for management of oxidative stress and diabetes. J Ethnopharmacol. 2021;268:113667. doi:10.1016/j.jep.2020.113667
45. Rolo AP, Palmeira CM. Diabetes and mitochondrial function: role of hyperglycemia and oxidative stress. Toxicol Appl Pharmacol. 2006;212(2):167–178. doi:10.1016/j.taap.2006.01.003
46. Bashan N, Kovsan J, Kachko I, Ovadia H, Rudich A. Positive and negative regulation of insulin signaling by reactive oxygen and nitrogen species. Physiol Rev. 2009;89(1):27–71. doi:10.1152/physrev.00014.2008
47. Kangralkar VA, Patil SD, Bandivadekar RM. Oxidative stress and diabetes: a review. Int J Pharm Appl. 2010;1(1):38–45.
48. Rouzbehan S, Moein S, Homaei A, Moein MR. Kinetics of α-glucosidase inhibition by different fractions of three species of Labiatae extracts: a new diabetes treatment model. Pharm Biol. 2017;55(1):1483–1488. doi:10.1080/13880209.2017.1306569
49. Zhang H, Wang G, Beta T, Dong J. Inhibitory properties of aqueous ethanol extracts of propolis on alpha-glucosidase. Evid Based Compl Altern Med. 2015;2015:1–7. doi:10.1155/2015/587383
50. Ghadyale V, Takalikar S, Haldavnekar V, Arvindekar A. Effective control of postprandial glucose level through inhibition of intestinal alpha glucosidase by Cymbopogon martinii (Roxb.). Evid Based Compl Altern Med. 2012;2012:1–6. doi:10.1155/2012/372909
51. Hermans MP, Valensi P. Elevated triglycerides and low high-density lipoprotein cholesterol level as marker of very high risk in type 2 diabetes. Curr Opin Endocrinol Diabetes Obes. 2018;25(2):118–129. doi:10.1097/MED.0000000000000398
52. Moller DE. New drug targets for type 2 diabetes and the metabolic syndrome. Nature. 2001;414(6865):821–827. doi:10.1038/414821a
53. Buse JB, Tan MH, Prince MJ, Erickson PP. The effects of oral anti-hyperglycaemic medications on serum lipid profiles in patients with type 2 diabetes. Diabetes, Obes Metab. 2004;6(2):133–156. doi:10.1111/j.1462-8902.2004.00325.x
54. Verma S, Hussain ME. Obesity and diabetes: an update. Diabetes Metab Syndr Clin Res Rev. 2017;11(1):73–79. doi:10.1016/j.dsx.2016.06.017
55. Dabla PK. Renal function in diabetic nephropathy. World J Diabetes. 2010;1(2):48. doi:10.4239/wjd.v1.i2.48
56. Bernhardt WM, Schmitt R, Rosenberger C, et al. Expression of hypoxia-inducible transcription factors in developing human and rat kidneys. Kidney Int. 2006;69(1):114–122. doi:10.1038/sj.ki.5000062
57. Li K-X, Ji M-J, Sun H-J. An updated pharmacological insight of resveratrol in the treatment of diabetic nephropathy. Gene. 2021;780:145532. doi:10.1016/j.gene.2021.145532
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.