Back to Journals » Vascular Health and Risk Management » Volume 18
Descending Aortic Distensibility and Cardiovascular Outcomes: A Cardiac Magnetic Resonance Imaging Study
Authors Sood MR , Abdelmoneim SS , Dontineni N, Ivanov A, Lee E, Rubin M, Vittoria M, Meykler M, Ramachandran V , Sacchi T, Brener S, Klem I, Heitner JF
Received 17 February 2022
Accepted for publication 27 June 2022
Published 30 August 2022 Volume 2022:18 Pages 653—665
DOI https://doi.org/10.2147/VHRM.S359632
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Pietro Scicchitano
Michael R Sood,1,2 Sahar S Abdelmoneim,1 Nripen Dontineni,1 Alexander Ivanov,1 Ernest Lee,1 Michael Rubin,1 Michael Vittoria,1 Marcella Meykler,1 Vidhya Ramachandran,1 Terrence Sacchi,1 Sorin Brener,1 Igor Klem,3 John F Heitner1,4
1Division of Cardiology, New York-Presbyterian Hospital, Brooklyn, NY, USA; 2Division of Cardiology, Mount Sinai South Nassau, Oceanside, NY, USA; 3Duke University, Raleigh Durham, NC, USA; 4Division of Cardiology, New York University-Langone Health, Brooklyn, NY, USA
Correspondence: Michael R Sood, Division of Cardiology, Mount Sinai South Nassau, Oceanside, NY, USA, Email [email protected]
Background: Aortic distensibility (AD) is an important determinant of cardiovascular (CV) morbidity and mortality. There is scant data on the association between AD measured within the descending thoracic aorta and CV outcomes.
Objective: We evaluated the association of AD at the descending thoracic aorta (AD desc) with the primary outcome of all-cause mortality, myocardial infarction (MI), stroke or coronary revascularization in patients referred for a cardiovascular magnetic resonance (CMR) study.
Methods: 928 consecutive patients [(mean age 60 ± 17; 33% with prior cardiovascular disease (CVD))] were evaluated. AD desc was measured at the cross-section of the descending thoracic aorta in the 4-chamber view (via steady-state free precession [SSFP] cine sequences) and was grouped into quintiles (with the 1st quintile corresponding to the least AD, i.e., the stiffest aorta). Cox proportional-hazards regression analysis were performed for the primary outcome.
Results: A total of 315 patients (34%) experienced the primary outcome during a median (25% IQR, 75% IQR) follow-up of 5.0 (0.56, 9.3) years. A decreased AD was significantly associated with hypertension, diabetes, renal disease, and dyslipidemia (p < 0.0001). A primary outcome occurred in 43% of patients with AD desc ≤ median compared to 25% with AD desc > median, p < 0.0001, and in 44% of patients with AD desc in the 1st quintile compared to 31% with AD desc in the other quintiles (p = 0.0004). Event free survival was incrementally reduced amongst quintiles (p < 0.0001). However, AD desc ≤ median was not an independent predictor of the primary endpoint after multivariable adjustment in the overall population [adjusted HR 1.09 (95% CI:0.82– 1.45), p = 0.518] or in the subgroup analysis of patients with or without prior CVD.
Conclusion: In this real-world cohort of 928 patients referred for CMR, AD desc is not an independent predictor of CV outcomes.
Keywords: cardiovascular magnetic resonance, aortic distensibility, descending aorta, AD, CMR
Introduction
Aortic stiffness (AS) has been shown to be a determinant of atherosclerotic disease and an independent predictor of cardiovascular (CV) mortality and morbidity.1–4 It has been demonstrated in various patient populations with risk factors such as hypertension, renal disease or in those with already established CV disease.1,5–8 Its prognostic utility, however, as a biomarker for screening sub-clinical cardiovascular disease (CVD) or modifying its treatments is not well established. AS is considered to be an endpoint to various mechanisms of endothelial or vascular injury that are involved in the formation of atherosclerosis, such as hypertension, dyslipidemia, diabetes mellitus, smoking, collagen and elastin turnover, as well as bone and mineral resorption.9 Currently, the European Society of Hypertension recommends measuring AS as a sub clinical marker of CVD and to guide blood pressure treatments.10
AS can be measured by various imaging modalities. Direct measurement is done by measuring aortic distensibility (AD), which is inversely related to AS and is a well-established parameter of local AS in population studies.11 It is calculated as the fractional change in volume or cross-sectional area for a given change in pressure. Indirect measurements are done by aortic pulse wave velocity (PWV), which has been the widely accepted non-invasive gold standard for the measurement of AS due to its cost effectiveness and precision.12
Although there is an abundance of data that correlates AS or AD to various risk factors, disease processes and mortality; hard CV outcome data in large real-world populations are limited along with the reproducibility of reference values. Furthermore, methods evaluating AD have relied on (ascending) aorta measurements or carotid-femoral PWV using tonometry.13 Thus, PWV, depending on where it can be practically applied to measure aortic properties, may reflect only local aortic or regional vessel properties and robust outcome data on the entire vascular system has remained incompletely explored.14–16
Cardiovascular magnetic resonance (CMR) is an imaging modality with high spatial and temporal resolution that can study various areas of the CV system in any dimensional plane and thus, can readily evaluate any aortic location. It has been utilized to evaluate the association between AD and CV risk factors in several published studies and has been shown as a predictor of CV morbidity.17–19 In the Dallas Heart study, CMR-derived ascending AD and aortic arch PWV was shown to have modest correlation with CV events in patients without CVD.19 However, like with PWV and CMR, a paucity of data exists on the value of the descending thoracic AD (AD desc) in predicting CV events.
We hypothesized that the CMR–derived direct measurement of AD desc might be associated with the primary outcome of all cause-mortality, myocardial infarction (MI), stroke, or coronary revascularization.
Methods
This is a retrospective cohort study using our CMR database containing 1315 consecutive patients referred for CMR from January 2006 through December 2011 at our institution. We included all referrals to our CMR center during this time frame, hence, participants with baseline CVD (defined as prior stroke, MI, or coronary revascularization) were also studied. Indications for CMR primarily included the assessment of non-ischemic cardiomyopathies (35%), and myocardial viability or ischemia (22%). Other less common indications were for the assessment of valvular disease (8%), arrhythmias (7%) or congenital heart disease (6%). About one third of the scans overlapped in referral categories and all scans assessed myocardial function.
All participants of this study signed informed consent for research purposes and met all standards and guidelines of the Declaration of Helsinki and Care guidelines. All patient data has been kept anonymous. The study was reviewed and approved by the Division of Cardiology, and Clinical Research at New York Presbyterian Brooklyn Methodist Hospital, New York. Due to the non-interventional nature of the study, an ethics committee review was waived.
Cardiac Magnetic Resonance Imaging Protocol
CMR imaging was performed with a 1.5 Tesla [Siemens Avanto Siemens HealthCare] whole body magnetic resonance scanner as previously described.20 Cine images utilizing the steady-state free precession sequences (SSFP) were acquired for cardiac imaging. The following imaging parameters were used: field of view of 270–340 mm with 25–50% over-sampling, 80–100% field of view in the phase direction, slice thickness of 4–6 mm, matrix size 1.4 mm × 1.3 mm and receiver bandwidth 930Hz/Pix, and temporal resolution was 20–40 msec. Four chamber views were acquired through standard 4-chamber acquisition with alignment in orthogonal planes of the short axis and two chamber views of the left ventricle (LV). SSFP cine CMR with ECG gating was performed to evaluate AD. Blood pressure measurements were obtained during scan time or closest to the time of CMR scan. Pulse pressure (PP) was calculated by the difference in systolic blood pressure (SBP) and diastolic blood pressure (DBP).
Evaluation of Cardiovascular (CV) Risk Factors
Before CMR, patients completed standardized questionnaires to obtain clinical demographics, CV traditional risk factors (smoking, high cholesterol, diabetes, and hypertension), prior history of coronary artery disease (CAD), prior history of stroke and medications. We defined a prior history of CAD as any MI or coronary revascularization (percutaneous coronary intervention [PCI] or coronary artery bypass surgery [CABG]). This included patients who underwent an elective PCI or CABG who did not present with the clinical syndrome of a MI but still had evident CAD. Non-obstructive or non-revascularized CAD was not included. We defined chronic kidney disease as patients who had abnormal renal function by creatinine level at the time of scan or who had a prior history of kidney disease. Claudication was defined as symptoms of exercise induced lower extremity pain. Prior CVD was defined as patients with prior CAD, symptomatic carotid disease with or without revascularization, stroke, or those with symptoms of claudication or lower extremity revascularization. These data were compiled in the CMR database and any missing information was also gathered from pertinent medical records and from their referring physicians.
Aortic and Cardiac Magnetic Resonance Analysis
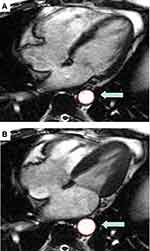
CMR measurements were performed as previously reported and in accordance with the recommendation of the task force of the Society for Cardiovascular Magnetic Resonance.20 Two research personnel conducted the CMR measurements. They were trained and monitored by 2 physicians for measurement consistencies and were blinded to patient outcomes. An average of three measurements for each parameter was obtained. A third research staff member compiled follow-up data. Precession software (Heart Imaging Technologies, LLC, and Durham, NC) was used for offline analysis. For LV mass calculations, endocardial and epicardial borders were traced manually in SSFP sequences at both end-systole and end-diastole (excluding papillary muscle). LV function was also calculated per task force of the Society of Cardiovascular Magnetic Resonance recommendation.20 We defined abnormal left ventricular ejection fraction (LVEF) as <45%. The cross-sectional area of the descending thoracic aorta was manually traced in a 4-chamber SSFP cine image as depicted in Figure 1. Measurements were done at the thoracic level consistent to the cardiac structure in the 4-chamber view in all patients. Descending aorta measurements included the following: maximal (A desc max) and minimal (A desc min) cross sectional lumen areas (cm2) of descending aorta; absolute changes in descending aortic areas: Δ A desc = (A desc max – A desc min). Descending AD (AD desc) was calculated as the relative change in cross-sectional area normalized for peripheral pulse pressure, PP (mmHg) so: AD desc= Δ A desc/(A desc min x PP) in 10−3 mmHg −1.21
Outcomes and Follow-Up
The primary outcome was defined as the composite of all-cause mortality, MI (defined as hospitalization for chest pain with the diagnosis of MI per prior definition),22 stroke, or coronary revascularization including CABG or PCI. Patients were followed up for the primary outcome during a median (25% IQR, 75% IQR) study follow up of 5.0 (0.56, 9.3) years. Telephone interviewers contacted patients to inquire about any CV event that occurred after the index CMR scan. Hospital chart review or NY state mortality records, if necessary, were available to confirm outcomes.
Statistical Analysis
Normal reference values of AD across various imaging modalities are limited. Ingna Voges et al provided normal reference values in percentile curves of the cross sectional thoracic aorta via CMR in 71 healthy children and young adults.23 Due to the lack of reference values, particularly of the descending aorta distensibility, continuous variables, mean and standard deviation were used if normally distributed, while median and range were used for skewed data. Categorical variables were reported as frequencies. Binomial confidence intervals were utilized to provide 95% confidence interval around the estimates. Baseline characteristics were compared between those with primary outcomes versus those without using a Student’s t-test for continuous variables and a chi-square test for categorical variables. AD desc distribution was descriptively displayed as continuous variable, ≤ median cutoff and as quintiles distribution (with the 1st quintile being the lowest distensibility or stiffest aorta). Absolute percentages of patients with the primary outcome versus those without and across the different AD desc quintiles were assessed by the Cochran-Armitage test.
Kaplan–Meier curves log-rank statistic was used to test differences between survival curves in patients with AD desc ≤median cutoff, or AD desc ≤ 1st quintile cutoff. Prediction of the primary outcome was assessed by using univariable (Log rank test) and Cox proportional hazards and multivariate regression analysis. The major following risk factors included in the model were: age, gender, obesity (BMI>30 kg/m2), dyslipidemia, hypertension, smoking, diabetes, and history for CVD, prior renal disease and an abnormal LVEF; as all of these are known to have a strong association with the primary outcome. Subgroup analysis was performed to evaluate the effect of prior history of CVD on the association between AD desc and the primary outcome. All statistical analyses were performed with JMP software version 10 (SAS Inc., Cary, North Carolina). All probability values were 2-sided. Statistical significance was set a prior at a two-tailed p <0.05.
Results
We evaluated 1315 patients who underwent CMR between 2006–2011 at our institution. Of those, 148 patients were excluded due to either lack of follow-up data, significant artifacts impeding measurements, or those with a history of prior aortic surgery or interventions. Blood pressure data for calculating AD desc for the remaining 1167 patients was available in 928 patients, who were included in the final analysis.
Clinical Characteristics
Baseline clinical characteristics of study population are shown in Table 1. The mean age was 60 years [age strata was distributed as follows: strata 1 (<46 years) included 195 (21%) patients; strata 2 (≥46 to <60 years) included 244 (26%) patients; strata 3 (≥60 to <72 years) included 221 (24%) patients and strata 4 (≥ 72 years) included 268 (29%) patients]. One third of the population had prior CVD, a quarter were diabetic, and 53% were over age 60.
 |
Table 1 Demographic and Clinical Characteristics of Study Population (n = 928) Categorized by Primary Outcome |
CMR Aortic Distensibility Data
Hemodynamic parameters and CMR aortic measurements values are presented in Table 2. AD desc (10−3 mmHg −1) distribution in the entire population was as follows (mean ± SD 3.50 ± 2.84; median 2.77 (25–75% IQR 1.38–4.84)). AD desc distribution in quintiles is shown in Table 3. The 1st quintile had the lowest AD desc value (i.e., least AD or stiffest aorta) while the 5th quintile had the highest AD desc value (highest AD).
 |
Table 2 Cardiac Magnetic Resonance Parameters of the Overall Study Population Categorized by Primary Outcome (n = 928) |
 |
Table 3 Distribution of Aortic Distensibility in Descending Aorta (AD Desc) Values in the Overall Population by Quintiles (n = 928) |
CMR Aortic Distensibility and Risk Factors
Average AD desc (10−3 mmHg −1) across different age strata (p <0.0001) is shown in Figure 2. An age-related significant decrease in AD desc was noted (r = − 0.50, p <0.0001). Additionally, AD desc correlated inversely with BMI (r = -0.11, p = 0.001). Men had less AD desc (10−3 mmHg −1) than females (3.26 ± 2.55 vs. 3.78 ± 3.13 mm, p = 0.006, respectively). There were no differences in AD desc based on race. Additionally, there was no significant changes between AD desc (10−3 mmHg −1) in current smokers vs. former or nonsmokers (3.56 ± 2.89 vs. 3.49 ± 2.84, p = 0.86, respectively).
 |
Figure 2 Mean AD across different age strata (p <0.0001). Data presented as mean and standard deviation (error bars). |
Patients with a history of hypertension had a less distensible aorta compared to those with a normal blood pressure (2.77 ± 2.36 vs. 4.72 ± 3.15, p <0.0001, respectively). Similar data was noted for AD desc in patients with diabetes, kidney disease and dyslipidemia [diabetics vs. nondiabetics: 2.53 ± 1.95 vs. 3.85 ± 3.03, p <0.0001]; chronic kidney disease vs. normal kidney function: 2.61 ± 2.20 vs. 3.62 ± 2.89, p <0.0001, respectively and dyslipidemia vs. no dyslipidemia: 2.89 ± 2.40 vs. 4.05 ± 3.08, p <0.0001, respectively.
Study Outcomes
The median (25%IQR, 75%IQR) study follow-up duration was 5.0 (0.49, 9.3) years. Of the 928 patients, 315 (33.9%) had one of the primary outcomes: 205 (22%) died, 60 (6.5%) patients had MI, 25 (3%) patients had stroke, 68 (7%) patients had PCI, and 21 (2%) patients had CABG (i.e, 694 events in 315 patients). Patients who experienced the primary outcome were older, more often male, hypertensive, diabetics, and had a prior history of CVD or other comorbidities. The baseline clinical characteristics of patients with and without a primary outcome are presented in Table 1.
CMR Aortic Distensibility and Outcomes
Patients who had a primary outcome were more likely to have lower LVEF and right ventricular ejection fraction (RVEF) and a higher LV scar percentage on CMR. We found that AD desc and the absolute changes in descending aortic cross-sectional areas (Δ A desc) were significantly lower in patients experiencing the primary outcome (Table 2), p <0.0001. Additionally, the primary outcome occurred in 43% of patients with AD desc ≤ median compared to 25% with AD desc > median, p <0.0001, and in 44% of patients with AD desc in the 1st quintile compared to 31% with AD desc in the other quintiles (p = 0.0004). In Kaplan–Meier analysis (Figure 3), patients with AD desc ≤ median had worse overall event-free survival versus those with AD desc > median (log rank X2 = 20.5, p <0.0001). Similar results are shown when AD desc quintiles were also utilized, as shown in Figure 3.
Table 4 illustrates univariate and multivariable Cox proportional hazards models in evaluating the association between clinical and CMR variables and the primary outcome in the overall population. Univariate analysis showed that AD desc ≤ median was associated with primary outcome [unadjusted HR of 1.82 (95% CI: 1.41–2.35), p <0.0001]. However, it was not an independent predictor of the primary endpoint in the multivariate model [adjusted HR 1.09 (95% CI: 0.82–1.45), p = 0.52].
 |
Table 4 Distribution of Aortic Distensibility in Descending Aorta (AD Desc) in the Overall Population by Quintiles Categorized by Primary Outcome (n = 928) |
Other significant predictors for the primary outcome in the multivariate model were age, prior CVD and abnormal LVEF as shown in Table 5.
 |
Table 5 Univariate and Multivariate Cox Proportional Hazards Regression Models for Clinical and Cardiac CMR Imaging Variables Associated with Primary Outcome in the Overall Population (n = 928) |
Subgroup Analysis
There was a statistically significant difference between mean AD desc in those with prior CAD vs. no CAD (2.88 ± 2.49 v.s 3.76 ± 2.94, p <0.0001, respectively) and prior CVD compared to no CVD (2.87 ± 2.47 vs. 3.83 ± 2.96, p <0.0001, respectively). AD desc was not an independent predictor of the primary outcome in patient with prior CVD [AD desc ≤ median adjusted HR 1.13 (95% CI: 0.76–1.68), p = 0.538] or without prior CVD [AD desc ≤ median adjusted HR 1.07 (95% CI: 0.71–1.60), p = 0.758]. Similar nonsignificant results for adjusted HR were shown when AD desc was used as a continuous variable or as AD desc ≤ 1st quintile (data not shown).
Discussion
We evaluated the relationship of AD in the descending thoracic aorta measured by CMR with the composite of all-cause mortality, MI, stroke, and coronary revascularizations. This study is the largest CV outcome study, to our knowledge, in assessing AD desc in a real-world referral population for CMR.
We have shown that decreased distensibility (a stiffer aorta) was associated with older age, hypertension, diabetes, renal disease, dyslipidemia, and the primary outcome but was not an independent predictor of the outcomes when adjusted for other known CV risk factors or the presence of prior CVD.
Since there is no defined cutoff for normal AD desc, we utilized multiple analyses including AD desc as a continuous variable, AD desc ≤ median cutoff, or AD desc ≤ 1st quintile cutoff and in all of these, there was no independent predictive value for AD vis-à-vis subsequent CV events. This was also true for the subgroup analysis in patients with and without CVD. This lack of independent association may reflect that descending AD already captures the impact of the classical risk factors to predict the development or progression of CVD and does not add prognostic information provided by these clinical variables. It is noteworthy, however, that our population is a real-world referral for CMR, and comorbid with 33% of the patients with CVD, and hence, the prognostic value of AD may be best studied in larger cohorts of healthier patients where CVD is not already present.
In this study, we measured AD in a unique aortic location - that of the descending thoracic aorta. It has been proposed that variations in AS may occur in different anatomical locations of the aorta in relation to various risk factors or histological changes (elongation and dilations and or fragmentation of elastin fibers within the media) or even as a part of normal aging.24–26 Furthermore, inherent dynamic differences in the properties at different aortic locations are proposed to be attributed to a higher proportion of collagen to elastin ratio more distally in the aorta compared to proximally. In addition, there have been suggested associations of oxidative stress such as smoking to affect the abdominal aorta, with mechanisms proposed to involve neutrophilic degradation of elastin.27–30 This may also explain why we observed no difference in AD desc in smokers vs. nonsmokers per the aortic location that we studied.
Our overall results vary with other publications on the association of non-invasive measured arterial stiffness [distensibility, and PWV]. In a recent study by Redheuil et al21 from the Multi-Ethnic Study of Atherosclerosis (MESA) cohort, decreased ascending aortic distensibility (AAD) assessed by CMR predicted all-cause mortality in a multi-ethnic population (n = 3675) free of CVD at the time of enrollment. Additionally, the authors reported that decreased AAD was associated with CV events in low to intermediate-CVD risk individuals with a HR of 5.3 (p = 0.03) for the first quintile of AAD in the fully adjusted model in studying proximal AD. The MESA study also reported similar observation (of increased risk but lacking statistical significance) in the subgroup of patients with high baseline CV risk profile (adjusted Hazards Ratio of 1.3 [95% CI 0.6–2.9]), p = 0.52. Similarly, “the Dallas Heart Study” by Maroules et al evaluated 2212 subjects without CVD (defined as no prior history of MI, stroke, or coronary revascularization by PCI or CABG) and reported that CMR-derived AAD is independently associated with non-fatal cardiac events (HR 1.45, P = <0.0005), while the associated composite CV events showed a HR 1.18, p = 0.08. Both the MESA and Dallas Heart are two of the largest reported ascending aorta distensibility studies using CMR imaging and both studied the proximal aorta. In our current study, we have shown that AD desc ≤ median cut off was not an independent predictor of the primary endpoint in the overall population [adjusted HR 1.09 (95% CI:0.82–1.45), p = 0.518] and in subgroup analysis of patients with or without prior CVD [adjusted HR 1.13 (95% CI: 0.76–1.68), p = 0.538 or adjusted HR 1.07 (95% CI: 0.71–1.60), p = 0.758, respectively]. Ultimately, we feel that the differences in population demographics, sample size, and the aortic location that we studied, may have attributed to these differences. Further studies should validate the prognostic impact of AD and explore its consistencies between various aortic locations.
The strengths of our study are the enrollment of a real-world, large referral population including those with prior CVD along with the hard CV outcomes that we analyzed. We also examined a unique aortic location in that of the descending thoracic aorta and measured AD desc using CMR in an easily accessible and reproducible, non-contrast, SSFP (traditional cine image) of the 4-chamber view. Our study also highlights a statistically significant distribution of AD at the descending thoracic aorta analyzed into its median and age distribution in a real world and comorbid population and exhibits a linear distribution among quintiles of decreasing AS with having a primary outcome and reduced event free survival (Tables 3 and 4; Figures 2 and 3, respectively). Given the lack of reference values available, these may be a resource for future studies.
Study Limitations
The study has several limitations inherent to the clinical retrospective study design, limited term follow-up (5-year average), the technical acquisition methods of AD desc (though simplified via a 4-chamber view of CMR scans), the lack of measured inter-observer variability of the 2 volunteers conducting measurements, as well as the sample size. We also used the peripheral (brachial) PP rather than central aortic pressure to calculate AD. This may have resulted in impedance mismatch due to the differences in the elastic properties between thoracic aorta and brachial artery or from localized peripheral vascular disease. Such mismatch effect is theoretically expected to decrease as age increases (notably our mean population age was 60 years).31,32 The number of events may be low especially in the subgroup analyses (where a wider 95% CI was observed). Nevertheless, our single center CMR study remains the largest reported sample of AD measured at the descending aorta for CV outcomes in a real-world referral population to date.
Despite documenting cardiac medications at study entry (prior to the index CMR), we were unable to monitor the compliance of medication adherence and hence we were unable to explore the ongoing dynamic changes of AD in response to cardiac medications. Prior studies have suggested an improvement in AS with certain medication classes and demonstrated improved aortic wall compliance and clinical outcomes after treatment with beta-blockers and ACE inhibitors. These medications may affect AS directly by reducing the hemodynamic afterload and lowering the PP.33,34
Conclusions
In this study, we examined a unique CMR-derived method for AD at the descending thoracic aorta (AD desc) of 928 patients with the primary outcome of all-cause mortality, MI, stroke, or coronary revascularization. We found that a decreased AD desc (a stiffer aorta) was associated with older age, hypertension, diabetes, renal disease, and dyslipidemia. AD desc was significantly lower in patients experiencing the primary outcome as was the event free survival also lower (AD desc ≤ median; or quintile 1 compared to others; or incrementally amongst quintiles). However, AD desc ≤ median was not found to be an independent predictor of the primary endpoint after multivariable adjustment in the overall population or in the subgroup analysis of patients with or without prior CVD.
Clinical Competencies
AD is an imaging biomarker to atherosclerosis leading to CV events. Decreased AD (a stiffer aorta) was associated with older age, hypertension, diabetes, renal disease, and dyslipidemia. A decrease in AD desc was significantly associated with the primary outcome (all-cause mortality, MI, stroke, or coronary revascularization) in the unadjusted model, but not in the fully adjusted model.
Translational Outlook
This study suggests that measurements of AD is readily accessible during a CMR study and can potentially be used as an imaging biomarker to CV health. Future outlook includes the potential use of AD as a tool to assess and modify anti-atherogenic therapies to improve CV outcomes when AS is detected. Larger, multicenter, and prospective studies are recommended.
Abbreviations
AS, aortic stiffness; CV, cardiovascular; CVD, cardiovascular disease; AD, aortic distensibility; PWV, pulse wave velocity; CMR, cardiovascular magnetic resonance; MI, myocardial infarction; SSFP, steady-state free precession; PP, pulse pressure; SBP, systolic blood pressure; DBP, diastolic blood pressure; CAD, coronary artery disease; PCI, percutaneous coronary intervention; CABG, coronary artery bypass surgery; LVEF, left ventricular ejection fraction; AD desc, descending aortic distensibility; RVEF, right ventricular ejection fraction; MESA, Multi-Ethnic Study of Atherosclerosis; AAD, ascending aortic distensibility; CVA, cerebrovascular event.
Disclosure
The authors report no conflicts of interest.
References
1. Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness, A systemic review and metaanalysis. JACC. 2010;10:1318–1327.
2. Laurent S, Boutouyrie P, Asmar R, et al.Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2010;37(5):1236–1241.
3. Malayeri AA, Natori S, Bahrami H, et al. Relation of aortic wall thickness and distensibility to cardiovascular risk factors (from the Multi-Ethnic Study of Atherosclerosis [Mesa]). Am J Cardiol. 2008;102:491–496. doi:10.1016/j.amjcard.2008.04.010
4. Laurent S, Boutouyrie P, Asmar R, et al. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37:1236–1241. doi:10.1161/01.HYP.37.5.1236
5. Laurent S, Katsahian S, Fassot C, et al. Aortic stiffness is an independent predictor of fatal stroke in essential hypertension. Stroke. 2003;34(5):1203–1206. doi:10.1161/01.STR.0000065428.03209.64
6. Covic A, Haydar AA, Bhamra-Ariza P, Gusbeth-Tatomir P, Goldsmith DJ. Aortic pulse wave velocity and arterial wave reflections predict the extent and severity of coronary artery disease in chronic kidney disease patients. J Nephrol. 2005;18(4):388–396.
7. Blacher J, Guerin AP, Pannier B, Marchais SJ, Safar ME, London GM. Impact of aortic stiffness on survival in end-stage renal disease. Circulation. 1999;99(18):2434–2439. doi:10.1161/01.cir.99.18.2434
8. Clin Kidney J. Less arterial stiffness in kidney transplant recipients than chronic kidney disease patients matched for renal function. eCollection. 2021;14(4):1244–1254. doi:10.1093/ckj/sfaa120
9. DeLoach SS, Townsend RR. Vascular stiffness: its measurement and significance for epidemiologic and outcome studies. Clin J Am Soc Nephrol. 2008;3:184–192. doi:10.2215/CJN.03340807
10. Safar ME, London GM. Therapeutic studies, and arterial stiffness in hypertension: recommendations of the European Society of Hypertension. The Clinical Committee of Arterial Structure and Function. Working Group on Vascular Structure and Function of the European Society of Hypertension. J Hypertens. 2000;18:1527–1535. doi:10.1097/00004872-200018110-00001
11. O’Rourke MF, Kelly RP. Wave reflection in the systemic circulation and its implications in ventricular function. J Hypertens. 1993;11:327–337. doi:10.1097/00004872-199304000-00001
12. Laurent S, Cockcroft J, Van Bortel L, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588–2605. doi:10.1093/eurheartj/ehl254
13. Khoshdel AR, Carney SL, Nair BR, Gillies A. Better management of cardiovascular diseases by pulse wave velocity: combining clinical practice with clinical research using evidence-based medicine. Clin Med Res. 2007. doi:10.3121/cmr.2007.708
14. Kass DA. Ventricular arterial stiffening: integrating the pathophysiology. Hypertension. 2005;46:185–193. doi:10.1161/01.HYP.0000168053.34306.d4
15. Devos DG, Rietzschel E, Heyse C, et al. MR pulse wave velocity increases with age faster in the thoracic aorta than in the abdominal aorta. J Magn Reson Imaging. 2015;41:765–772. doi:10.1002/jmri.24592
16. Hughes SM, Dixon LJ, McVeigh GE. Arterial stiffness and pulse wave velocity: problems with terminology. Circulation. 2004;109:
17. Markl M, Wallis W, Brendecke S, Simon J, Frydrychowicz A, Harloff A. Estimation of global aortic pulse wave velocity by flow-sensitive 4D MRI. Magn Reson Med. 2010;63(6):1575–1582. doi:10.1002/mrm.22353
18. Markl M, Wallis W, Strecker C, Gladstone BP, Vach W, Harloff A. Analy-sis of pulse wave velocity in the thoracic aorta by flow-sensitive four-dimensional MRI: reproducibility and correlation with characteristics inpatients with aortic atherosclerosis. J Magn Reson Imaging. 2012;35(5):1162–1168. doi:10.1002/jmri.22856
19. Maroules CD, Khera A, Ayers C, et al. Cardiovascular outcome associations among cardiovascular magnetic resonance measures of arterial stiffness: the Dallas heart study. J Cardiovasc Magn Reson. 2014;16:33. doi:10.1186/1532-429X-16-33
20. Schulz-Menger J, Bluemke DA, Bremerich J, et al. Standardized image interpretation and post processing in cardiovascular magnetic resonance: Society for Cardiovascular Magnetic Resonance (SCMR) board of trustees task force on standardized post processing. J Cardiovasc Magn Reson. 2013;15:35. doi:10.1186/1532-429X-15-35
21. Redheuil A, Wu CO, Kachenoura N, et al. Proximal aortic distensibility is an independent predictor of all-cause mortality and incident CV events: the Mesa study. J Am Coll Cardiol. 2014;64:2619–2629. doi:10.1016/j.jacc.2014.09.060
22. Thygesen K, Alpert JS, Jaffe AS, et al. Fourth universal definition of myocardial infarction (2018). Circulation. 2018;138:e618–e651. doi:10.1161/CIR.0000000000000617
23. Voges I, Jerosch-Herold M, Hedderich J, et al. Normal values of aortic dimensions, distensibility, and pulse wave velocity in children and young adults: a cross-sectional study. J Cardiovasc Magn Reson. 2012;14:77. doi:10.1186/1532-429X-14-77
24. Nichols WW. Aging, high blood pressure and disease in humans. In: McDonald’s Blood Flow in Arteries. London: Edward Arnold; 1990:398–420.
25. Stoiber L, Mahfoud F, Zamani SM, et al. Renal sympathetic denervation restores aortic distensibility in patients with resistant hypertension: data from a multi-center trial. Clin Res Cardiol. 2018;107:642–652. doi:10.1007/s00392-018-1229-z
26. Redheuil A, Yu WC, Wu CO, et al. Reduced ascending aortic strain and distensibility: earliest manifestations of vascular aging in humans. Hypertension. 2010;55:319–326. doi:10.1161/HYPERTENSIONAHA.109.141275
27. Aune D, Schlesinger S, Norat T, Riboli E. Tobacco smoking and the risk of abdominal aortic aneurysm: a systematic review and meta-analysis of prospective studies. Sci Rep. 2018;8:14786. doi:10.1038/s41598-018-32100-2
28. McCulloch MA, Mauras N, Canas JA, et al. Magnetic resonance imaging measures of decreased aortic strain and distensibility are proportionate to insulin resistance in adolescents with type 1 diabetes mellitus. Pediatr Diabetes. 2015;16:90–97. doi:10.1111/pedi.12241
29. Resnick LM, Militianu D, Cunnings AJ, Pipe JG, Evelhoch JL, Soulen RL. Direct magnetic resonance determination of aortic distensibility in essential hypertension: relation to age, abdominal visceral fat, and in situ intracellular free magnesium. Hypertension. 1997;30:654–659. doi:10.1161/01.HYP.30.3.654
30. Pannier B, Guerin AP, Marchais SJ, Safar ME, London GM. Stiffness of capacitive and conduit arteries: prognostic significance for end-stage renal disease patients. Hypertension. 2005;45:592–596. doi:10.1161/01.HYP.0000159190.71253.c3
31. Soulat G, Gencer U, Kachenoura N, et al. Changes in segmental pulse wave velocity of the thoracic aorta with age and left ventricular remodelling. An MRI 4D flow study. J Hypertens. 2020;38(1):118–126. doi:10.1097/HJH.0000000000002224
32. Ferreira AV, Viana MC, Mill JG, Asmar RG, Cunha RS. Racial differences in aortic stiffness in normotensive and hypertensive adults. J Hypertens. 1999;17:631–637. doi:10.1097/00004872-199917050-00006
33. Asmar R. Effect of antihypertensive agents on arterial stiffness as evaluated by pulse wave velocity: clinical implications. Am J Cardiovasc Drugs. 2001;1:387. doi:10.2165/00129784-200101050-00008
34. Mitchell GF, Dunlap ME, Warnica W, et al. Long-term trandolapril treatment is associated with reduced aortic stiffness: the prevention of events with angiotensin-converting enzyme inhibition hemodynamic substudy. Hypertension. 2007;49(6):1271–1277. doi:10.1161/HYPERTENSIONAHA.106.085738
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.