Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 14
Dermatological Considerations in the Diagnosis and Treatment of Marginal Zone Lymphomas
Authors Ronchi A, Sica A , Vitiello P, Franco R
Received 5 January 2021
Accepted for publication 23 February 2021
Published 8 March 2021 Volume 2021:14 Pages 231—239
DOI https://doi.org/10.2147/CCID.S277667
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Andrea Ronchi,1 Antonello Sica,2 Paola Vitiello,3 Renato Franco1
1Pathology Unit, Department of Mental and Physical Health and Preventive Medicine, Università degli Studi della Campania “Luigi Vanvitelli”, Naples, 80138, Italy; 2Oncology and Haematology Unit, Department of Precision Medicine, University of Campania Luigi Vanvitelli, Naples, 80131, Italy; 3Dermatology Unit, Department of Mental and Physical Health and Preventive Medicine, Università degli Studi della Campania “Luigi Vanvitelli”, Naples, 80131, Italy
Correspondence: Renato Franco
Pathology Unit, Department of Mental and Physical Health and Preventive Medicine, Università degli Studi della Campania “Luigi Vanvitelli”, Via Luciano Armanni 5, Naples, 80138, Italy
Tel +390815664084
Email [email protected]
Abstract: Primary cutaneous marginal zone lymphoma (PC-MZL) is a B-cell lymphoma arising in the skin. Although it is a rare disease, PC-MZL accounts for 20– 40% of all primary cutaneous B-cell lymphoma in Western Countries. The aetiology and the pathogenesis of PC-MZL are poorly understood, as it generally lacks the chromosomal translocations most typically present in marginal zone lymphomas of other sites. The diagnosis of PC-MZL may be challenging, due to the rarity of the disease, and needs the competence of different professional figures, including the dermatologist and the pathologist. Furthermore, the management of the patient after the diagnosis is complex and involves the dermatologist, the haematologist, the surgeon, the radiotherapist and the radiologist. The aim of this review is to describe the clinical and histological findings for the diagnosis of PC-MZL, and the state of art for the management of the patient.
Keywords: marginal zone lymphoma, cutaneous lymphoma, Borrelia burgdorferi, immunohistochemistry, dermoscopy
Introduction
Primary cutaneous B-cell lymphomas (PC-BCLs) are a heterogeneous group of non-Hodgkin lymphomas originally arising in the skin.1 As most primary cutaneous lymphomas are T-cell neoplasms, PC-BCLs are rare, accounting for 25% of all primary cutaneous lymphomas. Most frequent PC-BCLs are primary cutaneous follicle centre lymphoma, primary cutaneous marginal zone lymphoma (PC-MZL), and primary cutaneous diffuse large B-cell lymphoma, leg type (Figure 1).1 The group includes other extremely rare histotypes, like intravascular large B-cell lymphoma, and provisional entity like Epstein-Barr Virus (EBV)-positive mucocutaneous ulcer.1 However, other B-cell lymphomas may anecdotally arise primarily in the skin, as well as systemic lymphomas may involve the skin during their course.2
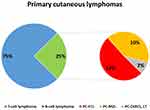 |
Figure 1 Frequency distribution of primary cutaneous lymphomas. |
PC-MZL was included in the large group of extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT) by the last 2017 World Health Organization revision of the classification of haematological neoplasms.1 PC-MZL accounts for about 20–40% of all PC-BCLs in Western Countries, with an estimated incidence of 0.4 per 1,000,000 per year in the United States.3 PC-MZL derives from post-germinal centre memory B-cells but its aetiology is substantially unknown. Some evidences about immunoglobulin variable region gene analysis support the existence of a link between a chronic antigenic stimulation and the development of a neoplastic lymphoid clone.4 Different associations have been reported to date: infectious disease (including Borrelia burgdorferi, Helicobacter pylori, Hepatitis C and EBV), medications (including methotrexate, cyclosporine, antidepressant), influenza (flu) or viral hepatitis A vaccination.5–9 Moreover, associations with autoimmune diseases (including rheumatoid arthritis, Hashimoto thyroiditis, Sjogren syndrome, polyarteritis nodosa, ulcerative colitis) have been described.10 In this setting, chronic infection by Borrelia burgdorferi was hypothesised to have a role in the pathogenesis of PC-MZL at least in some geographic locations, but its effective role is still debatable Indeed, some series have demonstrated Borrelia infection in up to 40% cases, but the results have not been confirmed by other series.5 Furthermore, anecdotal cases associated to tattoos and vaccinations have been reported.11,12
PC-MZLs generally lack the chromosomal translocations most typically present in MALT lymphomas of other sites. However, some cases have been reported showing both the translocations t(14;18)(q32;q21) IGH/BCL2 and t(14;18)(q32;q21) IGH/MALT1.13
PC-MZL is an indolent lymphoma with rare dissemination to extra-cutaneous sites and excellent prognosis.1 Cutaneous relapses may occur in about 20–30% of cases, more commonly in patients with multifocal skin involvement, and spontaneous regression was also recorded.14,15 Extra-cutaneous spread or transformation to high-grade lymphoma is exceptional.1
Dermatological and Dermoscopical Findings
PC-MZL usually presents as solitary or multiple not ulcerated skin lesions represented by red to violaceous papules, or more rarely plaques and nodules (Figure 2A).16 Although some paediatric cases have been recorded, the lesions are usually localized on the trunk and arms of middle-aged adults.16 Systemic symptoms are generally absent. Dermoscopy could play an adjuvant role for the diagnosis of PC-MZL, showing a salmon-coloured background and serpentine blood vessels (Figure 2B).17–19 However, dermoscopic differential diagnosis is broad and includes many entities, like arthropod bites, amelanotic melanoma, basal cell carcinoma and others. Consequently, histological evaluation is mandatory for a definite diagnosis. The excisional biopsy is more representative and should be always preferred, but an incisional punch biopsy could be performed in some instances. As PC-BCLs often involve the deep tissue, the biopsy should always include the hypodermic fat tissue.
The clinical diagnosis may be challenging, and the differential diagnosis includes cutaneous pseudolymphomas, as well as other primary or secondary cutaneous B-cell o T-cell lymphomas.20 Pseudolymphomatous folliculitis (PLF), a poorly defined entity among the cutaneous pseudolymphomas, presents clinically as a solitary dome-shaped or flat to elevated nodule, located on the face, the scalp and the trunk.21 Primary cutaneous follicle centre lymphoma presents as solitary or – less commonly – grouped erythematous or violaceous papules, plaques and/or nodules, usually located on the trunk and head-neck.22 Primary cutaneous diffuse large B-cell lymphoma, leg type is a rare aggressive PC-BCL, presenting as rapidly growing erythematous or cyanotic nodules and/or plaques, usually located on one or both legs.23 “B-Symptoms” (fever, weight loss, night sweats) and extra-cutaneous diffusion are common.1 Differential diagnosis of PC-MZL also includes Lupus Erythematosus Tumidus (LET), Jessner’s lymphocytic infiltrate and granuloma faciale. LET is characterized by non-scarring, erythematous, succulent, urticaria-like plaques without surfaces changes.24 Jessner’s lymphocytic infiltrate is characterized by multiple asymptomatic erythematous papules or plaques with arciform or annular pattern, commonly located on the face, neck and upper trunk.25 Both Jessner’s lymphocytic infiltrate and LET probably represent part of the spectrum of cutaneous lupus. Granuloma faciale is characterized by single or multiple erythematous papules, plaques or nodules on the face.26
Other lymphomas may rarely occur at the skin as primary localization or secondary involvement by a systemic disease.2 Lymphoplasmacytic lymphoma is a low-grade B-cell lymphoma rarely involving the skin, with a substantial subset of cases being associated with Waldenstrom macroglobulinaemia.16 Although most patients are asymptomatic, anemia or blood hyperviscosity may be possible.27 Cutaneous localizations are rare and present as purple, sometimes ulcerated, nodules. In case of association with Waldenstrom macroglobulinaemia, diffuse urticarial rash and IgM paraproteinemia may arise (Schnitzler syndrome).28 Rare cases of cutaneous involvements have been reported in patients affected by multiple myeloma or plasma cell leukaemia.29 In these cases, single or multiple violaceous cutaneous nodules have been described, but erythematous plaques may also be observed.29 In plasma cell myeloma, hyperkeratotic spicules may occur, mainly on the face. Primary cutaneous CD4-positive small/medium T-cell lymphoma is a rare indolent disease with insidious clinical evolution, classically presenting a solitary asymptomatic nodule, plaque or tumour on the face, the neck or the trunk.30
Pathological Findings
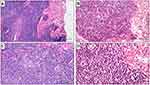
PC-BCLs as a group share some common histological findings, which may allow distinguishing them from T-cells lymphomas on a morphological basis. The overall architecture is usually nodular rather than band-like, the papillary dermis is spared (a “Grenz-zone” is present), and the epidermotropism and/or folliculotropism is absent.16 PC-MZL usually – but not always – shares these common “B-cell” histological findings. PC-MZL always involves the reticular dermis, sparing the papillary dermis and epidermis, and often involves the hypodermis. The overall architecture is more often nodular, but it may also be diffuse. Ulceration of the epidermis is exceptional.16 Periadnexal infiltration is often present, but lympho-epithelial lesions are uncommon are not critical for the diagnosis.23 Lymphoid follicles characterized by reactive germinal center and preserved mantle zone are frequently present. The follicles may play an important role for diagnostic purpose, as they may show germinal center colonization by marginal zone cells, and partial destruction of follicular dendritic cell meshwork. The lymphoid population is variably mixed, including centrocyte-like marginal zone B cells, monocytoid B-cells, lymphoplasmacytic cells, cells resembling centroblasts and immunoblasts, and reactive T cells. Plasma cells are variably present, more often at the periphery of the nodules, but Dutcher bodies are infrequent.31 A variable amount of inflammatory cells may be admixed to the neoplastic population, including T-cell lymphocytes, histiocytes, mast cells, and eosinophils.32 Histological findings are shown in Figure 3. Some morphological variants of PC-MZL have been described, including small cell lymphocytic variant, monocytoid variant and variant with diffuse plasmacytic differentiation.33–39 Morphological findings of PC-MZL lack specificity and the diagnosis may be one of the most challenging in the setting of cutaneous lymphoid neoplasms. The lympho-epithelial lesions, which play an important role in diagnosis of mucosa-associated marginal zone lymphomas, are useless in case of PC-MZL. Indeed, they are often absent, and on the other hand, they may be present in reactive disorders. The reactive inflammatory – lymphoid and not lymphoid – population may outnumber the neoplastic cells, and reactive germinal centers in the context of the neoplasm may simulate an inflammatory disease. On the other way, cases with prominent lympho-plasmacytoid or plasmacytoid differentiation may simulate lymphoplasmacytic lymphoma or myeloma. Consequently, histological diagnosis of PC-MZL is challenging and always relies on the comprehensive integration of morphological and immunohistochemical findings and clonal analysis.
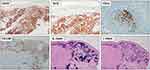
Immunohistochemically, PC-MZL is characterized by the expansion of the marginal zone cellular population, which is positive for CD20 and Bcl2. The reactive germinal centers are positive for Bcl6 and CD10 and negative for Bcl2. CD21 and CD23 immunostains may be helpful to highlight the partial destruction of follicular dendritic cell meshwork. Plasma cells are more often polyclonal by testing Immunoglobulin (Ig) light chain immunostains. Myeloid cell nuclear differentiation antigen (MNDA) has recently emerged as a useful marker in MZL, but its diagnostic value in case of PC-MZL is still poorly investigated. Verdanet et al have recently reported MNDA positivity in 4 out of 13 cases of PC-MZL (30.8%).40 Primary cutaneous plasmacytoid dendritic cells have been hypothesised to have a diagnostic role, as large clusters have been observed in PC-MZL rather than in reactive B-cell infiltrates.41 Actually, two groups of PC-MZL are defined on the basis of the expression of surface immunoglobulins: the “class-switched” group expresses IgG or, more rarely, IgA and IgE, and is characterized by a nodular and scattered distribution of B-cells, and many T-cells, mainly Th2. The “non-class-switched” group expresses IgM and is characterized by a diffuse distribution of B-cells, and a less represented T-cell population.42 A variable number of IgG-positive PC-MZLs are IgG4-positive, ranging from 13% to 40% in different series, and are not associated with systemic IgG4-related disease.31 Hypermutation and antigenic pressure has been demonstrated in such subset of PC-BCL.43 Immunohistochemical findings are shown in Figure 4. The most useful immunohistochemical markers are listed in Table 1.
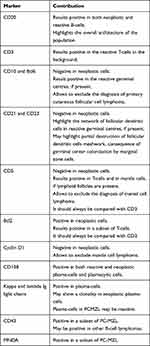 |
Table 1 Main Contribution of Immunohistochemical Markers to Diagnosis of PCMZL |
Patient Management
As PC-MZL is an indolent lymphoma, systemic spread and bone marrow infiltration are rare events. However, instrumental staging is mandatory at the time of the first diagnosis, using superficial and abdominal lymph node ultra-sound (US), and total-body computed tomography (CT).16 Although combined CT and positron emission tomography (CT/PET) is actually the gold standard for staging purpose in case of primary cutaneous follicle centre lymphoma and primary cutaneous diffuse large B-cell lymphoma, leg type, its introduction as a routine staging test in case of PC-MZL is still debated.44 Bone marrow biopsy is not mandatory, but it may be useful in the case of single or multiple cell line cytopenia.22 PC-MZL staging yet requires a careful history and clinical examination and laboratory tests such as: blood count, LDH, Erythrocyte Sedimentation Rate (ESR), C-reactive protein (CRP), beta-2 microglobulin, Serum Protein Electrophoresis (SPE), HIV antibodies, HBV antibodies, AST, ALT, creatinine, amylase, lipase, bilirubin, HCV Antibodies.45–52 Moreover, Borrelia burgdorferi serology is recommended in endemic areas.5,7 Tumor Node Metastases (TNM) classification of cutaneous lymphoma other than mycosis fungoides/Sézary syndrome is an available staging system for PC-MZL (Table 2).52 However, T stage has no prognostic value in PC-MZL, while the presence of a single cutaneous lesion has proved an independent prognostic significance on disease-free survival.53 In addition, the International Extranodal Lymphoma Study Group (IELSG) recently proposed a specific prognostic score for patients with indolent PC-BCLs, demonstrating that elevated LDH, more than 2 skin lesions, and nodular lesions may affect the progression-free survival.54 However, no factor showed any meaningful difference in overall survival.54
 |
Table 2 ISCL/EORTC Staging System for Primary Cutaneous Lymphomas |
Treatment
PC-MZL is an indolent tumour with excellent prognosis, and overtreatment should be avoided. Several treatment options are available depending on disease features (size and site of skin involvement) and patient findings (age and general health). A “wait-and-see” strategy can be followed in selected patients. This strategy consists in the observation, treating the patient only in the rare cases of systemic symptoms.55 According to European Organization for Research and Treatment of Cancer/International Society of Cutaneous Lymphoma (EORTC/ISCL) consensus recommendation, solitary and localized lesions can be treated by radiotherapy, surgery alone or surgery followed by radiotherapy, with curative intent. These treatments usually are sufficient for complete remission (>95%). Surgical excision and radiotherapy represent the first-line therapy in case of solitary lesion.
When used as first-line therapy, radiotherapy may be sufficient to achieve complete remission of the neoplasm. A total dose ranging between 20 and 36 Gy is recommended by the actual guidelines, with good results in terms of response to therapy and tolerability of toxicity.56 However, increasing evidences support the efficacy of lower dosages, which greatly reduce side effects.57 Indeed, the classic protocols of radiotherapy are increasingly modifying the intensity of treatments to obtain the best response with a reduced toxicity. Several studies proposed the use of low-dose radiotherapy (LDRT) (4 Gy in 2 sessions or 8 Gy in 2 sessions), showing high response rates with reduction of toxicity.58 LDRT may represent a good treatment choice mainly in case of relapsed neoplasm or multifocal disease.58 However, some Authors proposed LDRT as first-line therapy with a good rate of remission and low toxicity, reserving standard dose RT only in case of relapse.59
Topical therapies play a minor role for treatment of PC-MZL. Topical corticosteroids with high potency represent a potential alternative in case of plaques with mild infiltration, while intralesional injections may be used in case of more thickened skin lesions.60 However, disease recurrences and adverse effects have been reported in these cases.60 Intralesional therapy using interferon-α (IFN-α) and rituximab may be used as second-line treatment or in the patients with multiple cutaneous lesions.61,62 However, there treatments may be burdened by pain at injection site and potential systemic reaction.62 Genetically modified viruses (adenovirus interferon-γ) are being tested as intralesional therapy.63
In patients with Borrelia burgdorferi infection, antibiotic treatment (doxycycline 100 mg bid 3 weeks or cefotaxime i.v.) should be performed, but not all cases respond to therapy.64
Systemic therapies are usually unnecessary in PC-MZL and it should be limited to cases with systemic spread. These cases are exceeding rare, and consequently data about the use of systemic therapy in PC-MZL are lacking. However, the most useful drug in this setting is rituximab, alone or in combination with chlorambucil, fludarabine, cyclophosphamide and vincristine in CVP, bendamustine.65 Moreover, new therapeutic options are being evaluated for the treatment of relapsed or refractory indolent lymphomas, such as lenalidomide, ibrutinib, idelalisib.66–69
Conclusion
PC-MZL is an indolent lymphoma with an excellent prognosis, and the extra-cutaneous secondary dissemination is exceeding rare. The diagnosis may be challenging, from both a clinical and histological point of view, and differential diagnosis is wide, including benign and malignant diseases. Surgery and radiotherapy represent the best therapy in most cases.
Abbreviations
PC-MZL, primary cutaneous marginal zone lymphoma; PC-BCL, primary cutaneous B-cell lymphoma; EBV, Epstein-Barr Virus; MALT, mucosa-associated lymphoid tissue; PCDLBCL, LT, primary cutaneous diffuse large B-cell lymphoma, leg type; CD, cluster of differentiation; Ig, immunoglobulin; MNDA, myeloid cell nuclear differentiation antigen; LDH, Lactate Dehydrogenase; PET, positron emission tomography; CT, computed tomography; US, ultra sound; ESR, Erythrocyte Sedimentation Rate; CRP, C-reactive protein; SPE, Serum Protein Electrophoresis; HIV, Human Immunodeficiency Virus; HBV, hepatitis B virus; AST, aspartate transaminase; ALT, alanine transaminase; HCV, Human Immunodeficiency Virus; IELSG, International Extranodal Lymphoma Study Group; EORTC/ISCL, European Organization for Research and Treatment of Cancer/International Society of Cutaneous Lymphoma.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Willemze R, Cerroni L, Kempf W, et al. The 2018 update of the WHO-EORTC classification for primary cutaneous lymphomas. Blood. 2019;133(16):1703–1714. doi:10.1182/blood-2018-11-881268
2. Pinter-Brown LC. Diagnosis and management of cutaneous B-cell lymphoma. Dermatol Clin. 2015;33(4):835–840. doi:10.1016/j.det.2015.05.003
3. Bradford PT, Devesa SS, Anderson WF, Toro JR. Cutaneous lymphoma incidence patterns in the United States: a population-based study of 3884 cases. Blood. 2009;113(21):5064–5073. doi:10.1182/blood-2008-10-184168
4. Perez M, Pacchiarotti A, Frontani M, et al. Primary cutaneous B-cell lymphoma is associated with somatically hypermutated immunoglobulin variable genes and frequent use of VH1-69 and VH4-59 segments. Br J Dermatol. 2010;162(3):611–618. doi:10.1111/j.1365-2133.2009.09576.x
5. Perrone S, D’Elia GM, Annechini G, Pulsoni A. Infectious aetiology of marginal zone lymphoma and role of anti-infective therapy. Mediterr J Hematol Infect Dis. 2016;8(1):e2016006. doi:10.4084/mjhid.2016.006
6. Ponzoni M, Ferreri AJ. Bacteria associated with marginal zone lymphomas. Best Pract Res Clin Haematol. 2017;30(1–2):32–40. doi:10.1016/j.beha.2017.01.001
7. Foster LH, Portell CA. The role of infectious agents, antibiotics, and antiviral therapy in the treatment of extranodal marginal zone lymphoma and other low-grade lymphomas. Curr Treat Options Oncol. 2015;16(6):28. doi:10.1007/s11864-015-0344-6
8. Bende RJ, van Maldegem F, van Noesel CJ. Chronic inflammatory disease, lymphoid tissue neogenesis and extranodal marginal zone B-cell lymphomas. Haematologica. 2009;94(8):1109–1123. doi:10.3324/haematol.2009.005983
9. Suarez F, Lortholary O, Hermine O, Lecuit M. Infection-associated lymphomas derived from marginal zone B cells: a model of antigen-driven lymphoproliferation. Blood. 2006;107(8):3034–3044. doi:10.1182/blood-2005-09-3679
10. Fallah M, Liu X, Ji J, Försti A, Sundquist K, Hemminki K. Autoimmune diseases associated with non-Hodgkin lymphoma: a nationwide cohort study. Ann Oncol. 2014;25(10):2025–2030. doi:10.1093/annonc/mdu365
11. Chandler JB, Waldman R, Sloan SB, Rose MG, Wong EY. Cutaneous marginal zone lymphoma following anthrax vaccination. Ann Hematol. 2020. doi:10.1007/s00277-020-04336-4
12. Shinohara MM, Nguyen J, Gardner J, Rosenbach M, Elenitsas R. The histopathologic spectrum of decorative tattoo complications. J Cutan Pathol. 2012;39(12):1110–1118. doi:10.1111/cup.12023
13. Palmedo G, Hantschke M, Rütten A, et al. Primary cutaneous marginal zone B-cell lymphoma may exhibit both the t(14;18)(q32;q21) IGH/BCL2 and the t(14;18)(q32;q21) IGH/MALT1 translocation: an indicator for clonal transformation towards higher-grade B-cell lymphoma? Am J Dermatopathol. 2007;29(3):231–236. doi:10.1097/DAD.0b013e31804795a6
14. Tang X, Tang J, Liu W, Wang L. Primary cutaneous marginal zone B-cell lymphoma with unusual manifestation and spontaneous regression. Indian J Dermatol Venereol Leprol. 2020;86(4):428–431. doi:10.4103/ijdvl.IJDVL_516_19
15. Franco R, Fernández-Vázquez A, Mollejo M, et al. Cutaneous presentation of follicular lymphomas. Mod Pathol. 2001;14(9):913–919. doi:10.1038/modpathol.3880411
16. Vitiello P, Sica A, Ronchi A, Caccavale S, Franco R, Argenziano G. Primary cutaneous B-cell lymphomas: an update. Front Oncol. 2020;10:651. doi:10.3389/fonc.2020.00651
17. Piccolo V, Mascolo M, Russo T, Staibano S, Argenziano G. Dermoscopy of primary cutaneous B-cell lymphoma (PCBCL). J Am Acad Dermatol. 2016;75(4):e137–e139. doi:10.1016/j.jaad.2016.02.1217
18. Piccolo V, Russo T, Agozzino M, et al. Dermoscopy of cutaneous lymphoproliferative disorders: where are we now? Dermatology. 2018;234:131–136. doi:10.1159/000490412
19. Geller S, Marghoob AA, Scope A, Braun RP, Myskowski PL. Dermoscopy and the diagnosis of primary cutaneous B-cell lymphoma. J Eur Acad Dermatol Venereol. 2018;32(1):53–56. doi:10.1111/jdv.14549
20. Baldassano MF, Bailey EM, Ferry JA, Harris NL, Duncan LM. Cutaneous lymphoid hyperplasia and cutaneous marginal zone lymphoma: comparison of morphologic and immunophenotypic features. Am J Surg Pathol. 1999;23(1):88–96. doi:10.1097/00000478-199901000-00010
21. Arai E, Okubo H, Tsuchida T, Kitamura K, Katayama I. Pseudolymphomatous folliculitis: a clinicopathologic study of 15 cases of cutaneous pseudolymphoma with follicular invasion. Am J Surg Pathol. 1999;23:1313–1319. doi:10.1097/00000478-199911000-00001
22. Wilcox RA. Cutaneous B-cell lymphomas: 2019 update on diagnosis, risk stratification, and management. Am J Hematol. 2018;93:1427–1430. doi:10.1002/ajh.25224
23. Cho-Vega JH, Vega F, Rassidakis G, et al. Primary cutaneous marginal zone B cell lymphoma. Am J Clin Pathol. 2006;125:S38–S49. doi:10.1309/CVFYBQNMX1PKNAA7
24. Patsinakidis N, Kautz O, Gibbs BF, Raap U. Lupus erythematosus tumidus: clinical perspectives. Clin Cosmet Investig Dermatol. 2019;12:707–719. doi:10.2147/CCID.S166723
25. Rémy-Leroux V, Léonard F, Lambert D, et al. Comparison of histopathologic-clinical characteristics of Jessner’s lymphocytic infiltration of the skin and lupus erythematosus tumidus: multicenter study of 46 cases. J Am Acad Dermatol. 2008;58(2):217–223. doi:10.1016/j.jaad.2007.09.039
26. Lallas A, Argenziano G, Apalla Z, et al. Dermoscopic patterns of common facial inflammatory skin diseases. J Eur Acad Dermatol Venereol. 2014;28(5):609–614. doi:10.1111/jdv.12146
27. Tees MT, Flinn IW. Chronic lymphocytic leukemia and small lymphocytic lymphoma: two faces of the same disease. Expert Rev Hematol. 2017;10(2):137–146. doi:10.1080/17474086.2017.1270203
28. Gusdorf L, Lipsker D. Schnitzler syndrome: a review. Curr Rheumatol Rep. 2017;19(8):46. doi:10.1007/s11926-017-0673-5
29. Torne R, Su WP, Winkelmann RK, Smolle J, Kerl H. Clinicopathologic study of cutaneous plasmacytoma. Int J Dermatol. 1990;29(8):562–566. doi:10.1111/j.1365-4362.1990.tb03469.x
30. Gru AA, Wick MR, Eid M. Primary cutaneous CD4+ small/medium T-cell lymphoproliferative disorder-clinical and histopathologic features, differential diagnosis, and treatment. Semin Cutan Med Surg. 2018;37(1):39–48. doi:10.12788/j.sder.2018.006
31. Carlsen ED, Swerdlow SH, Cook JR, et al. Class-switched primary cutaneous marginal zone lymphomas are frequently IgG4-positive and have features distinct from IgM-positive cases. Am J Surg Pathol. 2019;43:1403–1412. doi:10.1097/PAS.0000000000001363
32. Cerroni L, Signoretti S, Höfler G, et al. Primary cutaneous marginal zone B-cell lymphoma: a recently described entity of low-grade malignant cutaneous B-cell lymphoma. Am J Surg Pathol. 1997;21:1307–1315. doi:10.1097/00000478-199711000-00005
33. Magro CM, Olson LC. Small cell lymphocytic variant of marginal zone lymphoma: a distinct form of marginal zone lymphoma derived from naïve B cells as a cutaneous counterpart to the naïve marginal zone lymphoma of splenic origin. Ann Diagn Pathol. 2018;34:116–121. doi:10.1016/j.anndiagpath.2018.02.006
34. LeBoit PE, McNutt NS, Reed JA, et al. Primary cutaneous immunocytoma a B‐cell lymphoma that can easily be mistaken for cutaneous lymphoid hyperplasia. Am J Surg Pathol. 1994;18:969. doi:10.1097/00000478-199410000-00001
35. Rijlaarsdam JU, van der Putte SCJ, Berti E, et al. Cutaneous immunocytomas: a clinicopathologic study of 26 cases. Histopathology. 1993;23:117–125. doi:10.1111/j.1365-2559.1993.tb00469.x
36. Turbiner Geyer J, Ferry JA, Longtine JA, et al. Characteristics of cutaneous marginal zone lymphomas with marked plasmacytic differentiation and a T cell‐rich background. Am J Clin Pathol. 2010;133:59–69. doi:10.1309/AJCPW64FFBTTPKFN
37. Magro CM, Yang A, Fraga G. Blastic marginal zone lymphoma: a clinical and pathological study of 8 cases and review of the literature. Am J Dermatopathol. 2013;35:319–326. doi:10.1097/DAD.0b013e318267495f
38. Walsh NM, Lano IM, Green P, et al. AL amyloidoma of the skin/subcutis: cutaneous amyloidosis, plasma cell dyscrasia or a manifestation of primary cutaneous marginal zone lymphoma? Am J Surg Pathol. 2017;41:1069–1076. doi:10.1097/PAS.0000000000000861
39. Ueberdiek S, Kempf W, Kretschmer L, Schon MP, Mitteldorf C. AL‐amyloidoma of the skin – a rare manifestation of primary cutaneous marginal zone lymphoma. Am J Dermatopathol. 2019;41:518–521. doi:10.1097/DAD.0000000000001368
40. Verdanet E, Dereure O, René C, et al. Diagnostic value of STMN1, LMO2, HGAL, AID expression and 1p36 chromosomal abnormalities in primary cutaneous B cell lymphomas. Histopathology. 2017;71(4):648–660. doi:10.1111/his.13279
41. Kempf W, Kerl H, Kutzner H. CD123-positive plasmacytoid dendritic cells in primary cutaneous marginal zone B-cell lymphoma: a crucial role and a new lymphoma paradigm. Am J Dermatopathol. 2010;32(2):194–196. doi:10.1097/DAD.0b013e3181aff9b3
42. Edinger JT, Kant JA, Swerdlow SH. Cutaneous marginal zone lymphomas have distinctive features and include 2 subsets. Am J Surg Pathol. 2010;34(12):1830–1841. doi:10.1097/PAS.0b013e3181f72835
43. Franco R, Camacho FI, Fernández-Vázquez A, et al. IgV(H) and bcl6 somatic mutation analysis reveals the heterogeneity of cutaneous B-cell lymphoma, and indicates the presence of undisclosed local antigens. Mod Pathol. 2004;17(6):623–630. doi:10.1038/modpathol.3800106
44. Albano D, Durmo R, Treglia G, Giubbini R, Bertagna F. 18F-FDG PET/CT or PET Role in MALT Lymphoma: an open issue not yet solved - a critical review. Clin Lymphoma Myeloma Leuk. 2020;20(3):137–146. doi:10.1016/j.clml.2019.10.006
45. Merli M, Frigeni M, Alric L, et al. Direct-acting antivirals in hepatitis C virus-associated diffuse large B-cell lymphomas. Oncologist. 2019;24(8):e720–e729. doi:10.1634/theoncologist.2018-0331
46. Tonziello G, Pisaturo M, Sica A, et al. Transient reactivation of occult hepatitis B virus infection despite lamivudine prophylaxis in a patient treated for non-Hodgkin lymphoma. Infection. 2013;41(1):225–229. doi:10.1007/s15010-012-0305-y
47. Pisaturo M, Guastafierro S, Filippini P, et al. Absence of occult HCV infection in patients experiencing an immunodepression condition. Infez Med. 2013;21(4):296–301.
48. Coppola N, Pisaturo M, Guastafierro S, et al. Increased hepatitis C viral load and reactivation of liver disease in HCV RNA-positive patients with onco-haematological disease undergoing chemotherapy. Dig Liver Dis. 2012;44(1):49–54. doi:10.1016/j.dld.2011.07.016
49. Merli M, Defrancesco I, Visco C, et al. Direct-acting antivirals in relapsed or refractory hepatitis C virus-associated diffuse large B-cell lymphoma. Leuk Lymphoma. 2020;28:1–7.
50. Bagaglio S, Uberti-Foppa C, Sagnelli C, et al. HIV-1 recombinant forms in immigrants regularly residing in Milan, northern Italy. Infection. 2020;48(4):553–558. doi:10.1007/s15010-020-01434-3
51. Sica A, Casale D, Rossi G, et al. The impact of the SARS-CoV-2 infection, with special reference to the haematological setting. J Med Virol. 2020;93(1):223–233. doi:10.1002/jmv.26197
52. Kim YH, Willemze R, Pimpinelli N, et al. TNM classification system for primary cutaneous lymphomas other than mycosis fungoides and Sezary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the cutaneous lymphoma task force of the European Organization of Research and Treatment of cancer (EORTC). Blood. 2007;110:
53. Zinzani PL, Quaglino P, Pimpinelli N, et al. Prognostic factors in primary cutaneous B-cell lymphoma: the Italian study group for cutaneous lymphomas. J Clin Oncol. 2006;24(9):1376–1382. doi:10.1200/JCO.2005.03.6285
54. Mian M, Marcheselli L, Luminari S, et al. CLIPI: a new prognostic index for indolent cutaneous B cell lymphoma proposed by the international extranodal lymphoma study group (IELSG 11). Ann Hematol. 2011;90(4):401–408. doi:10.1007/s00277-010-1083-1
55. Reginelli A, Urraro F, Sangiovanni A, et al. Extranodal lymphomas: a pictorial review for CT and MRI classification. Acta Biomed. 2020;91(8–S):34–42. doi:10.23750/abm.v91i8-S.9971
56. Willemze R, Hodak E, Zinzani PL, Specht L, Ladetto M; ESMO Guidelines Committee. Primary cutaneous lymphomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(Suppl4):iv30–iv40. doi:10.1093/annonc/mdy133
57. Ulutin HC, Oztürk B, Ongürü O, Kuzhan O, Arpaci F, Ozet A. Treatment of primary cutaneous B-cell lymphoma with radiotherapy. Radiat Med. 2005;23(4):292–295.
58. Oertel M, Elsayad K, Weishaupt C, Steinbrink K, Eich HT. De-escalated radiotherapy for indolent primary cutaneous B-cell lymphoma. Strahlenther Onkol. 2020;196(2):126–131. doi:10.1007/s00066-019-01541-7
59. Goyal A, Carter JB, Pashtan I, et al. Very low-dose versus standard dose radiation therapy for indolent primary cutaneous B-cell lymphomas: a retrospective study. J Am Acad Dermatol. 2018;78(2):408–410. doi:10.1016/j.jaad.2017.07.053
60. Perry A, Vincent BJ, Parker SR. Intralesional corticosteroid therapy for primary cutaneous B-cell lymphoma. Br J Dermatol. 2010;163(1):223–225. doi:10.1111/j.1365-2133.2010.09798.x
61. Sica A, Vitiello P, Papa A, et al. Use of rituximab in NHL malt type pregnant in I° trimester for two times. Open Med (Wars). 2019;14:757–760. doi:10.1515/med-2019-0087
62. Peñate Y, Hernández-Machín B, Pérez-Méndez LI, et al. Intralesional rituximab in the treatment of indolent primary cutaneous B-cell lymphomas: an epidemiological observational multicentre study. The Spanish working group on cutaneous lymphoma. Br J Dermatol. 2012;167:174–179. doi:10.1111/j.1365-2133.2012.10902.x
63. Dreno B, Urosevic-Maiwald M, Kim Y, et al. TG1042 (Adenovirus-interferon-γ) in primary cutaneous B-cell lymphomas: a Phase II clinical trial. PLoS One. 2014;9(2):e83670. doi:10.1371/journal.pone.0083670
64. Monari P, Farisoglio C, Calzavara Pinton PG. Borrelia Burgdorferi-associated primary cutaneous marginal-zone B-cell lymphoma: a case report. Dermatology. 2007;215(3):229–232. doi:10.1159/000106580
65. Valencak J, Weihsengruber F, Rappersberger K, et al. Rituximab monotherapy for primary cutaneous B-cell lymphoma: response and follow-up in 16 patients. Ann Oncol. 2009;20(2):326–330. doi:10.1093/annonc/mdn636
66. Leonard JP, Trneny M, Izutsu K, et al. AUGMENT: a phase III study of lenalidomide plus rituximab versus placebo plus rituximab in relapsed or refractory indolent lymphoma. J Clin Oncol. 2019;37(14):1188–1199. doi:10.1200/JCO.19.00010
67. Sica A, Sagnelli C, Papa A, et al. An anecdotal case report of chronic lymphatic leukemia with del(11q) treated with ibrutinib: artificial nourishment and physical activity program. Int J Environ Res Public Health. 2020;17(6):1929. doi:10.3390/ijerph17061929
68. Visco C, Di Rocco A, Evangelista A, et al. Outcomes in first relapsed-refractory younger patients with mantle cell lymphoma: results from the MANTLE-FIRST study. Leukemia. 2020. doi:10.1038/s41375-020-01013-3
69. Wagner-Johnston ND, Schuster SJ, deVos S, et al. Outcomes of patients with up to 6 years of follow-up from a phase 2 study of idelalisib for relapsed indolent lymphomas. Leuk Lymphoma. 2020;10:1–11. doi:10.1080/10428194.2020.1855344
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.