Back to Journals » Nature and Science of Sleep » Volume 13
Depression of Non-Neuronal Cholinergic System May Play a Role in Co-Occurrence of Subjective Daytime Sleepiness and Hypertension in Patients with Obstructive Sleep Apnea Syndrome
Authors Meng Z, Sun B, Chen W, Zhang X, Huang M, Xu J
Received 15 September 2021
Accepted for publication 18 November 2021
Published 14 December 2021 Volume 2021:13 Pages 2153—2163
DOI https://doi.org/10.2147/NSS.S339038
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ahmed BaHammam
Zili Meng,1,* Bing Sun,1,* Wei Chen,1,* Xilong Zhang,2 Mao Huang,2 Jing Xu1
1Department of Respiratory and Critical Care Medicine, The Affiliated Huaian No. 1 People’s Hospital of Nanjing Medical University, Huaian, Jiangsu, People’s Republic of China; 2Department of Respiratory and Critical Care Medicine, The First Affiliated Hospital with Nanjing Medical University, Nanjing, Jiangsu, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jing Xu
Department of Respiratory and Critical Care Medicine, The Affiliated Huaian No. 1 People’s Hospital of Nanjing Medical University, 6 Beijing Road West, Huaian, Jiangsu, 223300, People’s Republic of China
Tel +86-13912080023
Fax +86-517 83724440
Email [email protected]
Purpose: Simultaneous occurrence of hypertension and excessive daytime sleepiness (EDS) is very common in obstructive sleep apnea syndrome (OSAS), although no study has specifically addressed this issue. The present study explored the risk factors for co-occurrence of OSAS-related EDS and hypertension.
Patients and Methods: A total of 161 OSAS patients were studied after undergoing an eight-hour in-laboratory polysomnography for one night. The OSAS severity assessment depends on the number of breathing disturbances per hour of sleep. EDS was defined using the Epworth Sleepiness Scale (ESS) scores of ≥ 13. Hypertension was defined according to direct cuff blood pressure (BP) measurements. Beat-to-beat R-R interval data were incorporated in polysomnography for heart rate variability analysis. The low-frequency/high-frequency band ratio was used to reflect sympathovagal balance. The study participants were divided into four groups based on the presence of EDS and/or hypertension: EDS with hypertension (n = 53), EDS without hypertension (n = 27), no EDS with hypertension (n = 38), and no EDS or hypertension (n = 43). Clinical, polysomnographic and heart rate data were compared and studied among the four groups. Plasma acetylcholine (ACh) levels were assessed to explore the effects of the non-neuronal cholinergic system and the co-occurrence of EDS and hypertension.
Results: Patients with EDS and hypertension had more severe OSAS severity indices compared to control patients. Increased cardiac sympathovagal imbalance and nocturnal hypoxemia regulated the presence of EDS and hypertension. Further plasma biomarker analysis revealed that both ESS scores and BP levels were associated with significantly elevated plasma norepinephrine, interleukin-6 and superoxide dismutase levels and significantly decreased ACh levels. Logistic regression analyses showed that ACh was the only factor significantly associated with co-occurrence of EDS and hypertension after controlling for confounders using odds ratio of 0.932, with a 95% confidence interval of 0.868 to 1.000 (P = 0.049).
Conclusion: The results suggested that OSAS coupled with both EDS and hypertension is a more severe phenotype of the respiratory disorder. The presence of EDS and hypertension was accompanied by sympathovagal imbalance, and co-occurrence of these two conditions may be related to decreased plasma ACh levels.
Keywords: acetylcholine, subjective excessive daytime sleepiness, hypertension, obstructive sleep apnea
Introduction
Obstructive sleep apnea syndrome (OSAS) is the most common type of sleep breathing disorder and is characterized by cyclic episodes of complete or partial upper airway obstruction during sleep. Both cross-sectional and longitudinal studies have demonstrated that OSAS is an independent risk for hypertension.1–3 Mechanisms where OSAS may contribute to hypertension include excessive daytime sympathetic vasoconstrictor activity combined with overproduction of superoxide ion and inflammatory effects on resistance vessels.4,5
Excessive daytime sleepiness (EDS) is also common in OSAS patients and is considered an important health problem, leading to road accidents, reduced cognitive performance and psychosocial morbidity. A series of studies have suggested that OSAS coupled with hypertension is associated with higher incidence of EDS, as measured using the Epworth Sleepiness Scale (ESS).6,7 Continuous positive airway pressure (CPAP) therapy is more effective at reducing blood pressure (BP) and preventing hypertension in OSAS patients with EDS than in those without EDS.8,9 These clinical studies have established an association between EDS and hypertension or have possibly suggested that EDS might in some way be related to the pathogenesis of hypertension and that an interaction between hypertension and EDS in OSAS patients may exist. However, no existing study has explored the underlying reasons for co-occurrence of OSAS-related EDS and hypertension.
In OSAS patients, marked increases in sympathetic drive during sleep can persist into the daytime and may contribute to a sustained increase in BP.5,10 Lombardi et al demonstrated that the increased low-to-high frequency power ratio of heart rate variability (HRV) was significantly higher in OSAS patients with EDS than in those without EDS, suggesting that sympathoexcitation may contribute to both OSAS-related EDS and hypertension.11 Other studies have suggested that the neuronal cholinergic system (NCS) is implicated in the pathogenesis of hypertension.12 Suzuki et al13 have speculated that a deficient NCS is accompanied by impaired cognitive performance in OSAS patients and a significant role in wakefulness and breathing for ACh was demonstrated by Otuyama et al in individuals with sleep-disordered breathing.14 These reports revealed that NCS was responsible for regulating the presence of EDS and hypertension. As an interaction has been shown between an NCS and non-neuronal cholinergic system (NNCS),15 an investigation of whether NNCS is also associated with EDS and hypertension in patients with OSAS is needed.
The objective of the present study was to examine whether there were common underlying pathophysiologic characteristics for subjective EDS and hypertension in patients with OSAS. Specifically, it was hypothesized that the NNCS and/or sympathetic system may be correlated with the co-occurrence of OSAS-related EDS and hypertension.
Methods
Study Population
For this study, 1183 patients with suspected sleep-disordered breathing were recruited following an overnight polysomnographic (PSG) examination at the Sleep Center of The Affiliated Huaian No. 1 People’s Hospital of Nanjing Medical University. All participants underwent a detailed clinical interview with a questionnaire about medical history, sleep habits and general health and anthropometric data were also collected. The exclusion criteria included insomnia, narcolepsy, use of medications that could affect blood pressure (BP), BP of >180/120 mmHg, age less than 18 or older than 70 years, atrial fibrillation, autonomic nervous system disease (eg, Raynaud disease, erythromelalgia, and hemiatrophy) that might influence continuous BP measurements, prior hospitalization for cardiac or respiratory issues less than six weeks prior to recruitment. None of the patients were being treated with CPAP at the time they were enrolled in the study. No patients were on cholinergic drugs, sedatives, or hypnotics. Patients with OSAS had to meet an apnea/hypopnea index (AHI) criterion of more than five events per hour. Based on these criteria, a total of 161 OSAS patients were included in the study and further divided into four groups: EDS with hypertension (EDS+/hypertension+, n = 53), EDS without hypertension (EDS+/hypertension−, n = 27), no EDS with hypertension (EDS−/hypertension+, n = 38), and no EDS or hypertension (EDS−/hypertension−, n = 43) (Figure 1). All enrolled patients provided written informed consent before study participation. The study protocol was approved by the Scientific Research and Technology Ethics Committee of Huaian No. 1 People’s Hospital.
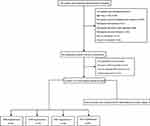 |
Figure 1 Flow chart of the study design and analysis. |
Subjective Daytime Sleepiness
Subjective daytime sleepiness was assessed using the Epworth Sleepiness Scale (ESS), which is the most widely used clinical tool to quantify individual feelings of lethargy and was also used as a criterion for screening OSAS by the American Academy of Sleep Medicine (AASM).16 The scale ranged from 0 to 24, including eight different daytime situations. To qualify for the study, a clinical cut-off point for a total ESS score of 13 was used to define EDS.17
PSG and BP Parameters
All potential participants underwent a full night PSG (SOMNOscreen plus system, SOMNOmedics, Randersacker, Germany) performed by experienced technicians in the sleep laboratory. The OSAS diagnoses and severity were manually analyzed according to the AASM guidelines.16 The following PSG parameters were evaluated: AHI, arousal index (Al), oxygen desaturation index (ODI), the percentage of sleep time with oxygen saturation less than 90% (T90), and the mean (MSpO2) and lowest (LSpO2) SpO2 values during sleep stage. Each participant had their BP assessed when they were referred to the sleep laboratory, where cuff BP values were the average of three consecutive readings during a 5- min period after resting in the supine position for at least 10 min. Hypertension was defined as a systolic BP exceeding 140 mmHg or a diastolic BP exceeding 90 mmHg.18 Non-invasive beat-to-beat blood pressure monitoring was synchronized with PSG using a pulse transit time (PTT)-based method (SOMNOscreen plus system, SOMNOmedics, Randersacker, Germany), where PTT was defined as transmission time of the arterial pulse pressure wave from the aortic valve into the periphery. The stiffness and tension in the arterial walls are major factors determining the speed of the pulse wave transmission, which in turn depends to a significant extent on BP. The PTT calibration was implemented using a cuff measurement of each patient after a supine resting period of 10 min. Using this one-point calibration, accurate BP values were calculated for each PTT value and synchronized with PSG.19 Several reports have shown that BP calculated from PTT using a one-point calibration correlates significantly with BP measured by the cuff method.20 Awake BP was calculated as the average of BP values taken during a supine resting period of 10 min in the awake state, while asleep BP was the average of BP values during the nighttime period.
Sympathovagal Balance
Beat-to-beat RR interval data was incorporated into the PSG and short-term frequency domain analysis as a primary method to study HRV. Spectral-domain analyses were performed on 5-min stationary series. All stationary segments were subjected to spectral analysis using a fast Fourier transform algorithm and were then displayed in different power spectrums as high band (HF), low band (LF) and very low band (VLF). The efferent vagal activity is a major contributor to the HF component, while sympathetic activity is related to spectral power in the LF band. The LF/HF ratio reflects the sympathovagal balance, while an increased LF/HF ratio indirectly indicates sympathetic dominance.21
Plasma Sample Collection and Clinical Assays
To explore the relevant molecular biomarkers for co-occurrence of EDS and hypertension, four to five mL peripheral venous blood samples were obtained from 61 patients prior to PSG and used for further analysis. Blood collected from the indwelling catheter was transferred to an EDTA-containing tube and refrigerated until centrifugation. Plasma was frozen at −80°C until use.
ELISA kits were used for quantitative in vitro determination of serum concentrations of the following potential plasma risk factors for EDS and hypertension in OSAS patients: norepinephrine (NE) and ACh, which reflected sympathetic and NNCS activities, respectively; interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α), which reflected inflammatory stress; and endothelin-1 (ET-1) and superoxide dismutase (SOD), which represented endothelial dysfunction and oxidative stress, respectively (Biovision, Milpitas, CA, USA [for ACh]; Cloud-Clone Corp., Wuhan, China [for NE and SOD]; Neobioscience, Shenzhen, China [for IL-6 and TNF-α]; and MultiSciences, Hangzhou, China [for ET-1]). The minimum detectable dose (MDD) for ACh was 0.5 pg/mL and the assay ranged between 3 and 160 pg/mL, MDD below 24.4 pg/mL for NE with a range between 61.7 and 5000 pg/mL, MDD of 0.055 ng/mL for SOD, with a range between 0.156 and 10 ng/mL, MDD of 1.56 pg/mL for IL-6 with a range between 3.125 and 200 pg/mL, MDD of 0.11 pg/mL for ET-1, with a range between 0.16 and 20 pg/mL and MDD of 7.8 pg/mL for TNF-α, with a range between 15.6 and 1000 pg/mL.
Statistical Analysis
The Kolmogorov–Smirnov and Levine tests were used to assess the normality and homogeneity of the distributions, respectively. The values of normally distributed samples were presented as the mean ± standard error and were compared by ANOVA test. Samples with skewed distributions were expressed as medians, and the Kruskal–Wallis rank-sum test was performed to compare these data. The comparisons between groups after ANOVA or Kruskal–Wallis test were statistically evaluated using post-hoc tests. The chi-square test was performed for comparisons of categorical variables. Multiple linear regression analysis was performed to investigate the relevant clinical risk factors for EDS and hypertension.
To explore the possible risk factors for co-occurrence of OSAS-related EDS and hypertension, correlation analysis among plasma indicators, ESS scores and BP values was performed, followed by logistic regression model analysis with NE, ACh, IL-6, TNF-α, ET-1, and SOD plasma levels as predictors and co-occurrence of EDS and hypertension as outcomes. The relevant sleep parameters, demographic, and anthropometric data were entered into the models as covariables using an enter method. All data analyses were performed using SPSS version 20 statistical software (IBM Corp., Armonk, NY, USA). P values <0.05 were considered statistically significant.
Results
Characteristics of the Study Population and PSG Parameters
Table 1 represents a summary of demographic, clinical, and sleep characteristics of all OSAS patients. A total of 161 participants were made up of 136 men and 25 women aged 23 to 68 years, had a mean BMI of 29.3 ± 4.2 kg/m2, an AHI of 58.4 ± 25.8 events/h, ESS scores of 11.8 ± 5.9, and mean cuff SBP of 138.2 ± 14.9 mmHg. In the study population, 49.7% of the OSAS patients presented with EDS, 56.5% had hypertension and 32.9% exhibited both conditions. At the first pretreatment PSG, 87.6% of the participants presented with severe OSAS. Sleep onset latency differed between the groups with and without EDS. Patients in the EDS+/hypertension+ group had significantly worse sleep-related respiratory parameters AHI, ODI, AI, MSpO2 and T90 compared with the other three subgroups (all P < 0.05), while no significant difference were observed between the EDS−/hypertension+ and EDS+/hypertension− groups and both were worse than the EDS−/hypertension− group. Moreover, LF/HF showed a similar trend. In contrast, other data, including age, tobacco use, alcohol consumption, presence of coronary heart disease, diabetes, and TST, were comparable among the four subgroups.
 |
Table 1 Demographic, Clinical, Sleep Characteristics and LF/HF Ratio of Four OSAS Subgroups |
Clinical Predictors of EDS and Hypertension
Table 2 represents the clinical risk factors for EDS and hypertension. After adjusting for age, gender, neck and waist circumferences, BMI, and tobacco and alcohol use in multiple linear regression models, ESS scores were significantly associated with MSpO2 (β = −0.252, P = 0.003) and LF/HF ratio (β = 0.194, P = 0.005). Awake SBP was negatively correlated with LSpO2 (β = −0.230, P = 0.031) and positively with LF/HF ratio (β = 0.229, P = 0.007). Results were similar when the model was conducted with asleep SBP as the dependent variable for T90 (β = 0.293, P = 0.027) and LF/HF ratio (β = 0.214, P = 0.009), where AI was not associated with ESS scores or BP values (all P > 0.05).
 |
Table 2 The Associations Between ESS Scores, BP Parameters, PSG and Demographic Variables, as Well as LF/HF Ratio in Patients with OSAS |
Plasma Biomarker Result Analysis
Due to the unbalanced distribution among the four groups of patients who provided the plasma biomarker data, 61 patients (EDS+/hypertension+, n = 33; EDS+/hypertension−, n = 0; EDS−/hypertension+, n = 13; EDS−/hypertension−, n = 15) were reanalyzed to represent the entire cohort. The baseline data for these patients are presented in Table 3. Unadjusted correlation analyses were performed among ESS scores, awake SBP, and plasma biomarkers. Both higher ESS scores and awake BP levels were associated with significantly elevated plasma NE (ESS: r = 0.272, P = 0.034; awake SBP: r = 0.497, P < 0.001), IL-6 (ESS: r = 0.321, P = 0.012; awake SBP: r = 0.448, P < 0.001) and SOD (ESS: r = 0.371, P = 0.003; awake SBP: r = 0.324, P = 0.011) levels. Plasma ACh level was negatively correlated with ESS scores (r = −0.442, P < 0.001) and with awake SBP (r = −0.501, P < 0.001). The ET-1 was only associated with awake SBP (r = 0.329, P = 0.010), but not with ESS scores (r = 0.158, P = 0.223), while TNF-α level was not associated with either ESS scores or awake BP value (both P > 0.05) (Table 3). When EDS+/hypertension+ (ESS scores of ≥13 and awake SBP of ≥140 mmHg) was modeled as a binary outcome, logistic regression models revealed that co-occurrence of both EDS and hypertension was associated with decreased plasma ACh levels (odds ratio = 0.971, 95% confidence interval: 0.948 to 0.994, P = 0.016) compared to OSAS without the two conditions as (Model 1 in Table 4). After adjusting for the potentially relevant baseline data of (age, etc.) and PSG parameters (AHI, etc.), the regression model results were similar (Model 2 and 3 in Table 4). In contrast, the remaining plasma biomarkers did not have significantly increased odds of co-occurrence with EDS and hypertension.
 |
Table 3 Demographic and Sleep Characteristics, Plasma Biomarkers and the Correlations Between ESS Cores and BP Values in Patients with OSAS |
 |
Table 4 Predictors of the Co-Occurrence of EDS and Hypertension |
Discussion
Previous studies have reported conflicting results for the association between EDS and hypertension in OSAS patients. Kapur et al found that the association between sleep-disordered breathing and hypertension is stronger in individuals who have reported EDS than in those who have not.6 Goldstein et al predicted that patients with EDS will have a higher BP and will be more likely to develop hypertension after five years compared to patients who have shown few symptoms of daytime sleepiness.22 Conversely, Tam et al found that the ESS scores in normotensive moderate-to-severe OSAS patients were significantly higher than those in hypertensive patients.23 After examining 1649 OSAS patients, Roure et al have found that BP parameters were similar in individuals with and without EDS.24 This inconsistency might be due to the OSAS severity, participant age, and/or the study sample size. In addition, ESS is a simple, self-administered and subjective sleepiness questionnaire for evaluating the propensity to sleep during the day, which could introduce a certain degree of misclassification. Therefore, the methods for assessing EDS or the clinical cut-off point to define EDS might also explain these conflicting results. The present study aimed to explore the potential risk factors for co-occurrence of subjective EDS and hypertension, so accurate identification of EDS was very important. According to the ESS, a score between 0 and 10 was within the normal range, a score of 10 represented the upper limit of normal, while scores of 16 or more, indicating of daytime sleepiness, were found only in patients with OSAS of at least moderate severity.25 A plethora of studies have relied on a clinical total ESS score cut-off point exceeding 10 to define subjective EDS, but a previous study has reported that some participants with EDS where ESS scores exceed 10 had an AHI of less than five events per hour,26 suggesting that this cut-off value has a limited predictability for patients with suspected OSAS. This dichotomization for both clinical screening and research purposes still has some limitations, and supporting evidence for this particular cut-off point is limited.27 According to the original description by Aurora et al,17 an established correlation was found between subjective assessments of sleepiness, where ESS scores of ≥13 optimally predicted a mean sleep latency of <8 min on a multiple sleep latency test (MSLT). Based on theoretical considerations, a clinical cut-off point for a total ESS score of 13 was used to define EDS in the current study. Like the previous studies, ESS scores positively correlated with BP values in the present study. In addition, OSAS patients with EDS and hypertension exhibited even more severe sleep disorders than those with hypertension or EDS alone. Additionally, contributions from indices of sympathetic activity, oxidative stress, inflammation, or endothelial dysfunction to the relationship between hypertension and EDS were not found. In contrast, non-neuronal ACh deficiency was associated with co-occurrence of subjective EDS and hypertension.
The OSAS-related hypertension is thought to be induced by multifactorial mechanisms, mainly including sympathetic hyperactivity caused by repeated intermittent hypoxia, which might even occur during daytime normoxia.28 On the other hand, a pilot study by Donadio et al demonstrated that the mechanism that induced EDS in OSAS was related to the degree of daytime sympathetic hyperactivity.29 Lombardi et al11 reported that both LF and HF power tended to be lower in OSAS with EDS, although not significantly. In this study, the EDS group had a significantly higher nighttime LF/HF ratio compared to non-EDS patients, where the increased LF/HF ratio indirectly indicated the prevalence of sympathetic over parasympathetic cardiac modulation. These clinical findings suggested that sympathetic activity associated with EDS in OSAS may allow the identification of one of the mechanisms potentially associated with an increased cardiovascular risk in patients with this condition. Unlike the study by Bisogni et al30 where neither LF power nor LF/HF differed between OSAS patients with and without subjective EDS. Similarly, Sforza et al31 found that all frequency-domain data for the HRV did not reveal any differences between non-sleepy and sleepy participants (assessed by ESS scores) in a study of 825 elderly individuals. The present study observed that both ESS scores and BP values were significantly associated with LF/HF ratio after adjusting for the potential confounders in linear regression models. No association was found in logistic regression models between co-occurrence of two phenomena and increased sympathetic activity assessed using plasma NE levels. No significant contribution to arousal in EDS was found, while nocturnal hypoxemia was shown to be the most correlative clinical predictor of subjective EDS. One explanation for these disparate results is that EDS evaluations were performed by different methods. The ESS rating embodies a patient’s overall or average propensity or trait for falling asleep, whereas the MSLT uses physiological data to assess a state or rate of falling sleep.17 Given that objectively and subjectively measured EDS may reflect distinct aspects of sleepiness, some disagreements in findings are to be expected, so it is speculated that objective EDS, which is measured by the MSLT and is sensitive to the arousal system, is closely associated with sympathetic nervous system overactivity.11,32 In contrast, subjective EDS, which is assessed by ESS and reflects daytime alertness and sleepiness, is correlated with the severity of nocturnal hypoxemia and is closely associated with NNCS depression.
Extensive research effort has resulted in a substantial body of evidence that is consistent with the role of central cholinergic mechanisms that were implicated in the pathogenesis of hypertension.33 Evidence has shown that hypertensive individuals and those in the early stages of hypertension also have an increased sympathetic and a reduced cardiac vagal drive.34 It can also be inferred that from normotension to mild and more severe degrees of BP elevation, there is a gradual reduction in bradycardic and tachycardic responses to baroreceptor stimulation and deactivation, which is a major mechanism of heart rate control.35 These responses were largely reduced or even abolished via administration of atropine,36 which was likely explained by the hypertension-related alteration of the parasympathetic division of the autonomic nervous system. These studies have provided evidence that both NCS and NNCS may be involved in the development of hypertension. On the other hand, activation of ACh receptors by ACh itself and by nicotinamide promotes cortical activation and wakefulness.37 According to Eggers et al, ACh degradation by acetylcholinesterase (AChE) in the brainstem was lower in patients with Alzheimer’s disease presenting with sleepiness than in patients without sleep disturbances.38 Donepezil, a reversible AChE inhibitor, has been approved to enhance cholinergic transmission. When administered to patients presenting with opiate-type dementia, it can improve daytime sleepiness symptoms, as assessed by ESS scores,39 in narcolepsy patients, donepezil has also been reported to improve ESS scores,40 and it can also alleviate subjective EDS in patients with OSAS.41 Neural ACh therefore plays a significant role in wakefulness physiology and reduced ACh levels may be associated with subjective sleepiness in patients with OSAS.
These studies are the first to report that subjective daytime sleepiness is associated with reduced plasma ACh levels. Similarly, a pilot study by Pak et al showed that lower levels of plasma choline metabolites are linked with subjective sleepiness.42 In contrast, Reale et al observed a significant reduction in choline in patients with OSAS compared with healthy controls, suggesting NNCS dysfunction may be associated with OSAS. However, no correlation was found between plasma choline levels and ESS scores.43 Causes of the discrepancies in these studies remain unclear. Also, we cannot explain the association mechanism between lower plasma ACh levels and self-reported sleepiness. According to the study by Saw et al, neuronal cholinergic tone is diminished because of the impaired ability of the NNCS to either produce or release ACh.15 Malow et al have revealed that intermittent peripheral stimulation of the vagus nerve could improve subjective EDS in epilepsy patients.44 These results imply a cross-talk mechanism between the NNCS and NCS that regulates both. For NCS impairment induced by OSAS-related neuroinflammation, an intriguing hypothesis that arises from these findings is that decreased plasma ACh level is the result of NCS dysfunction or vice versa.
Limitations
Although this work revealed important discoveries, it was a preliminary study with some limitations that should be addressed. There may have been patient bias, as most of the study participants had an AHI of >30. Second, the study had a small sample size and only 61 blood samples were collected and analyzed. Plasma biomarkers should be obtained and evaluated from more participants, which may better define the relationship between EDS and hypertension.
Conclusions
This study showed that depression of NNCS is associated with co-occurrence of hypertension and self-reported EDS in patients with OSAS. Future prospective studies that incorporate large samples and different EDS measures are needed to explore the possible mechanisms of the two different EDS dimensions, as well as the common molecular OSAS pathways associated with objective EDS and hypertension.
Ethics Approval and Consent to Participate
The Scientific Research and Technology Ethics Committee of the Affiliated Huaian No. 1 People’s Hospital of Nanjing Medical University approved the study. Informed written consent was obtained from all participants prior to the study. All procedures performed in the studies involving human participants were in accordance with the 1964 Helsinki declaration.
Acknowledgments
The authors are grateful to the patients of the study. The authors also extend their appreciation to the colleagues at the Sleep Center of The Affiliated Huaian No. 1 People’s Hospital of Nanjing Medical University.
Funding
The study was supported by the National Natural Science Foundation of China (81900084).
Disclosure
The authors declare no conflicts of interest for this work.
References
1. Marin JM, Agusti A, Villar I, et al. Association between treated and untreated obstructive sleep apnea and risk of hypertension. JAMA. 2012;307(20):2169–2176. doi:10.1001/jama.2012.3418
2. Lavie P, Herer P, Hoffstein V. Obstructive sleep apnoea syndrome as a risk factor for hypertension: population study. BMJ. 2000;320(7233):479–482. doi:10.1136/bmj.320.7233.479
3. Peppard PE, Young T, Palta M, et al. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342(19):1378–1384. doi:10.1056/NEJM200005113421901
4. Phillips BG, Somers VK. Hypertension and obstructive sleep apnea. Curr Hypertens Rep. 2013;5(5):43–52. doi:10.1007/s11906-010-0112-8
5. Weiss JW, Tamisier R, Liu Y. Sympathoexcitation and arterial hypertension associated with obstructive sleep apnea and cyclic intermittent hypoxia. J Appl Physiol. 2015;119(12):1449–1454. doi:10.1152/japplphysiol.00315.2015
6. Kapur VK, Resnick HE, Gottlieb DJ, et al. Sleep disordered breathing and hypertension: does self-reported sleepiness modify the association? Sleep. 2008;31(8):1127–1132.
7. Feng J, He QY, Zhang XL, Chen BY. Epworth Sleepiness Scale may be an indicator for blood pressure profile and prevalence of coronary artery disease and cerebrovascular disease in patients with obstructive sleep apnea. Sleep Breath. 2012;16(1):31–40. doi:10.1007/s11325-011-0481-5
8. Robinson GV, Smith DM, Langford BA, Davies RJ, Stradling JR. Continuous positive airway pressure does not reduce blood pressure in nonsleepy hypertensive OSA patients. Eur Respir J. 2006;27(6):1229–1235. doi:10.1183/09031936.06.00062805
9. Robinson GV, Langford BA, Smith DM, Stradling JR. Predictors of blood pressure fall with continuous positive airway pressure (CPAP) treatment of obstructive sleep apnoea (OSA). Thorax. 2008;63(10):855–859. doi:10.1136/thx.2007.088096
10. Weiss JW, Tamisier R, Liu Y. Sympathoexcitation and arterial hypertension associated with obstructive sleep apnea and cyclic intermittent hypoxia. J Appl Physiol. 2015;119(12):1449–1454. doi:10.1152/japplphysiol.00315
11. Lombardi C, Parati G, Cortelli P, et al. Daytime sleepiness and neural cardiac modulation in sleep-related breathing disorders. J Sleep Res. 2010;17(3):263–270. doi:10.1111/j.1365-2869.2008.00659.x
12. Lataro RM, Silva CA, Tefé-Silva C, Prado CM, Salgado HC. Acetylcholinesterase inhibition attenuates the development of hypertension and inflammation in spontaneously hypertensive rats. Am J Hypertens. 2015;28(10):1201–1208.
13. Suzuki K, Miyamoto M, Miyamoto T, et al. Parkinson’s disease and sleep/wake disturbances. Curr Neurol Neurosci Rep. 2015;15(3):8. doi:10.1007/s11910-015-0525-5
14. Otuyama LJ, Rizzi CF, Piovezan RD, et al. The cholinergic system may play a role in the pathophysiology of residual excessive sleepiness in patients with obstructive sleep apnea. Med Hypotheses. 2013;81(3):509–511. doi:10.1016/j.mehy.2013.06.024
15. Saw EL, Kakinuma Y, Fronius M, Katare R. The non-neuronal cholinergic system in the heart: a comprehensive review. J Mol Cell Cardiol. 2018;125:129–139.
16. Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;8(5):597–619. doi:10.5664/jcsm.2172
17. Aurora RN, Caffo B, Crainiceanu C, Punjabi NM. Correlating subjective and objective sleepiness: revisiting the association using survival analysis. Sleep. 2011;34(12):1707–1714. doi:10.5665/sleep.1442
18. Perloff D, Grim C, Flack J, et al. Human blood pressure determination by sphygmomanometry. Circulation. 1993;88(5Pt1):2460–2470. doi:10.1161/01.cir.88.5.2460
19. Bilo G, Zorzi C, Ochoa Munera JE, Torlasco C, Giuli V, Parati G. Validation of the Somnotouch-NIBP noninvasive continuous blood pressure monitor according to the European Society of Hypertension International Protocol revision 2010. Blood Press Monit. 2015;20(5):291–294. doi:10.1097/MBP.0000000000000124
20. Gesche H, Grosskurth D, Küchler G, et al. Continuous blood pressure measurement by using the pulse transit time: comparison to a cuff-based method. Eur J Appl Physiol. 2012;112:309–315. doi:10.1007/s00421-011-1983-3
21. Fred S, Ginsberg JP. An overview of heart rate variability metrics and norms. Front Public Health. 2017;5:258. doi:10.3389/fpubh.2017.00258
22. Goldstein IB, Sonia AI, David S. Relationship between daytime sleepiness and blood pressure in healthy older adults. Am J Hypertens. 2004;17(9):787–792. doi:10.1016/j.amjhyper.2004.05.009
23. Tam W, Ng SS, To KW, Hui DS. The interaction between hypertension and obstructive sleep apnea on subjective daytime sleepiness. J Clin Hypertens. 2019;21(3):390–396. doi:10.1111/jch.13485
24. Roure N, Gomez S, Mediano O, et al. Daytime sleepiness and polysomnography in obstructive sleep apnea patients. Sleep Med. 2008;9(7):727–731. doi:10.1016/j.sleep.2008.02.006
25. Johns MW, New A. Method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep. 1992;14(6):540–545. doi:10.1093/sleep/14.6.540
26. Kapur VK, Baldwin CM, Resnick HE, Gottlieb DJ, Nieto J. Sleepiness in patients with moderate to severe sleep-disordered breathing. Sleep. 2005;28(4):472–477. doi:10.1093/sleep/28.4.472
27. Trimmel K, Ebrowska M, Bck M, et al. Wanted: a better cut-off value for the Epworth Sleepiness Scale. Wien Klin Wochenschr. 2018;130(9–10):349–355. doi:10.1007/s00508-017-1308-6
28. Venkataraman S, Vungarala S, Covassin N, Somers VK. Sleep apnea, hypertension and the sympathetic nervous system in the adult population. J Clin Med. 2020;9(2):591. doi:10.3390/jcm9020591
29. Donadio V, Liguori R, Vetrugno R, et al. Daytime sympathetic hyperactivity in OSAS is related to excessive daytime sleepiness. J Sleep Res. 2007;16(3):327–332. doi:10.1111/j.1365-2869.2007.00602.x
30. Bisogni V, Pengo MF, Drakatos P, et al. Excessive daytime sleepiness does not correlate with sympathetic nervous system activation and arterial stiffening in patients with mild-to-moderate obstructive sleep apnoea: a proof-of-principle study. Int J Cardiol. 2017;236:458–461. doi:10.1016/j.ijcard.2017.01.149
31. Sforza E, Pichot V, Martin MS, Barthélémy JC, Roche F. Prevalence and determinants of subjective sleepiness in healthy elderly with unrecognized obstructive sleep apnea. Sleep Med. 2015;18(8):981–986. doi:10.1016/j.sleep.2015.03.010
32. Bonnet MH, Arand DL. Impact of motivation on multiple sleep latency test and maintenance of wakefulness test measurements. J Clinical Sleep Med. 2005;1(4):386–390. doi:10.5664/jcsm.26367
33. Kubo T. Cholinergic mechanism and blood pressure regulation in the central nervous system. Brain Res Bull. 1998;46(6):475–481. doi:10.1016/s0361-9230(98)00041-0
34. Mancia G, Grassi G. The autonomic nervous system and hypertension. Circ Res. 2014;114(11):1804–1814. doi:10.1161/CIRCRESAHA.114.302524
35. Grassi G, Cattaneo BM, Seravalle G, et al. Baroreflex control of sympathetic nerve activity in essential and secondary hypertension. Hypertension. 1998;31:68–72. doi:10.1161/01.hyp.31.1.68
36. Mancia G, Bonazzi O, Pozzoni L, et al. Baroreceptor control of atrioventricular conduction in man. Circ Res. 1979;44:752–758. doi:10.1161/01.res.44.6.752
37. Watson CJ, Baghdoyan HA, Lydic R. Neuropharmacology of sleep and wakefulness: 2012 update. Sleep Med Clin. 2012;7(3):469–486. doi:10.1016/j.jsmc.2012.06.010
38. Eggers C, Szelies B, Bauer B, et al. Imaging of acetylcholine esterase activity in brainstem nuclei involved in regulation of sleep and wakefulness. Eur J Neurol. 2010;14(6):690–693. doi:10.1111/j.1468-1331.2007.01737.x
39. Slatkin NE, Rhiner M. Treatment of opiate-related sedation: utility of the cholinesterase inhibitors. J Support Oncol. 2003;1(1):53–63.
40. Niederhofer H. Donepezil in the treatment of narcolepsy. J Clin Sleep Med. 2006;2(1):71–72. doi:10.1016/j.rehab.2011.07.496
41. Sukys-Claudino L, Moraes W, Guilleminault C, Tufik S, Poyares D. Beneficial effect of donepezil on obstructive sleep apnea: a double-blind, placebo-controlled clinical trial. Sleep Med. 2012;13(3):290–296. doi:10.1016/j.sleep.2011.09.014
42. Pak VM, Dai F, Keenan BT, Gooneratne NS, Pack AI. Lower plasma choline levels are associated with sleepiness symptoms. Sleep Med. 2017;44:89–96. doi:10.1016/j.sleep.2017.10.004
43. Reale M, Velluto L, Nicola MD, et al. Cholinergic markers and cytokines in OSA patients. Int J Mol Sci. 2020;21(9):3264. doi:10.3390/ijms21093264
44. Malow BA, Edwards J, Marzec M, Sagher O, Ross D, Fromes G. Vagus nerve stimulation reduces daytime sleepiness in epilepsy patients. Neurology. 2001;57(5):879–884. doi:10.1212/WNL.57.5.879
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
