Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 13
Delays in Insulin Initiation among Patients with Type 2 Diabetes Mellitus in Southeast China: A Retrospective, Real-World Study
Authors Chen P , Ma X, Chen H, Wang K, Zhou L
Received 1 April 2020
Accepted for publication 10 July 2020
Published 25 August 2020 Volume 2020:13 Pages 3059—3068
DOI https://doi.org/10.2147/DMSO.S256381
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Antonio Brunetti
Pin Chen,1 Xiao Ma,2 Hong Chen,2 Ke Wang,2 Li Zhou2
1Department of Endocrinology, 900 Hospital of the Joint Logistics Team, Fuzhou Clinical Medical College of Fujian Medical University, Fuzhou, People’s Republic of China; 2Lilly Suzhou Pharmaceutical, Shanghai, People’s Republic of China
Correspondence: Li Zhou
Lilly Suzhou Pharmaceutical, Shanghai, People’s Republic of China
Email [email protected]
Purpose: To describe the extent of delays in insulin initiation, analyze its impact on glycemic control, and explore factors influencing delayed insulin initiation among Chinese type 2 diabetes mellitus (T2DM) patients.
Methods: A real-world, retrospective cohort study with regional electronic health records from Fuzhou, southeast China was conducted among T2DM patients. Adult patients uncontrolled with oral antidiabetic drugs (OADs; HbA1c ≥ 7%) and initiated on insulin treatment were included. Time to insulin initiation was described. After propensity-score matching, Wilcoxon rank-sum test and chi-square test were used to compare follow-up HbA1c (first HbA1c 3 months after insulin initiation) between timely (initiated insulin within 6 months after OAD failure) and delayed (initiated after 6 months) insulin-initiation groups. Sensitivity analysis was also performed by linear and logistic regression. Factors associated with delayed insulin initiation were explored using logistic regression.
Results: A total of 940 patients were included, with mean±SD age 66.3± 11.9 years. In sum, 328 had HbA1c recorded 3 months after insulin initiation. After propensity-score matching (1:1 matching), 184 patients were included for further analysis. Median follow-up HbA1c was lower in the timely-initiation group than the delayed-initiation group (7.25% vs 8.25%, P=0.009). Patients in the timely initiation group also had higher odds of achieving HbA1c < 7% (OR=3.15, P=0.001). Results were confirmed by logistic regression. Hypertension, coronary artery disease, baseline HbA1c, and hospital level at insulin initiation were associated with delays in insulin initiation.
Conclusion: Timely insulin initiation after OAD failure is associated with better glycemic control.
Keywords: type 2 diabetes mellitus, therapeutic inertia, delayed insulin initiation, glycemic control, HbA1c
Introduction
The worldwide prevalence of diabetes mellitus (DM) is increasing, and the estimated burden is expected to be 693 million cases by the year 2045.1 The prevalence of DM was estimated to be 10.9% in Chinese adults in a 2013 national survey, and about half of treated patients (50.8%) had inadequate glycemic control.2,3 DM is associated with increased risks of retinopathy and nephropathy as well as a two- to fourfold-increased risk of cardiovascular diseases.4
The target of glucose control for most DM patients is glycosylated hemoglobin (HbA1c) <7% (53 mmol/mol). Although oral antidiabetic drugs (OADs) are administered, due to disease progression and deterioration of pancreatic β-cell function, some type 2 DM (T2DM) patients who are unable to control blood glucose with OADs often require treatment intensification with insulin to maintain target HbA1c levels.5 The American Diabetes Association (ADA), European Association for the Study of Diabetes (EASD), and China Diabetes Society (CDS) recommend treatment intensification if target HbA1c has not been achieved after 3 months. However, delays in treatment intensification with insulin are common among patients with T2DM.6,7 Therapeutic inertia, defined as the failure to initiate or intensify therapy in a timely manner, is one of the main reasons for uncontrolled hyperglycemia in T2DM according to evidence-based clinical guidelines.8 Delays in insulin initiation after OAD failure is common in patients with T2DM, and this reluctance toward insulin initiation could be due to patient-, physician-, or health-care system–related factors.9
Delays in treatment intensification can occur at all stages of T2DM treatment: from initiation of oral therapy after failure of lifestyle modification with proper diet and regular exercise, use of OADs and insulin as combination therapy, or intensification of insulin. Previous studies conducted in other countries have demonstrated the negative impact of delays in treatment intensification on glycemic control,10 but there is a paucity of data from China, even though T2DM has becoming a major public health challenge in China. As such, the purpose of this retrospective study using a regional electronic medical record database in southeast China was to provide real-world evidence of the extent of delays in insulin initiation, the impact of delays in insulin initiation on glycemic control after insulin initiation, and to explore the potential factors related to delays in insulin initiation among Chinese patients with T2DM.
Methods
Data Source
This study extracted data from a regional electronic health–record database in Fuzhou, southeast China. This regional database, a pilot of the China National Health Big Data project, contains major clinical information from multiple information systems of 37 hospitals. It includes information on >2 billion medical records belonging to 23 million patients from September 2001 to January 2018. The database contains major clinical information systems, such as hospital information systems, laboratory information systems, electronic medical records, and picture archiving and communication systems. Data collection, processing, and management was authorized by the Fuzhou Health Commission. All data were structured, standardized, and managed in an integrated platform.11 The database is not freely available, so review and approval were obtained from China Electronics Corporation data.
Study Design and Patient Population
In this retrospective cohort study, patients with T2DM (as defined by ICD10 or previously treated with OADs) aged ≥18 years who had been initiated on insulin treatment after failure of an OAD regimen were identified. Specifically, patients were included in the study if they had a minimum of one record of insulin prescription, OAD prescription prior to insulin initiation and a minimum of one record of preinitiation HbA1c ≥7%, and a minimum of one record of postinitiation HbA1c. Patients were excluded if insulin had been prescribed only transiently after OAD failure to control blood glucose during surgery or patients who had received glucagon-like peptide-1 receptor agonist (GLP-1RA). This study was approved by the China Ethics Committee of Registering Clinical Trials (ChiECRCT-20,180,224). The study was designed and conducted in accordance with the ISPE Guidelines for Good Pharmacoepidemiology Practice. This observational study used data previously collected, and did not impose any form of intervention. Therefore, informed consent was not required.
Definitions
The last OAD regimen was defined as the OADs used for treatment just before insulin initiation. The index date was defined as the date of OAD failure. If preinitiation HbA1c levels were always ≥7%, the index date (OAD failure) was the date of first HbA1c reading ≥7%. If HbA1c levels were not always ≥7%, the index date (OAD failure) was the date of first HbA1c ≥7% following the last HbA1c <7%. Preinitiation HbA1c, defined as HbA1c in the 3 months after the first prescription of the last OAD regimen to insulin initiation, was used to identify the index date.
In our study, delayed insulin initiation was defined as lack of insulin initiation within 6 months after OAD failure. The period between the index date and insulin initiation was considered the time to insulin initiation. Patients were divided into two groups according to time to insulin initiation: timely initiation (≤6 months) and delayed initiation (>6 months). HbA1c measured on the index date was considered baseline HbA1c. Follow-up HbA1c, defined as first HbA1c measured 3 months after insulin initiation, was used for comparison between the delayed-initiation and timely-initiation groups.
Study Outcomes
The study outcome was the extent of delays in insulin initiation after OAD-regimen failure in patients with uncontrolled DM. The number of patients initiated on insulin treatment within 3 months, 3–6 months, and after 6 months was estimated. Also, the impact of delays in insulin initiation on glycemic control was assessed. This was done by comparing follow-up HbA1c levels, and follow-up HbA1c-target attainment (<7%) between the delayed-initiation group and timely-initiation group after propensity-score matching (PSM). Furthermore, univariate and multivariate logistic/linear regression models were used for sensitivity analyses to investigate the association between therapeutic inertia and glycemic control. Factors associated with delays in insulin initiation were also assessed by multivariate logistic regression.
Statistical Analysis
Descriptive statistics (means, SD, medians, IQRs, frequency, and percentages) are used to report continuous and categorical variables. PSM was performed to balance the differences in confounding variables between the delayed-initiation and timely-initiation groups. Based on literature reviews and6,12 clinical and research experience, variables selected from baseline characteristics for PSM were age-group, sex, last OAD regimen, baseline HbA1c, hypertension, dyslipidemia, coronary artery disease, and hospital level at insulin initiation. A 1:1 greedy PSM algorithm using calipers of a specific width was deployed to compare patients between the delayed-initiation and timely-initiation groups. The best matches were defined as pairs with the highest digit match (0.0001) on PS. The matching algorithm proceeded sequentially to the next-highest digit match. No more matches were made below the lowest allowed digit of 0.1.
After PSM, Wilcoxon rank-sum and chi-square tests were performed to compare follow-up HbA1c levels and target-HbA1c attainment between the delayed- and timely-initiation groups, respectively. In order to further analyze the association between delays in insulin initiation and glycemic control, linear and logistic regression were used as sensitivity analyses, with delays in insulin initiation serving as the independent variable, continuous follow-up HbA1c levels or categorical follow-up HbA1c (<7% or ≥7%) as the dependent variable, and the same variables used for matching in PSM as covariates. Box–Cox transformation was used to achieve approximate normality of the distribution of continuous follow-up HbA1c levels to make them compatible for linear regression.13 HbA1c <7% was given the response profile “1” and HbA1c ≥7% the response profile of “0”.
The potential factors possibly associated with delays in insulin initiation — age-group, sex, last OAD regimen, baseline HbA1c, hypertension, dyslipidemia, coronary artery disease, stroke, therapeutic department, and hospital level — at insulin initiation were explored using the univariate and multivariate logistic regression models, and ORs with 95% CIs were calculated. Two-tailed P<0.05 was considered statistically significant. When conducting logistic regression, delayed-initiation patients were given the response profile “1” and timely-initiation patients the response profile “0”. All statistical analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC).
Results
Baseline Characteristics
A total of 940 patients were found eligible, of which 328 had HbA1c measurement available after insulin initiation (Figure 1). The mean±SD age of the patients included in the study was 66.3±11.9 years, and 51.5% were female. The majority (82.9%) of patients had been treated with two or more OADs prior to insulin initiation. Mean±SD baseline HbA1c was 9.4%±1.9%, with 49% of patients having an HbA1c ≥9% (Table 1). The 328 patients with available HbA1c measurements were subjected to PSM. After PSM matching, 184 patients (92 in each group) were included for further analysis.
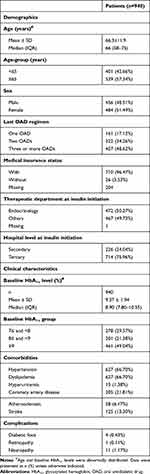 |
Table 1 Demographic and Clinical Characteristics of Enrolled Patients |
 |
Figure 1 Screening of eligible patients. |
Delays in Insulin Initiation in Chinese Patients
Among 940 T2DM patients who had failed to have blood glucose controlled on OAD treatment, 615 (65.4%), 64 (6.8%), and 261 (27.8%) were initiated on insulin within 3 months, 3–6 months, and after 6 months, respectively, which indicated that more than a quarter of patients experienced delays in insulin initiation (>6 months) in this study.
Impact of Delays in Insulin Initiation on Glycemic Control
The impact of delays in insulin initiation in both the delayed- and timely-initiation groups was assessed by considering glycemic control after insulin initiation. The 328 patients who had HbA1c records available for 3 months after insulin initiation were eligible for PSM. All baseline and clinical characteristics were well balanced after PSM (Supplementary Table 1). After PSM, 184 patients were used for comparison between the delayed- and timely-initiation groups, with 92 patients in each group. Among 184 PSM patients, Wilcoxon rank-sum test revealed median HbA1c levels to be significantly lower in the timely-initiation group than the delayed-initiation group (7.25% vs 8.25%, P=0.009), suggesting the beneficial impact of early initiation of insulin therapy (Table 2). In sum, 38% of patients in the timely-initiation group attained the treatment goal of HbA1c <7% after 3 months of insulin treatment, while the corresponding figure of patients attaining the treatment goal in the delayed-initiation group was only 16% (P=0.001) after PSM (Table 3). The sensitivity analyses were conducted among 328 patients using linear and logistic regression models to further depict the association between delays in insulin initiation and glycemic control and to adjust for confounders. Delays in insulin initiation served as the independent variable, and continuous (linear) or categorical follow-up HbA1c levels (logistic) served as the dependent variable. Since the distribution of continuous HbA1c levels were skewed, a Box–Cox transformation was performed before inclusion as a dependent variable in linear regression. Further, to rule out the effect of confounding factors, such as age, sex, last OAD regimen, baseline HbA1c, hypertension, dyslipidemia, and coronary artery disease, at baseline and hospital level at insulin initiation, these factors were adjusted in the regression model. Multivariate linear regression showed that there was no statistically significant difference in Box–Cox transformed mean follow-up HbA1c levels between the groups (P=0.193, Supplementary Table 2). Multivariate logistic regression revealed the timely-initiation group had higher odds of attaining the treatment goal (OR 2.52, 95% CI 1.26–5.04; P=0.009) than the delayed-initiation group (Supplementary Table 3).
 |
Table 2 Wilcoxon Rank-Sum Test of Median Follow-Up HbA1c Levels (%) Before and After PSM |
 |
Table 3 Chi-Square Test of Follow-Up HbA1c-Goal Attainment (<7%) Before and After PSM |
Factors Associated with Delays in Insulin Initiation
A logistic regression model was also used to explore potential factors associated with delays in insulin initiation. Multivariate logistic regression analysis revealed that presence of one OAD regimen (OR 1.59, 95% CI 1.04–2.41; P=0.03) or a double-OADs regimen (OR 1.44, 95% CI 1.03–2.03; P=0.034) prior to insulin initiation, HbA1c level 7%–8% (OR 3.09, 95% CI 2.16–4.41; P<0.001) or 8%–9% (OR 2.47, 95% CI 1.66–3.67; P<0.001), tertiaryclass hospital (OR 1.95, 95% CI 1.29–2.94; P=0.001), presence of hypertension (OR 1.69, 95% CI 1.17–2.45; P=0.005), and coronary artery disease (OR1.60, 95% CI 1.10–2.34; P= 0.015) were associated with a higher possibility of experiencing delays in insulin initiation (Supplementary Table 4).
Discussion
In this study, delays in insulin initiation (defined as lack of insulin initiation within 6 months after OAD failure) were observed in 27.8% of patients with T2DM. Since there is no standard threshold duration for defining delays in treatment intensification among patients with OAD-treatment failure, different studies have reported different thresholds. In our study, insulin was the only treatment-intensification regimen analyzed. Yu et al showed 57.5% of patients with poor glycemic control receiving monotherapy with metformin were intensified by add-on therapy, including insulin.14 Previously conducted studies have reported significant delays in insulin initiation after OAD failure.15–17 Rubino et al showed estimated delays in insulin initiation of 1.8 years in 25% and 4.9 years in 50% of T2DM patients after OAD failure.16 Trends in delayed insulin initiation and intensification have been observed globally as well. In the Western Pacific region, around 66% of T2DM patients had HbA1c >9% at initiation of insulin, despite 74% having been treated with two or more OADs,18 whereas in Middle East and north African regions, 67.6% had HbA1c >9% at insulin initiation, despite 68.3% of them having been treated with two or more OADs.19 The disparity in the published literature might be due to different definitions used for delays in intensification and also the intensification regimens used. Our study findings substantiate the proportion of delays in insulin treatment in real-world health-care settings in current clinical practice in China.
In our study, an HbA1c level of 7% (53 mmol/mol) or higher was considered the baseline for patient selection. However, other studies have considered HbA1c levels of ≥6.5 or 8%.20,21 Moreover, our study considered treatment intensification as addition of insulin alone after OAD failure. In contrast, other studies have considered addition of another OAD after metformin to intensify the treatment regimen20 or addition of a third OAD or injectable drug to the previous OAD regimen.21 Also, in our study there were many insulin-experienced patients without HbA1c ≥7% prior to insulin initiation (excluded), which indicated that Chinese health-care providers might intensify treatment by referring to fasting or postprandial blood–glucose levels other than HbA1c. Therefore, future research may consider these as supplementary indicators to HbA1c when judging OAD-regimen failure to control blood glucose.
The main analyses revealed that after PSM, median follow-up HbA1c was lower and the percentage of patients achieving the HbA1c target of <7% was higher in the timely-initiation group than the delayed-initiation group. The result of HbA1c-goal attainment was confirmed by sensitivity multivariate logistic regression, but the result of average follow-up HbA1c was different between the Wilcoxon rank-sum test and sensitivity linear regression (Box–Cox transformation). The reason is that the Wilcoxon rank-sum test tests the difference in median follow-up HbA1c level, while linear regression tests the difference in mean Box–Cox transformed follow-up HbA1c level. Also, because of the skewed distribution of follow-up HbA1c levels, medians would be a more robust indicator of central tendency and a better choice to describe average follow-up HbA1c. Our findings are in line with other studies.6,12 Fu and Sheehan used 6 months as the threshold to define delays in insulin initiation, and reported that HbA1c dropped from 9.4% to 7.9% in patients who had had insulin initiation within 6 months of OAD-treatment failure, while, the corresponding change in HbA1c was from 9.0% to 8.2%. The mean change in HbA1c was also significantly greater in the timely-initiation group (−0.33%, 95% CI −0.41% to −0.25%) within 1 year of follow-up, which is in line with our current study. Other studies have also shown large delays in treatment intensification to be associated with poor glycemic levels and increased incidence of vascular complications.16,22,23
Early initiation of insulin is beneficial in recovery and preservation of β-cell function, achieving tight glycemic control, altering disease progression, and preventing cardiovascular risk.24,25 This was highlighted by Goodall et al, who compared the clinical consequences of delayed insulin initiation versus timely initiation. This study reported increased life expectancy (approximately 0.61 years), quality-adjusted life expectation (0.34 years), and also significant reductions in DM-related complications among timely insulin–initiated T2DM patients.15 Another study demonstrating real-world clinical and economic outcomes among early versus delayed insulin initiation reported that 32% of patients that had insulin initiated early showed significant reductions in HbA1c, concluding that early insulin initiation may be cost-effective in controlling hyperglycemia compared to delayed initiation.25
This study also explored potential factors influencing delays in insulin initiation. A low HbA1c level at the index date was one of the factors associated with higher odds of experiencing delays in insulin initiation. Further, HbA1c >9% was the strongest influencing factor for insulin initiation. Our findings are comparable with other similar studies.10,21,26 Additionally, patients receiving one or more OAD regimens in our study were associated with higher odds of experiencing delayed initiation of insulin. This might be because physicians are more likely to increase the dose or add another OAD to intensify treatment in Chinese patients treated with one or two OADs with poor glycemic control. However, patients receiving three or more OADs with poor glycemic control were more likely to be intensified with insulin. Similar findings have been observed in other studies with patients receiving two or more OADs at the time of insulin initiation with HbA1c ≥9%.18,19
In our study, the presence of comorbidities, such as coronary artery diseases and hypertension, was associated with higher odds of delays in insulin initiation. This could be because patient visits are time-constrained, and the physician and patient may have prioritized to treat a comorbidity, which might have led to delays in treatment intensification.28 All these comorbidities have been reported in previous studies to be linked to a likelihood of delays in intensification of therapy.27,29-31 Furthermore, in our study health-care providers in tertiary-care hospitals were less likely to ensure timely initiation of insulin than in secondary hospitals. This could be because patients visit tertiary hospitals mostly when their glucose level is high or they have been unable to be controlled in lower-tier hospitals. This suggests that the clinical situation of patients in tertiary-care hospitals may be different. However, previous studies have shown that health-care providers in tertiary-are hospitals tend to intensify treatment with insulin after OAD failure, because of better knowledge of DM management, updated recommendation guidelines, and less concern about hypoglycemic events.30,31 Furthermore, studies suggest that specialists are more likely to use insulin therapies32 and that they tend to initiate insulin treatment sooner than primary-care physicians.33 This probably reflects a referral bias, with specialists managing the most advanced and complex patients and hence those with the worst glycemic control. However, in this study we had no relevant data to assess differences in delays in insulin initiation between specialists and primary-care physicians.
The cause of delays in insulin initiation is complex. It is challenging for both health-care providers and patients to overcome. On the part of the patient, consequences of hypoglycemia, inconvenience of self-injection and monitoring blood glucose, concerns of weight gain, or the unacceptability of insulin injection could lead to rejection and nonadherence to treatment with insulin. Bailey et al suggested that a consideration of clinical and organizational context is necessary to reinforce timely administration of insulin, especially with respect to time constraints for diagnosis and management of comorbidities, health-care costs, and appreciation of patient concerns.
One of the strengths of this study is that the database considered covers multiple and different levels of hospitals within the city, which allowed for better capturing of medical information and patient visits. In this study, PSM was used and was well balanced to correlate with the timing of insulin initiation and HbA1c outcomes. Univariate and multivariate logistic/linear regression models were additionally used to test the robustness of the PSM results.
Limitations
Our study has limitations that should be taken into consideration. As it was an observational study, we cannot conclude that the association between delayed insulin initiation and poor outcomes is causal, although we included a number of covariates in PSM and regression, which should reduce but not necessarily eliminate residual confounding of unmeasured variables. For instance, such factors as poor economic conditions, poor adherence to treatment, and low health literacy could delay insulin initiation and also make the patients’ ability to control their blood glucose worse, which means that delayed insulin could be a mediated index variable between these factors and follow-up HbA1c. Additionally, the data obtained were confined primarily to Fuzhou, capital of Fujian Province in southeast China, and hence the study findings might not be an ideal representation of clinical practice in other parts of China.
Conclusion
This retrospective cohort analysis showed that more than a quarter (27.8%) of patients with T2DM had delayed initiation of insulin. Delays in timely insulin initiation were associated with poor glycemic control. More insightful studies are required to investigate the long-term benefits of timely insulin initiation and to establish reasons for delays in insulin initiation. This in turn will help health-care providers raise awareness of timely initiation of insulin therapy among patients unresponsive to OADs.
Ethical Approval and Informed Consent
This study was approved by the China Ethics Committee of Registering Clinical Trials (ChiECRCT-20,180,224). Ethical approval was obtained from the Chinese Clinical Trial Registry (ChiCTR), and the ChiECRCT was responsible for the review process. The approval statement reads as follows. This is a retrospective research based on electronic medical records of secondary and tertiary hospitals in Fuzhou. As there is no direct risk toward the objectives, informed consent is not required. The research conforms with medical research ethics and is approved to be conducted in accordance with the research protocol.
Acknowledgments
The authors acknowledge Chunming Li and Hongxin Zhao from Shanghai Synyi Medical Technology for the statistical analysis. The authors also acknowledge medical writing support provided by Dr Arjun B Y, Dr Anuradha Nalli and Dr Kaushik Subramanian from Indegene, India.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data, took part in drafting the article or revising it critically for important intellectual content, gave final approval to the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
Li Zhou, Xiao Ma, and Hong Chen are employees of Lilly. Ke Wang was an employee of Lilly at the time of manuscript preparation. Pin Chen has nothing to disclose. Li Zhou reports personal fees from Eli Lilly and Company during the conduct of the study. The authors report no other potential conflicts of interest for this work.
References
1. Cho NH, Shaw JE, Karuranga S, et al. IDF diabetes atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271–281. doi:10.1016/j.diabres.2018.02.023
2. Wang L, Gao P, Zhang M, et al. Prevalence and ethnic pattern of diabetes and prediabetes in China in 2013. JAMA. 2017;317(24):2515–2523. doi:10.1001/jama.2017.7596
3. Hu C, Jia W. Diabetes in China: epidemiology and genetic risk factors and their clinical utility in personalized medication. Diabetes. 2018;67(1):3–11. doi:10.2337/dbi17-0013
4. American Diabetes Association. 8. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2018. Diabetes Care. 2018;41(Suppl 1):S73–S85. doi:10.2337/dc18-S008.
5. Marín-Peñalver JJ, Martín-Timón I, Sevillano-Collantes C, Del Cañizo-Gómez FJ. Update on the treatment of type 2 diabetes mellitus. World J Diabetes. 2016;7(17):354–395. doi:10.4239/wjd.v7.i17.354
6. Fu AZ, Sheehan JJ. Change in HbA1c associated with treatment intensification among patients with type 2 diabetes and poor glycemic control. Curr Med Res Opin. 2017;33(5):853–858. doi:10.1080/03007995.2017.1292231
7. Society CD.Chinese guideline for the prevention and treatment of type 2 diabetes mellitus (2017 edition). Chin J Diabetes Mellitus. 2018;10(1):4–67.
8. Khunti K, Gomes MB, Pocock S, et al. Therapeutic inertia in the treatment of hyperglycaemia in patients with type 2 diabetes: a systematic review. Diabetes Obes Metab. 2018;20(2):427–437. doi:10.1111/dom.13088
9. Khunti K, Millar-Jones D. Clinical inertia to insulin initiation and intensification in the UK: a focused literature review. Prim Care Diabetes. 2017;11(1):3–12. doi:10.1016/j.pcd.2016.09.003
10. Reach G, Pechtner V, Gentilella R, Corcos A, Ceriello A. Clinical inertia and its impact on treatment intensification in people with type 2 diabetes mellitus. Diabetes Metab. 2017;43(6):501–511. doi:10.1016/j.diabet.2017.06.003
11. Wang X, He Y, Wang T, et al. Lipid-lowering therapy and low-density lipoprotein cholesterol (LDL-C) goal achievement in high-cardiovascular-risk patients in Fuzhou, China. J Cardiovasc Pharmacol Ther. 2020;1074248419899298. doi:10.1177/1074248419899298.
12. Ruiz-Negrón N, Wander C, McAdam-Marx C, Pesa J, Bailey RA, Bellows BK. Factors associated with diabetes-related clinical inertia in a managed care population and its effect on hemoglobin A1c goal attainment: a claims-based analysis. J Manag Care Spec Pharm. 2019;25(3):304–313. doi:10.18553/jmcp.2019.25.3.304
13. Matsumoto T, Ohnishi H, Sato T, et al. Insulin resistance is associated with longitudinal changes of cardiac repolarization heterogeneity in apparently healthy subjects. Cardiol Ther. 2019;8(2):239–251. doi:10.1007/s40119-019-0140-7
14. Yu S, Schwab P, Bian B, Radican L, Tunceli K. Use of add-on treatment to metformin monotherapy for patients with type 2 diabetes and suboptimal glycemic control: A U.S. database study. J Manag Care Spec Pharm. 2016;22(3):272–280. doi:10.18553/jmcp.2016.22.3.272
15. Goodall G, Sarpong EM, Hayes C, Valentine WJ. The consequences of delaying insulin initiation in UK type 2 diabetes patients failing oral hyperglycaemic agents: a modelling study. BMC Endocr Disord. 2009;9:19. doi:10.1186/1472-6823-9-19
16. Rubino A, McQuay LJ, Gough SC, Kvasz M, Tennis P. Delayed initiation of subcutaneous insulin therapy after failure of oral glucose-lowering agents in patients with Type 2 diabetes: a population-based analysis in the UK. Diabet Med. 2007;24(12):1412–1418. doi:10.1111/j.1464-5491.2007.02279.x
17. Nichols GA, Koo YH, Shah SN. Delay of insulin addition to oral combination therapy despite inadequate glycemic control: delay of insulin therapy. J Gen Intern Med. 2007;22(4):453–458. doi:10.1007/s11606-007-0139-y
18. Jabbar A, Mohamed WMIBW, Ozaki R, et al. Patterns and trends in insulin initiation and intensification among patients with type 2 diabetes mellitus in the Western Pacific region. Curr Med Res Opin. 2018;34(9):1653–1662. doi:10.1080/03007995.2018.1484712
19. Jabbar A, Abdallah K, Hassoun A, et al. Patterns and trends in insulin initiation and intensification among patients with type 2 diabetes mellitus in the Middle East and North Africa region. Diabetes Res Clin Pract. 2019;149:18–26. doi:10.1016/j.diabres.2019.01.017
20. Romanelli RJ, Chung S, Pu J, Nimbal V, Zhao B, Palaniappan L. Comparative effectiveness of early versus delayed metformin in the treatment of type 2 diabetes. Diabetes Res Clin Pract. 2015;108(1):170–178. doi:10.1016/j.diabres.2014.12.019
21. Mata-Cases M, Franch-Nadal J, Real J, et al. Therapeutic inertia in patients treated with two or more antidiabetics in primary care: factors predicting intensification of treatment. Diabetes Obes Metab. 2018;20(1):103–112. doi:10.1111/dom.13045
22. Khunti K, Wolden ML, Thorsted BL, Andersen M, Davies MJ. Clinical inertia in people with type 2 diabetes: a retrospective cohort study of more than 80,000 people. Diabetes Care. 2013;36(11):3411–3417. doi:10.2337/dc13-0331
23. Calvert MJ, McManus RJ, Freemantle N. Management of type 2 diabetes with multiple oral hypoglycaemic agents or insulin in primary care: retrospective cohort study. Br J Gen Pract. 2007;57(539):455–460.
24. Mashitisho MLI, Mashitisho BG. Early insulin therapy in patients with type 2 diabetes mellitus. J Endocrinol Metabol Diabetes S Af. 2016;21(1):13–15. doi:10.1080/16089677.2016.1160539
25. Bhattacharya R, Zhou S, Wei W, Ajmera M, Sambamoorthi U. A real-world study of the effect of timing of insulin initiation on outcomes in older medicare beneficiaries with type 2 diabetes mellitus. J Am Geriatr Soc. 2015;63(5):893–901. doi:10.1111/jgs.13388
26. McEwen LN, Bilik D, Johnson SL, et al. Predictors and impact of intensification of antihyperglycemic therapy in type 2 diabetes: translating research into action for diabetes (TRIAD). Diabetes Care. 2009;32(6):971–976. doi:10.2337/dc08-1911
27. Khunti K, Nikolajsen A, Thorsted BL, Andersen M, Davies MJ, Paul SK. Clinical inertia with regard to intensifying therapy in people with type 2 diabetes treated with basal insulin. Diabetes Obes Metab. 2016;18(4):401–409. doi:10.1111/dom.12626
28. Parchman ML, Pugh JA, Romero RL, Bowers KW. Competing demands or clinical inertia: the case of elevated glycosylated hemoglobin. Ann Fam Med. 2007;5(3):196–201. doi:10.1370/afm.679
29. Chaudhry SI, Berlowitz DR, Concato J. Do age and comorbidity affect intensity of pharmacological therapy for poorly controlled diabetes mellitus? J Am Geriatr Soc. 2005;53(7):1214–1216. doi:10.1111/j.1532-5415.2005.53370.x
30. Schwab P, Saundankar V, Bouchard J, et al. Early treatment revisions by addition or switch for type 2 diabetes: impact on glycemic control, diabetic complications, and healthcare costs. BMJ Open Diabetes Res Care. 2016;4(1):e000099. doi:10.1136/bmjdrc-2015-000099
31. Vitry AI, Roughead EE, Preiss AK, et al. Influence of comorbidities on therapeutic progression of diabetes treatment in Australian veterans: a cohort study. PLoS One. 2010;5(11):e14024. doi:10.1371/journal.pone.0014024
32. Khunti S, Davies MJ, Khunti K. Clinical inertia in the management of type 2 diabetes mellitus: a focused literature review. Br J Diabetes. 2015;15(2):65–69. doi:10.15277/bjdvd.2015.019
33. Higgins V, Piercy J, Roughley A, et al. Trends in medication use in patients with type 2 diabetes mellitus: a long-term view of real-world treatment between 2000 and 2015. Diabetes Metab Syndr Obes. 2016;9:371–380. doi:10.2147/DMSO.S120101
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
