Back to Journals » Infection and Drug Resistance » Volume 14
Delayed SARS-CoV-2 Clearance in Patients with Obesity
Authors Zhang X, Lin B, Yang G, Liu L, Lu J, Lu Z, Xue Y
Received 7 May 2021
Accepted for publication 13 July 2021
Published 22 July 2021 Volume 2021:14 Pages 2823—2827
DOI https://doi.org/10.2147/IDR.S319029
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Xiujun Zhang,1,2 Bin Lin,3 Gang Yang,4 Longgen Liu,1,2 Jianchun Lu,1,2 Zhaohui Lu,4 Yuan Xue1,2
1Institute of Hepatology, The Third People’s Hospital of Changzhou, Changzhou, People’s Republic of China; 2Department of Liver Diseases, The Third People’s Hospital of Changzhou, Changzhou, People’s Republic of China; 3Department of Infectious Diseases, The First People’s Hospital of Jintan, Changzhou, People’s Republic of China; 4Department of Respiratory Diseases, The Second People’s Hospital of Wuhu, Wuhu, People’s Republic of China
Correspondence: Zhaohui Lu; Yuan Xue Email [email protected]; [email protected]
Objective: The present study aimed to investigate the correlation between obesity and virus persistence in patients with COVID-19.
Design and Methods: A total of 57 patients with laboratory-confirmed COVID-19 were admitted to two clinical centers, and data were analyzed retrospectively. Among them, 18 patients with body mass index (BMI) ≥ 25 kg/m2 were diagnosed with obesity, and dynamics of viral replication were compared.
Results: Eighteen patients were diagnosed with obesity. The correlations between BMI and white blood cell, C-reactive protein, and cycle threshold (Ct) values of ORF1ab were not significant (all P > 0.05). On day 7 after admission, virus clearance was achieved in 13 (33.3%) patients with BMI < 25 kg/m2 and 5 (27.8%) patients with BMI ≥ 25 kg/m2 (χ2 = 0.176, P =0.68). On day 14, the RNA tests were negative in 37 (94.9%) patients with BMI < 25 kg/m2 and 13 (72.2%) patients with BMI ≥ 25 kg/m2 (χ2 = 5.865, P = 0.03). Multivariable analysis showed that only BMI ≥ 25 kg/m2 (P = 0.02) was the independent risk factor for virus clearance on day 14.
Conclusion: Obesity may affect the clearance of SARS-CoV-2, and BMI should be assessed in patients with COVID-19, although they are not seriously ill.
Keywords: SARS-CoV2, COVID-19, body mass index, obesity, virus clearance
Introduction
SARS-CoV-2 infection, also known as 2019-nCoV disease (COVID-19), poses a global threat to public health.1 The virus with high infectivity can be transmitted person-to-person, and the number of patients with COVID-19 is constantly rising worldwide.
Although the pathogenesis of COVID-19 remains largely unknown, virus replication is speculated to contribute to the inflammatory process.2 Considering that angiotensin-converting enzyme 2 (ACE2) receptor in host cells and the receptor-binding domain in SARS-Cov-2 spike protein are essential for viral entry and replication, ACE2 inhibitor and neutralizing antibodies are potential antiviral drugs with promising effects.3–6 Moreover, knowledge about the correlation between viral load and other indicators of infection is limited. Also, it lacks indicators predicting the early clearance of viral load during infection.
In addition to viral replication and antiviral therapy, obesity plays a vital role in the pathogenesis of COVID-19.7 Data from a large academic hospital system showed that obesity was an independent risk factor for mortality.8 Patients who were overweight had increased odds of developing severe COVID-19.9–12 One explanation is that the expression of human ACE2 in adipose tissue is higher than that in lung tissue.13 Patients with obesity may exhibit reduced lung function, poor response to mechanical ventilation, and several complications.14 Accumulating evidence has focused on obesity and the poor outcomes in patients with severe COVID-19.15 However, to the best of our knowledge, data on the dynamics of viral replication in patients with obesity are limited, especially those who are not seriously ill.
Herein, we investigated the correlation between virus clearance and obesity in patients with COVID-19.
Materials and Methods
Patients
A total of 57 adult patients with laboratory-confirmed COVID-19 were admitted to the Third People’s Hospital of Changzhou and the Second People’s Hospital of Wuhu. In this retrospective study, data from these patients were collected from January– March 20, 2021. COVID-19 was diagnosed according to the exposure history, abnormal computed tomography (CT) scan, and the positive RNA test.1 All the patients were Asian and obesity was defined as body mass index (BMI) ≥ 25 kg/m2, according to the International Obesity Task Force.16,17
The study was approved by the Ethics Committee of the Third People’s Hospital of Changzhou, according to the Declaration of Helsinki, 2013 (02A-A20200004). Furthermore, written informed consent was obtained from all participants before the study.
Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR) Assay
The assay was performed at the Changzhou Center for Disease Control and Prevention (CDC) and Wuhu CDC using a commercial kit (Biogerm Medical Biotechnology Co., Shanghai, China). Positive RNA test indicated that the cycle threshold (Ct) values of ORF1ab and N genes were < 40, which were indispensable for diagnosis.18 According to the manufacturer’s instructions, for patients with 37 < Ct < 40, the RNA test was repeated immediately, followed by the collection of throat swabs at an interval of 24 h.
Statistical Analysis
Analyses were performed using SPSS 23.0 software (Chicago, IL, USA). Data are expressed as median (interquartile range, IQR) for continuous variables and frequencies for categorical values and were compared using Kruskal–Wallis test or chi-square test. In addition, a correlation analysis was performed using Pearson’s correlation test, and multivariate analysis was conducted using logistic regression analysis to assess the probability of virus clearance. A two-sided P < 0.05 is considered statistically significant.
Results
Demographics and Clinical Characteristics of Patients with COVID-19
No patient developed severe pneumonia, and all patients survived. The cohort consisted of 29/57 males (median age, 45.0 years (IQR 32.5–55.5)). 41/57 (71.9%) patients presented fever, and most of them (32/41, 78.0%) had a short duration of fever (< 7 days).
5/57 patients showed an elevated level (< 85 U/L) of alanine transaminase (ALT), and 18 had leucopenia (white blood cell count < 4×109/L) on admission. CT scan showed that most patients (43/57, 75.4%) had bilateral pneumonia, and 11 (19.3%) had unilateral pneumonia.
The median BMI was 23.7 (IQR 21.6–25.8) kg/m2. Among the 57 patients, 18 were diagnosed with obesity. As shown in Table 1, basal Ct (ORF1ab) and Ct (N) were similar between patients with BMI < 25 kg/m2 and BMI ≥ 25 kg/m2 (P = 0.25 and 0.22, respectively), and no significant difference was detected in the comorbidity between the two groups (all P > 0.05).
 |
Table 1 Characteristics of Patients with BMI≥25 Kg/m2 and BMI<25 Kg/m2 |
Correlation Between BMI and Other Laboratory Parameters
The correlations between BMI and ALT, white blood cell (WBC), C-reactive protein, D-dimer, and Ct (ORF1ab) were not significant (all P > 0.05). Ct (ORF1ab) was positively correlated with Ct (N) (γ = 0.957, P < 0.01) and white blood cell count (γ = 0.285, P = 0.03), while it was inversely correlated with D-dimer levels (γ = −0.450, P < 0.01).
Independent Risk Factors for Virus Clearance on Days 7 and 14
On day 7 after admission, virus clearance was achieved in 13 (33.3%) patients with BMI < 25 kg/m2 and 5 (27.8%) patients with BMI ≥ 25 kg/m2 (χ2 = 0.176, P = 0.68). No independent risk factor was identified for virus clearance.
On day 14, the RNA tests were negative in 37 (94.9%) patients with BMI < 25 kg/m2 and 13 (72.2%) patients with BMI ≥ 25 kg/m2 (χ2 = 5.865, P = 0.03) (Figure 1). The BMI of patients with negative RNA tests was lower than that of patients with positive RNA tests [23.6 (IQR 21.1–25.3) vs 26.5 (IQR 23.7–28.3), Z = 2.298, P = 0.022] (Supplementary Table 1). As shown in Table 2, the univariate logistic analysis demonstrated that BMI ≥ 25 kg/m2 (P = 0.02) was associated with virus clearance on day 14. Subsequently, multivariable analysis showed that only BMI ≥ 25 kg/m2 (P = 0.02) was the independent risk factor for virus clearance on day 14.
 |
Table 2 Risk Factors for SARS-CoV-2 Clearance on Day 14 |
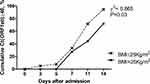 |
Figure 1 Dynamics of cycle threshold values in patients with BMI < 25 kg/m2 and BMI ≥ 25 kg/m2. Abbreviation: Ct, cycle threshold. |
Discussion
In the present study, we evaluated the correlation between obesity and virus clearance and analyzed the predictors for virus clearance during SARS-CoV2 infection. Multivariable analysis showed that BMI ≥ 25 kg/m2 is the independent risk factor for virus clearance on day 14.
A high frequency of obesity was detected among patients with COVID-19 admitted to the intensive care units.8,16,19 Patients with high BMI could develop a severe or critical illness, although the pathogenesis is yet to be elucidated. As ACE2, the receptor of SARS-CoV-2 is highly expressed in adipose tissue, and patients with obesity might have a large number of ACE2-expressing cells.13 To the best of our knowledge, BMI is not calculated routinely in clinical practice, and it lacks studies with a large sample size with respect to obesity and viral persistence. In the present study, data from two clinical centers showed that BMI is the independent risk factor for virus clearance on day 14. Thus, the current results could provide a useful reference for the treatment and prevention strategies in COVID-19.
Several factors may affect the delayed SARS-CoV-2 shedding, including chronic rhinosinusitis and atopy.6 We compared the comorbidities in patients with positive and negative RNA tests on day 14, and did not find any significant effect on viral persistence. In addition, glucocorticoids have been applied in clinical practice, but the effects remain controversial.20 Since glucocorticoid is not an independent risk factor for virus clearance, we did not find any evidence that glucocorticoid affects virus clearance on days 7 and 14.
The present study has some limitations. First, it is a retrospective study with a small sample-size due to which the confidence interval is large in the logistic regression analysis. Thus, large-scale, multicenter studies are required to confirm the current findings. Second, the mechanisms underlying the role of obesity in prolonged SARS-CoV-2 RNA shedding are not explored, especially in patients not critically ill.
Conclusions
BMI ≥ 25 kg/m2 is an independent risk factor for virus clearance on day 14, suggesting that obesity might affect the clearance of SARS-CoV-2. Thus, BMI should be assessed in patients with COVID-19, even if they are not seriously ill.
Abbreviations
BMI, body mass index; Ct, cycle threshold; COVID-19, 2019-nCoV disease; RT-PCR, reverse transcription PCR; ACE2, angiotensin converting enzyme 2; CT, computed tomography; CDC, Center for Disease Control and Prevention; ALT, alanine aminotransferase; WBC, white blood cell.
Data Sharing Statement
The data are available from the corresponding author upon request.
Ethical Approval
The study was approved by the Ethics Committee of the Third People’s Hospital of Changzhou, according to the Declaration of Helsinki, 2013.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by the Natural Science Foundation of Jiangsu Province (BK20180183) and the Science and Technology Project of Jintan (KJ201925).
Disclosure
The authors declare that there are no conflicts of interest.
References
1. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi:10.1016/S0140-6736(20)30211-7
2. Chen Y, Liu Q, Guo D. Emerging coronaviruses: genome structure, replication, and pathogenesis. J Med Virol. 2020;92(4):418–423. doi:10.1002/jmv.25681
3. Yousefi B, Valizadeh S, Ghaffari H, Vahedi A, Karbalaei M, Eslami M. A global treatments for coronaviruses including COVID-19. J Cell Physiol. 2020;235(12):9133–9142. doi:10.1002/jcp.29785
4. Verderese JP, Stepanova M, Lam B, et al. Neutralizing monoclonal antibody treatment reduces hospitalization for mild and moderate COVID-19: a real-world experience. Clin Infect Dis. 2021. doi:10.1093/cid/ciab579
5. RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397(10285):1637–1645. doi:10.1016/S0140-6736(21)00676-0.
6. Recalde-Zamacona B, Tomas-Velazquez A, Campo A, et al. Chronic rhinosinusitis is associated with prolonged SARS-CoV-2 RNA shedding in upper respiratory tract samples: a case-control study. J Intern Med. 2021;289(6):921–925. doi:10.1111/joim.13237
7. Kassir R. Risk of COVID-19 for patients with obesity. Obes Rev. 2020;21(6). doi:10.1111/obr.13034
8. Klang E, Kassim G, Soffer S, Freeman R, Levin MA, Reich DL. Morbid obesity as an independent risk factor for COVID-19 mortality in hospitalized patients younger than 50. Obesity (Silver Spring). 2020;28(9):1595–1599. doi:10.1002/oby.22913
9. Busetto L, Bettini S, Fabris R, et al. Obesity and COVID-19: an Italian snapshot. Obesity (Silver Spring). 2020;28(9):1600–1605. doi:10.1002/oby.22918
10. Cai Q, Chen F, Wang T, et al. Obesity and COVID-19 severity in a designated hospital in Shenzhen, China. Diabetes Care. 2020;43(7):1392–1398. doi:10.2337/dc20-0576
11. Hajifathalian K, Kumar S, Newberry C, et al. Obesity is associated with worse outcomes in COVID-19: analysis of Early Data From New York City. Obesity (Silver Spring). 2020;28(9):1606–1612. doi:10.1002/oby.22923
12. Gao M, Piernas C, Astbury NM, et al. Associations between body-mass index and COVID-19 severity in 6.9 million people in England: a prospective, community-based, cohort study. Lancet Diabetes Endocrinol. 2021;9(6):350–359. doi:10.1016/S2213-8587(21)00089-9
13. Li MY, Li L, Zhang Y, Wang XS. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect Dis Poverty. 2020;9(1):45. doi:10.1186/s40249-020-00662-x
14. Caci G, Albini A, Malerba M, Noonan DM, Pochetti P, Polosa R. COVID-19 and obesity: dangerous liaisons. J Clin Med. 2020;9(8):2511. doi:10.3390/jcm9082511
15. Soeroto AY, Soetedjo NN, Purwiga A, et al. Effect of increased BMI and obesity on the outcome of COVID-19 adult patients: a systematic review and meta-analysis. Diabetes Metab Syndr. 2020;14(6):1897–1904. doi:10.1016/j.dsx.2020.09.029
16. Zheng KI, Gao F, Wang XB, et al. Letter to the Editor: obesity as a risk factor for greater severity of COVID-19 in patients with metabolic associated fatty liver disease. Metabolism. 2020;108:154244. doi:10.1016/j.metabol.2020.154244
17. Goda A, Masuyama T. Obesity and Overweight in Asian People. Circ J. 2016;80(12):2425–2426. doi:10.1253/circj.CJ-16-1087
18. Zou L, Ruan F, Huang M, et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382(12):1177–1179. doi:10.1056/NEJMc2001737
19. Simonnet A, Chetboun M, Poissy J, et al. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity (Silver Spring). 2020;28(7):1195–1199. doi:10.1002/oby.22831
20. Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395(10223):473–475. doi:10.1016/S0140-6736(20)30317-2
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
