Back to Journals » Drug Design, Development and Therapy » Volume 16
Dehydrozingerone Alleviates Hyperalgesia, Oxidative Stress and Inflammatory Factors in Complete Freund’s Adjuvant-Induced Arthritic Rats
Authors Liu C, Li Y, Wen C, Yan Z, Olatunji OJ , Yin Z
Received 15 May 2022
Accepted for publication 12 August 2022
Published 8 September 2022 Volume 2022:16 Pages 3015—3022
DOI https://doi.org/10.2147/DDDT.S374827
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Anastasios Lymperopoulos
Chunhong Liu,1,2 Yetian Li,3 Chaoling Wen,4 Zheng Yan,2 Opeyemi Joshua Olatunji,5 Zongsheng Yin3
1The First Affiliated Hospital of Anhui Medical University, Hefei, 230022, People’s Republic of China; 2The Second People’s Hospital of Wuhu City, Wuhu, 230022, People’s Republic of China; 3Department of Orthopaedics, The First Affiliated Hospital of Anhui Medical University, Hefei, 230022, People’s Republic of China; 4Anhui Traditional Chinese Medicine College, Wuhu, 241001, People’s Republic of China; 5Traditional Thai Medical Research and Innovation Center, Faculty of Traditional Thai Medicine, Prince of Songkla University, Hat Yai, 90110, Thailand
Correspondence: Zongsheng Yin, Department of Orthopaedics, The First Affiliated Hospital of Anhui Medical University, Hefei, Anhui, 230022, People’s Republic of China, Email [email protected]
Purpose: Rheumatoid arthritis (RA) is a chronic autoimmune disease with severe inflammatory responses. Dehydrozingerone (DHZ) is a potent bioactive compound found in the rhizomes of Zingiber officinale, and it has been reported as an excellent anti-inflammatory and antioxidant agent. This study evaluated the anti-arthritic effects of DHZ in complete Freund’s adjuvant (CFA)-induced arthritis.
Methods: CFA administered rats were intragastrically treated with DHZ (100 mg/kg) for 28 days, and arthritis severity was assessed via body weight, arthritic score, paw edema and hyperalgesia. Serum inflammation biomarkers, oxidative stress markers, inflammatory cytokines and liver function enzymes were evaluated.
Results: The results indicated that DHZ significantly ameliorated arthritis severity as shown by reduced arthritic score, thymus and spleen indexes, paw circumference, paw withdrawal threshold and latency as well as increased body weight gain. Furthermore, DHZ treatment persuasively reduced serum levels of alkaline phosphatase (ALP), aspartate transaminase (AST), alanine transaminase (ALT), rheumatoid factor (RF), C-reactive protein (CRP), tumor necrosis factor alpha (TNF-α), interleukin-1β and 6 (IL-1β and IL-6), malondialdehyde (MDA), vascular endothelial growth factor (VEGF) and transforming growth factor β (TGF-β). In addition, DHZ observably increased serum superoxide dismutase (SOD) and glutathione (GSH) levels in treated rats.
Conclusion: These findings suggest that DHZ possesses anti-RA effect properties via modulating the inflammatory responses and oxidative stress.
Keywords: dehydrozingerone, anti-arthritis, complete Freund’s adjuvant, inflammation, oxidative stress, rheumatoid arthritis
Introduction
Rheumatoid arthritis (RA) is a chronic autoimmune and systemic inflammatory disease that causes irreversible damages to the joint, resulting in significant disability, musculoskeletal deficits, painful joints and destructive bone erosions.1,2 RA prevalence is reported to be approximately 2% of the global population.2 Several efforts have been made to decipher the etiology of RA, although still poorly understood, several critical factors including genetic and environmental factors, chronic inflammation, development of autoantibodies and oxidative stress are involved in the pathophysiology of RA.3–5 RA etiology is very complex, and it involves several factors occurring simultaneously including disordered innate immunity, dysregulated cytokine networks, activation of osteoclast and chondrocyte activation, macrophage activation leading to the production of matrix metalloproteinases and pro-inflammatory cytokines and joint damage. Although there is no known cure for RA, however, non-steroidal anti-inflammatory drugs are used in alleviating RA symptoms; unfortunately, these drugs cannot halt RA progression or protect against joint erosion.6 However, disease-modifying anti-rheumatic drugs (DMARDs) are relatively expensive and have several unpleasant side effects.3,7 Like several other devastating diseases, alternative therapies, especially herbal therapies/natural compounds, have been employed by RA patients due to their perceived safety, effectiveness and availability.7,8
Dehydrozingerone (DHZ) is a bioactive phenolic compound found in the rhizomes of Zingiber officinale. Previous studies have reported several pharmacological properties of DHZ, including anti-inflammatory, antioxidant, antiobesity, anticancer, neuroprotective, tyrosinase inhibitory, antidepressant and antifungal effects.9–12 However, despite the numerous reports of the therapeutic effects of DHZ, there are no reports on whether DHZ possesses therapeutic efficacy on RA. As such, the present study evaluated the therapeutic effects of DHZ in rat models of CFA-induced arthritis.
Materials and Methods
Chemicals and Reagents
Complete Freund’s adjuvant (CFA) and DHZ were procured from Sigma-Aldrich (St Louis, MO, USA). Heat-inactivated Bacillus Calmette-Guérin (BCG) was supplied by Rebio Scientific (Shanghai, China). ELISA kits for inflammatory mediators, proinflammatory cytokines, and oxidative stress parameter analyses were purchased from MultiScience (Hangzhou, Zhejiang, China) and Keygen Biotech (Nanjing, Jiangsu, China), respectively. All other chemicals used were of analytical grade.
Animals, CIA Induction and Treatment
Seven weeks old male Sprague-Dawley rats (170 ± 10 g) were purchased from Tianqin Biotechnology (Changsha, Hunan, China) and kept in standard animal husbandry conditions (temperature of 20 ± 2℃, relative humidity 55 ± 10% and 12/12 h light–dark schedules). All the rats were fed a standard chow diet and water ad libitum and allowed to acclimatize to the conditions of the environment for 7 days. The animal ethics committee of The First Affiliated Hospital of Anhui Medical University approved the experimental procedures (Approval number: HDSFDXWuHuyy-20,210,918). In addition, the animal experiment was performed in line with the National Institutes of Health guidelines (No. 8023, revised 1978) for animal care and use.
Heat-inactivated BCG was grinded in IFA and homogenized with water to obtain 20 mg/mL of CFA. Arthritis was induced in all the rats except the normal control via subcutaneous injection of 0.1 mL of CFA at the plantar surface of the left hind paw. After 7 days, a booster CFA injection was administered to the animals at the base of tail. The animals allotted into three groups with six rats per group and treated as follows:
Group 1: Normal control rats (NCR) intragastrically administered with 0.5% sodium carboxymethyl cellulose solution (CMC-Na)
Group 2: CFA control rats (CFAR): RA rats intragastrically treated with 0.5%CMC-Na
Group 3: DHZ-treated CFA rats (CFA+DHZ): RA rats intragastrically administered with 100 mg/kg of DZ.
The dose of DHZ chosen in this experiment was according to previous studies.9,10 The animals were treated daily for 28 days, and body weight gain of the animals was routinely recorded.
Clinical Assessment of Arthritis
The severity of arthritis was assessed following previously reported method and was assessed on a scale of 0–4 as follows: 0 = absence of swelling, edema or erythema; 1 = slight edema or erythema; 2 = moderate swelling, edema and erythema from the ankle to the tarsal bone; 3 = severe swelling, edema and erythema from the ankle to the tarsal bone; 4 = ankylosis, edema, erythema or incapacity to bend the ankle to the entire leg.13
Pain Behavioral Tests
Pain score assessment was performed in all the rats after the treatment using thermal hyperalgesia on a preheated hot plate with temperature set at 52 ± 1℃. The rats were individually placed on the hotplate, and the latency to the first pain reaction including flinching, jumping, and/or licking of the claw was recorded. A cut-off time of 20s was set to prevent tissue damage.
Mechanical hyperalgesia was evaluated following a previously reported protocol using von Frey filaments of varying pressure sizes at the plantar area of the hind paw of the rats.14
Animal Sacrifice and Blood Sampling
On the 29th day, the rats were fasted overnight and anaesthetized with chloral hydrate, and blood samples were obtained via abdominal aorta and centrifuged to obtain the serum which were subsequently used for further biochemical analysis. The thymus and spleen were promptly removed and weighed. The index of thymus and spleen were calculated.
Biochemical Analysis
The serum obtained was used for the quantification of liver function enzymes including ALT, ALP and AST; oxidative stress parameters including MDA, SOD and GSH, proinflammatory cytokines including IL-1β, IL-6 and TNF-α, as well as vascular endothelial growth factor (VEGF), rheumatoid factor (RF), C-reactive protein (CRP) and transforming growth factor β (TGF-β) using biochemical and ELISA kits strictly in accordance with the manufacturers’ instructions.
Statistical Analysis
Statistical analysis was performed using one-way ANOVA coupled with Tukey post hoc (mean ± SD, n = 6) with GraphPad Prism 9.0 (GraphPad Software, NC, USA). P < 0.05 was considered statistically significant.
Results
Effect of DHZ on Physical Parameters in Rats
As shown in Figure 1A–C, the body weight gain in the CFAR group was significantly reduced, while the paw swelling and arthritis score was remarkably high when compared to the NCR group. Whereas DHZ treated RA rats showed improvement in their body weight gain, while the swelling rate and arthritis score were remarkably reduced compared with the CFAR group (Figure 1A–C). Furthermore, the circumference of the hind paw in the CFAR group was notably increased, while treatment with DHZ significantly alleviated the observed increase in the treated animals (Figure 1D).
Effect of DHZ on the Thermal Hyperalgesia in Rats
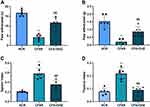
The anti-nociceptive effect of DHZ was assessed via plantar tests in polyarthritis rats. As indicated in Figure 2, substantial reduction in thermal hyperalgesia latency time as well as mechanical hyperalgesia was observed in the hind paws of CFAR rats when compared to the NCR (Figure 2A and B). In contrast to the CFAR group, significant increases were observed in the latency time of RA rats treated with DHZ, suggesting a reduction in thermal and mechanical hyperalgesia (Figure 2A and B).
Effect of DHZ on Spleen and Thymus Indexes in Rats
As shown in Figure 2C and D, there was remarkable hyperplasia of the spleen and thymus tissues of CFAR rats compared to the NCR rats. Compared with the CFAR group, the treatment of rats with DHZ significantly suppressed tissue hyperplasia in the treated rats.
Effects of DHZ on Proinflammatory Cytokines in Rats
As shown in Figure 3, the levels of proinflammatory cytokines, namely, IL-1β, TNF-α, IL-6 and TGF-β in the serum were significantly increased in the CFAR group compared to the NCR group. However, these proinflammatory cytokines were markedly suppressed in the DHZ treated animals when compared to the CFAR group (Figure 3A–D).
Effect of DHZ on Oxidative Stress Markers in Rats
From the results shown in Figure 4, it was inferred that the level of MDA in the serum level of CFAR was notably increased, while GSH and SOD were significantly reduced by 2.8 and 3.4 folds, respectively, when compared to NCR group. In contrast, treatment of RA rats with DHZ effectively reversed the MDA level and significantly increased the concentration of SOD and GSH in the serum of the treated rats compared with those of CFAR group (Figure 4A–C).
Effect of DHZ on Serum Biochemical Parameters
There were marked increases in the serum levels of AST, ALT, ALP, VEGF, CRP and RF in the CFAR group when compared to NCR rats. However, the administration of DHZ to RA rats significantly reverted the alterations in these parameters compared with CFAR rats (Figure 5A–F).
Discussion
Increasing evidences have indicated that RA is characterised by intense leukocyte infiltration in the bones and joints resulting in severe damages to the joint cartilage. Been an inflammatory related disorder, the mechanism involved in the development and progression of the disease involves a complex interplay between several inflammatory and oxidative stress mediators.15,16 The most common therapeutic approach for the treatment of RA involves the use of disease modifying antirheumatic drugs (such as methotrexate and sulfasalazine), glucocorticoids (such as prednisone and dexamethasone) and biological agents (such as tocilizumab and abatacept). Unfortunately, the long term prospects of these drugs are not encouraging for RA treatment due to serious side effects as well as huge financial burden associated with the use of these drugs, thus leaving a huge window for the discovery of new therapies from natural/alternative medicine.17,18 The pharmacological effects of DHZ including its antioxidant and anti-inflammatory activities suggested that DHZ may have beneficial role against RA. Therefore, the primary goal of this study was to evaluate the anti-rheumatoid arthritis effects of DHZ as well as its effects on oxido-inflammatory parameters of the CFA-induced arthritis rat models.
CFA-induced arthritis is one of the most common and widely accepted methods for mimicking RA in animal models due to the similarities with the clinical features of human arthritis.19,20 Consistent with previous studies, the CFA-induced arthritis rats displayed significant decrease in body weight gain, significantly increased paw bulbous swelling and redness, limp and paw edema as well as joint deformation, suggesting the development of spontaneous inflammation.7,16 Treatment with DHZ significantly suppressed the increased arthritis score as well as paw oedema and swelling in the treated RA rats.
The role of inflammation in RA is vividly indisputable and proinflammatory cytokines are believed to play critical roles in pathogenesis of the disease. Prevailing evidences have shown that these cytokines can instigate the infiltration of immune cells, thus leading to the release of matrix metalloproteinases that have been largely implicated in cartilage degradation in arthritis and osteoarthritis.3 In addition, they can also activate NF-κB pathway, leading to further up-regulation in the levels of proinflammatory cytokines, thus aggravating inflammatory cascade.6,18 In RA, macrophages, immune cells and T cells can also instigate further production of TNF-α and IL-6, thus magnifying inflammation.21–24 The results from this study indicated that DZ reduced the TNF-α, IL-1β and IL-6 serum levels in the treated RA rats, suggesting that the anti-inflammatory effects of DHZ may be beneficial to alleviate RA.
On the other hand, CFA administered rats showed a significant increase in serum oxidative stress biomarker (increased MDA level and obvious declines in SOD and GSH activities). Aside from inflammation, ROS and oxidative stress have been shown to contribute to the pathophysiology of RA.25,26 The activation of macrophages, monocytes and T cells in RA circumstances can lead to overproduction of ROS and oxidative stress, which is positively correlated with inflammation and joint destruction in RA.27 Considering the well-recognized connection between oxidative stress and chronic inflammation and the effect of ROS overproduction on cell destruction together with decreased antioxidant defense in RA,28,29 the antioxidant activity of DHZ may have proven beneficial in its anti-arthritic effects. Treatment of CFA administered rats with DHZ significantly increased CFA-induced decrease in serum GSH and SOD, while MDA level was markedly reduced suggesting that the antioxidant potentials of DHZ might have played a significant role in its anti-arthritic properties. These results were in accordance with previous reports on the antioxidant activity of DHZ through the attenuation of oxidative stress.12
Conclusion
In conclusion, the findings from this study indicated that DHZ possessed promising antiarthritic effects through its ability to ameliorate inflammatory cytokines and oxidative stress and improve antioxidant capacity in CFA induced arthritis. Further mechanistic studies are needed.
Funding
There is no funding to report.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Almutairi K, Nossent J, Preen D, Keen H, Inderjeeth C. The global prevalence of rheumatoid arthritis: a meta-analysis based on a systematic review. Rheumatol Int. 2021;41(5):863–877. doi:10.1007/s00296-020-04731-0
2. Walsh DA, McWilliams DF. Mechanisms, impact and management of pain in rheumatoid arthritis. Nat Rev Rheumatol. 2014;10:581–592. doi:10.1038/nrrheum.2014.64
3. Liu T, Su B. Styphnolobium japonicum (L.) Schott flower extract alleviates oxidative stress and inflammatory factors in the adjuvant-induced arthritis rat model. J Pain Res. 2021;14:2907–2919. doi:10.2147/JPR.S325988
4. Phull AR, Nasir B, Haq IU, Kim SJ. Oxidative stress, consequences and ROS mediated cellular signaling in rheumatoid arthritis. Chem Biol Interact. 2018;281:121–136. doi:10.1016/j.cbi.2017.12.024
5. Smolen JS, Aletaha D, Barton A, et al. Rheumatoid arthritis. Nat Rev Dis Primers. 2018;4:1–23. doi:10.1038/s41572-018-0001-z
6. Zuo J, Xia Y, Li X, Chen JW. Therapeutic effects of dichloromethane fraction of Securidaca inappendiculata on adjuvant-induced arthritis in rat. J Ethnopharmacol. 2014;153:352–358. doi:10.1016/j.jep.2014.02.015
7. Chen Y, Wang QW, Zuo J, Chen JW, Li X. Anti-arthritic activity of ethanol extract of Claoxylon indicum on Freund’s complete adjuvant-induced arthritis in mice. BMC Complement Altern Med. 2017;17:11. doi:10.1186/s12906-016-1500-7
8. Efthimiou P, Kukar M. Complementary and alternative medicine use in rheumatoid arthritis: proposed mechanism of action and efficacy of commonly used modalities. Rheumatol Int. 2010;30:571–586. doi:10.1007/s00296-009-1206-y
9. Moorkoth S, Prathyusha NS, Manandhar S, et al. Antidepressant-like effect of dehydrozingerone from Zingiber officinale by elevating monoamines in brain: in silico and in vivo studies. Pharmacol Rep. 2021;73:1273–1286. doi:10.1007/s43440-021-00252-0
10. Lee ES, Kang JS, Kim HM, et al. Dehydrozingerone inhibits renal lipotoxicity in high-fat diet-induced obese mice. J Cell Mol Med. 2021;25:8725–8733. doi:10.1111/jcmm.16828
11. Pathak N, Cheruku SP, Rao V, et al. Dehydrozingerone protects temozolomide-induced cognitive impairment in normal and C6 glioma rats besides enhancing its anticancer potential. Biotech. 2020;10:438.
12. Tirunavalli SK, Gourishetti K, Kotipalli RSS, et al. Dehydrozingerone ameliorates lipopolysaccharide induced acute respiratory distress syndrome by inhibiting cytokine storm, oxidative stress via modulating the MAPK/NF-kappa B pathway. Phytomedicine. 2021;92:153729. doi:10.1016/j.phymed.2021.153729
13. Brahn E, Banquerigo ML, Firestein GS, Boyle DL, Salzman AL, Szabo C. Collagen induced arthritis: reversal by mercaptoethylguanidine, a novel anti-inflammatory agent with a combined mechanism of action. J Rheumatol. 1998;25:1785–1793.
14. Wang P, Wen C, Olatunji OJ. Anti-inflammatory and antinociceptive effects of Boesenbergia rotunda polyphenol extract in diabetic peripheral neuropathic rats. J Pain Res. 2022;15:779. doi:10.2147/JPR.S359766
15. Behl T, Chadha S, Sehgal A, et al. Exploring the role of cathepsin in rheumatoid arthritis. Saudi J Biol Sci. 2022;29:402–410. doi:10.1016/j.sjbs.2021.09.014
16. Zuo J, Tao MQ, Wu XY, et al. Securidaca inappendiculata-derived xanthones protected joints from degradation in male rats with collagen-induced arthritis by regulating PPAR-γ signaling. J Inflamm Res. 2021;14:395–411. doi:10.2147/JIR.S295957
17. Ramiro S, Sepriano A, Chatzidionysiou K, et al. Safety of synthetic and biological DMARDs: a systematic literature review informing the 2016 update of the EULAR recommendations for management of rheumatoid arthritis. Ann Rheumatic Dis. 2017;76:1101–1136. doi:10.1136/annrheumdis-2016-210708
18. Bao Y, Peng J, Yang KL, et al. Therapeutic effects of Chinese medicine Di-Long (Pheretima vulgaris) on rheumatoid arthritis through inhibiting NF-κB activation and regulating Th1/Th2 balance. Biomed Pharmacother. 2022;147:112643. doi:10.1016/j.biopha.2022.112643
19. Ren SX, Zhang B, Lin Y, Ma DS, Li H. Mechanistic evaluation of antiarthritic activity of β-methylphenylalanine in experimental rats. Biomed Pharmacother. 2019;113:108730. doi:10.1016/j.biopha.2019.108730
20. Kamal RM, Sabry MM, Aly ZY, Hifnawy MS. Phytochemical and in-vivo anti-arthritic significance of Aloe thraskii Baker in combined therapy with methotrexate in adjuvant-induced arthritis in rats. Molecules. 2021;26:3660. doi:10.3390/molecules26123660
21. Lei M, Tao MQ, Wu YJ, et al. Metabolic enzyme triosephosphate isomerase 1 and nicotinamide phosphoribosyltransferase, two independent inflammatory indicators in rheumatoid arthritis: evidences from collagen-induced arthritis and clinical samples. Front Immunol. 2022;12:795626. doi:10.3389/fimmu.2021.795626
22. Wang DD, Wu XY, Dong JY, et al. Qing-Luo-Yin alleviated experimental arthritis in rats by disrupting immune feedback between inflammatory T cells and monocytes: key evidences from its effects on immune cell phenotypes. J Inflamm Res. 2021;14:7467–7486. doi:10.2147/JIR.S346365
23. Pan S, Wu YJ, Zhang SS, et al. The effect of α7nAChR signaling on T cells and macrophages and their clinical implication in the treatment of rheumatic diseases. Neurochem Res. 2022;47:531–544. doi:10.1007/s11064-021-03480-1
24. McInnes IB, Buckley CD, Isaacs JD. Cytokines in rheumatoid arthritis – shaping the immunological landscape. Nat Rev Rheumatol. 2016;12:63–68. doi:10.1038/nrrheum.2015.171
25. García-González A, Gaxiola-Robles R, Zenteno-Savín T. Oxidative stress in patients with rheumatoid arthritis. Rev Invest Clin. 2015;67:46–53.
26. Shokry AA, El-Shiekh RA, Kamel G, Bakr AF, Sabry D, Ramadan A. Anti-arthritic activity of the flavonoids fraction of ivy leaves (Hedera helix L.) standardized extract in adjuvant induced arthritis model in rats in relation to its metabolite profile using LC/MS. Biomed Pharmacother. 2022;145:112456. doi:10.1016/j.biopha.2021.112456
27. Mititelu RR, Pădureanu R, Băcănoiu M, et al. Inflammatory and oxidative stress markers-mirror tools in rheumatoid arthritis. Biomedicines. 2020;8:125. doi:10.3390/biomedicines8050125
28. El-Ghffar EA A, Eldahshan OA, Barakat A, Efferth T. The prophylactic effect of a Eugenia aquea extract against oxidative stress and inflammation associated with the development of arthritis in an adjuvant-induced arthritis rat model. Food Funct. 2018;9:6643. doi:10.1039/C8FO01570H
29. Tang Y, Xie D, Gong W, Wu H, Qiang Y. Pentahydroxy flavonoid isolated from Madhuca indica ameliorated adjuvant-induced arthritis via modulation of inflammatory pathways. Sci Rep. 2021;11:17971. doi:10.1038/s41598-021-97474-2
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.