Back to Journals » Patient Preference and Adherence » Volume 9
Defining medication adherence in individual patients
Authors Morrison A, Stauffer M, Kaufman A
Received 8 April 2015
Accepted for publication 7 May 2015
Published 1 July 2015 Volume 2015:9 Pages 893—897
DOI https://doi.org/10.2147/PPA.S86249
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Johnny Chen
Alan Morrison, Melissa E Stauffer, Anna S Kaufman
ScribCo, Effort, PA, USA
Background: The classification of patients as adherent or non-adherent to medications is typically based on an arbitrary threshold for the proportion of prescribed doses taken. Here, we define a patient as pharmacokinetically adherent if the serum drug levels resulting from his/her pattern of medication-taking behavior remained within the therapeutic range.
Methods: We used pharmacokinetic modeling to calculate serum drug levels in patients whose patterns of dosing were recorded by a medication event monitoring system. Medication event monitoring system data were from a previously published study of seven psoriasis patients prescribed 40 mg subcutaneous adalimumab at 14-day intervals for 1 year. Daily serum concentrations of adalimumab were calculated and compared with a known therapeutic threshold.
Results: None of the seven patients took adalimumab precisely every 14 days. Three patients who took adalimumab at intervals of 6–26 days could be classified as pharmacokinetically adherent, because their daily adalimumab serum concentration never fell below the therapeutic threshold. The four other patients, who took adalimumab at intervals of 7–93 days, could be classified as pharmacokinetically non-adherent, because their adalimumab serum concentration fell below the therapeutic threshold on 3.5%–71.3% of days.
Conclusion: Patients with varying patterns of adalimumab dosing could be classified as pharmacokinetically adherent or non-adherent according to whether or not their serum drug concentrations remained within the therapeutic range.
Keywords: pharmacokinetic adherence, drug therapy/utilization, drug administration schedule, patient compliance, adalimumab, pharmacokinetics
Introduction
Medication adherence is typically defined as a ratio of the number of drug doses taken to the number of doses prescribed over a given time period, eg, as the medication possession ratio (MPR).1 The canonical threshold for adherence is 80%, based on Haynes’ definition of adherence to antihypertensive medication as taking ≥80% of pills.2 This and similar thresholds are arbitrary.3 The use of such arbitrary categories of good and poor adherence is almost invariably unsupported by research documenting the appropriateness of the cutoff for a specific medication class or disease.4 In addition, the MPR is a unidimensional concept that does not differentiate between different patterns of non-adherence at any given MPR value. Hence, the MPR does not make use of the detailed information on timing of doses that is provided by a medication event monitoring system (MEMS).
Relationships between patterns of adherence – ie, distributions of time intervals between doses – and drug exposure (ie, serum levels) can be analyzed by pharmacokinetic modeling.5 Maclean et al used pharmacokinetic modeling to compute the serum drug levels resulting from different patterns of adherence and compared them to the therapeutic range of the drug.6 These authors proposed that patients with varying patterns of medication-taking behavior could be dichotomized according to whether or not their daily serum drug concentration remained within the therapeutic range.6 Maclean et al applied pharmacokinetic modeling to mathematically simulated clusters of medication-taking behaviors rather than to individual patients.6 In this paper, we apply pharmacokinetic modeling to MEMS data of individual patients prescribed adalimumab.
Methods
Data sources and variables
We used pharmacokinetic modeling to calculate the effect of different distributions of dosing intervals on serum levels of adalimumab. Adalimumab is a US Food and Drug Administration (FDA)-approved treatment for psoriatic and rheumatoid arthritis, Crohn’s disease, ankylosing spondylitis, plaque psoriasis, juvenile idiopathic arthritis, and ulcerative colitis.7,8 Pharmacokinetic parameters were obtained from published data for 30 patients with active rheumatoid arthritis receiving 40 mg adalimumab by subcutaneous injection every 14 days.9 Pharmacokinetic variables required for the model were the steady-state volume of distribution (Vd), the clearance (CL), and absorption rate constant (Ka). The values reported by Ternant et al were: Vd=10.8 L, CL=0.013 L/h, and Ka=0.0117/h.9
Adherence patterns, ie, timing of doses, were from West et al who electronically monitored self-administration of adalimumab in seven patients with moderate to severe psoriasis who participated in a 1-year open-label trial of educational materials.10 One patient (number 3) was lost to follow-up at approximately 190 days. The intervals (days) between doses are presented in Table 1. None of the seven patients exhibited perfect adherence, ie, took adalimumab precisely every 14 days. The interval between doses varied from a range of 6–18 days for patient number 7 to a range of 7–93 days for patient number 2 (Table 1).
  | Table 1 Dosing intervals and time below therapeutic threshold for patients 1–7 |
Several studies have shown that serum levels of adalimumab correlate with clinical outcomes.11–13 Serum concentrations correlated with clinical remission of inflammatory bowel disease and with clinical response in patients with rheumatoid arthritis.11–13 Trough levels of adalimumab of less than 4.9 μg/mL were associated with an absence of mucosal healing in patients with Crohn’s disease or ulcerative colitis.13 We therefore took 4.9 mg/L as the therapeutic threshold of adalimumab serum concentration. An upper limit for the therapeutic range, above which adalimumab becomes toxic, has not been described, and no dose-related adverse effects have been observed at doses up to 10 mg/kg (>10 times the 40 mg dose for an average adult weighing 70 kg) when injected intravenously.14,15
Pharmacokinetic modeling
Pharmacokinetic modeling analyses were performed using the SIM program of ADAPT (version 5; Biomedical Simulations Resource, University of Southern California, Los Angeles, CA, USA). The pharmacokinetic parameters were consistent with a one-compartment model.9 A one-compartment model (1compcl. for) was thus used for individual simulation with output error. The model incorporated a series of differential equations that calculated the serum concentration of adalimumab as a function of Vd, CL, and Ka.
Data files were created in ADAPT to replicate the dosing intervals for each patient in the West et al article.10 In our analyses, the dose at time 0 (day 1 in West et al) was 80 mg, and all subsequent doses were 40 mg. This dosing regimen was based on the modification of the recommended regimen16 implemented in the CHARM trial.17 Simulations were run for each of the seven patients in West et al using their individual dosing patterns. Serum concentrations were plotted versus time to show the effect of each patient’s pattern of dosing on adalimumab serum levels. The percentage of days below the therapeutic threshold was calculated by determining if the final serum concentration measurement for each day was below 4.9 mg/L. If so, then that day was categorized as below threshold. Otherwise, that day was categorized as above threshold. This calculation disregarded data on days 0–6 to account for the time it took to achieve the therapeutic serum concentration after the initial dose.
Results
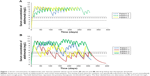
Serum concentrations of adalimumab are plotted against time in days for patients 1–7 in Figure 1. Three patients (numbers 3, 6, and 7) maintained serum concentrations above the therapeutic threshold of 4.9 mg/L for the entire observation period (Figure 1A). Serum levels fell below the threshold at least some days for patients 1, 2, 4, and 5 (Figure 1B). One patient (number 2) spent 71.3% of the days below the threshold, and two other patients, numbers 1 and 4, fell below the threshold on 23.2% and 31.8% of the days, respectively (Table 1). Patient number 5 fell below the therapeutic threshold only briefly, for 3.5% of the days at the end of the observation period (Table 1).
Discussion
The method described here – pharmacokinetic modeling of serum drug levels resulting from a patient’s particular dosing pattern – leads to three advances. First, it provides an objective means of defining a threshold for adherence – ie, a therapeutic drug serum level – whereas adherence has typically been defined in terms of an arbitrary cutoff – eg, an MPR. Second, it enables the detailed timing of doses provided by MEMS to be interpreted in terms of the clinical effects. Third, it enables identification of patients whose pattern of medication-taking behavior puts them at risk of therapeutic failure, so that they can be targeted for interventions designed to improve their adherence behavior. For example, patients whose MEMS data indicate their serum drug levels have fallen below the therapeutic range could be prompted in real time to take their next dose.
The pharmacokinetic modeling method represents a conceptual change in the definition of adherence. Instead of a unidimensional measure, in which adherence is defined as a point on a linear scale, adherence is now defined in terms of distributions of dosing intervals. We define pharmacokinetic adherence as any pattern of dosing that maintains a serum concentration within the therapeutic range. More broadly, medication adherence has traditionally been defined as whether a patient complies with a physician’s instructions. Instead, we define it as whether or not a pattern of patient behavior results in an unfavorable outcome, ie, whether or not the serum drug level remains within the therapeutic range.
In the present study, all of the seven patients sometimes took adalimumab at shorter dosing intervals than the prescribed 14 days. This would tend to lead to higher serum levels than maintained by the 14-day dosing interval. The levels achieved, however, never exceeded 11 mg/L, and no dose-related adverse effects have been observed at much higher levels of adalimumab.14,15 Hence, the upper limit of the therapeutic range, which may be highly relevant to some drugs, does not appear to be important for adalimumab.
While MEMS provides extraordinary detailed information about the timing of doses, there is an inherent limitation – the act of opening/closing the container does not necessarily mean that the patient actually consumed the medication. Other potential limitations of this study include the fact that the dosing intervals were from patients with psoriasis and may not be representative of medication-taking behavior in patients with other inflammatory diseases. In addition, pharmacokinetic parameters for adalimumab were from patients with rheumatoid arthritis, and may differ from values in patients with other inflammatory diseases, due to differences in drug metabolism. However, the FDA-approved maintenance dosing regimen is the same across all indications (40 mg every other week), which suggests that the variations in the pharmacokinetic parameters across different diseases are not expected to be greater than those among patients with the same disease. The pharmacokinetic models we employed did not take into account individual patient variables, such as weight and body mass index, which may affect serum drug levels, as this information was not available. These variables can, in principle, be incorporated in future pharmacokinetic modeling studies. Similarly, other factors that might affect serum drug levels, such as co-medications, were not considered in this analysis but could in principle be addressed by pharmacokinetic modeling. The pharmacokinetic modeling approach does not take into account the loss of clinical response due to development of antibodies to adalimumab, which occurs in some patients on long-term treatment.18 Antibody testing would be required to detect this. Finally, the validity of the method depends on knowledge of the relationship between serum drug levels and clinical outcomes. We took a serum concentration of adalimumab of 4.9 mg/L as a cutoff value – the trough level below which mucosal healing does not occur in patients with Crohn’s disease or ulcerative colitis.13 This is similar to the value of 5.05 mg/L, the median concentration reported in Crohn’s disease patients who did not achieve clinical remission.11 Other values reported in patients with rheumatoid arthritis are 5.4 mg/dL (the median serum concentration in non-responders)12 and 3.6 mg/dL (the serum concentration leading to a 50% decrease in CRP)9. The application of the latter two cutoffs would not change the assignment of patients except in the case of patient number 5, whose serum levels never fell below 3.7 (Figure 1B). For the remaining patients with serum levels below the therapeutic threshold, use of the cutoff value of 3.6 mg/L would simply decrease the proportion of days with serum concentrations below the threshold. Further research may be required to examine the relationship between serum threshold levels and clinical response.
In conclusion, this pharmacokinetic modeling study showed that patients with varying patterns of adalimumab dosing could be classified according to whether or not their serum drug concentrations remained within the therapeutic range. A dichotomy between pharmacokinetic adherence and non-adherence can be defined by an objective rather than arbitrary threshold. The concept of pharmacokinetic adherence can be applied to any drug for which pharmacokinetic parameters and a therapeutic range are known. This approach could be used to identify individual at-risk patients who could be targeted for intervention. Furthermore, studies of adherence – eg, estimation of a population adherence rate, studies of factors associated with adherence, and trials of interventions to improve adherence – could be based on an objective definition of adherence rather than an arbitrary threshold.
Acknowledgment
The authors thank Paul Hutson, PhD, of the University of Wisconsin-Madison, for guidance on the ADAPT simulations. The authors did not receive external funding for the research.
Author contributions
AM and MES conceived and designed the study, MES performed the analyses, all authors interpreted the data and critically revised the manuscript, and all authors approved the final version.
Disclosure
The authors have no conflicts of interest to disclose in this work.
References
Steiner JF, Prochazka AV. The assessment of refill compliance using pharmacy records: methods, validity, and applications. J Clin Epidemiol. 1997;50(1):105–116. | ||
Haynes RB. A critical review of the “determinants” of patient compliance with therapeutic regimens. In: Sackett DL, Haynes RB, editors. Compliance with therapeutic regimens. Baltimore, MD: Johns Hopkins University Press; 1976:26–39. | ||
Claxton AJ, Cramer J, Pierce C. A systematic review of the associations between dose regimens and medication compliance. Clin Ther. 2001; 23(8):1296–1310. | ||
Cramer JA, Roy A, Burrell A, et al. Medication compliance and persistence: terminology and definitions. Value Health. 2008;11(1):44–47. | ||
Kenna LA, Labbe L, Barrett JS, Pfister M. Modeling and simulation of adherence: approaches and applications in therapeutics. AAPS J. 2005;7(2):E390–E407. | ||
Maclean R, Pfister M, Zhou Z, et al. Quantifying the impact of nonadherence patterns on exposure to oral immunosuppressants. Ther Clin Risk Manag. 2011;2011(7):149–156. | ||
US Food and Drug Administration. HUMIRA (adalimumab) [prescribing information;]. Chicago: Abbott Laboratories; 2002. | ||
US Food and Drug Administration. FDA approves Humira to treat ulcerative colitis [press release]. Maryland, United States: US Food and Drug Administration; 2012. | ||
Ternant D, Ducourau E, Fuzibet P, et al. Pharmacokinetics and concentration-effect relationship of adalimumab in rheumatoid arthritis. Br J Clin Pharmacol. 2015;79(2):286–297. | ||
West C, Narahari S, O’Neill J, et al. Adherence to adalimumab in patients with moderate to severe psoriasis. Dermatol Online J. 2013;19(5): 18182. | ||
Chiu YL, Rubin DT, Vermeire S, et al. Serum adalimumab concentration and clinical remission in patients with Crohn’s disease. Inflamm Bowel Dis. 2013;19(6):1112–1122. | ||
Bartelds GM, Wijbrandts CA, Nurmohamed MT, et al. Clinical response to adalimumab: relationship to anti-adalimumab antibodies and serum adalimumab concentrations in rheumatoid arthritis. Ann Rheum Dis. 2007;66(7):921–926. | ||
Roblin X, Marotte H, Rinaudo M, et al. Association between pharmacokinetics of adalimumab and mucosal healing in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2014;12(1): 80–84. | ||
den Broeder A, van de Putte L, Rau R, et al. A single dose, placebo controlled study of the fully human anti-tumor necrosis factor-alpha antibody adalimumab (D2E7) in patients with rheumatoid arthritis. J Rheumatol. 2002;29(11):2288–2298. | ||
Weisman MH, Moreland LW, Furst DE, et al. Efficacy, pharmacokinetic, and safety assessment of adalimumab, a fully human anti-tumor necrosis factor-alpha monoclonal antibody, in adults with rheumatoid arthritis receiving concomitant methotrexate: a pilot study. Clin Ther. 2003;25(6):1700–1721. | ||
Clark M, Colombel JF, Feagan BC, et al. American gastroenterological association consensus development conference on the use of biologics in the treatment of inflammatory bowel disease, June 21–23, 2006. Gastroenterology. 2007;133(1):312–339. | ||
Colombel JF, Sandborn WJ, Rutgeerts P, et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn’s disease: the CHARM trial. Gastroenterology. 2007;132(1):52–65. | ||
Bartelds GM, Krieckaert CL, Nurmohamed MT, et al. Development of antidrug antibodies against adalimumab and association with disease activity and treatment failure during long-term follow-up. JAMA. 2011;305(14):1460–1468. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.