Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 15
Defining Chronic Mucus Hypersecretion Using the CAT in the SPIROMICS Cohort
Authors Stott-Miller M , Müllerová H , Miller B , Tabberer M, El Baou C , Keeley T, Martinez FJ , Han M, Dransfield M, Hansel NN, Cooper CB , Woodruff P, Ortega VE, Comellas AP , Paine R III, Kanner RE , Anderson W, Drummond MB , Kim V , Tal-Singer R , Lazaar AL
Received 11 June 2020
Accepted for publication 25 September 2020
Published 13 October 2020 Volume 2020:15 Pages 2467—2476
DOI https://doi.org/10.2147/COPD.S267002
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Marni Stott-Miller,1 Hana Müllerová,2 Bruce Miller,3 Maggie Tabberer,4 Céline El Baou,5 Tom Keeley,4 Fernando J Martinez,6 Meilan Han,7 Mark Dransfield,8 Nadia N Hansel,9 Christopher B Cooper,10 Prescott Woodruff,11 Victor E Ortega,12 Alejandro P Comellas,13 Robert Paine III,14 Richard E Kanner,14 Wayne Anderson,15 M Bradley Drummond,15 Victor Kim,16 Ruth Tal-Singer,17 Aili L Lazaar3
1GSK R&D, Epidemiology: Value, Evidence and Outcomes, Uxbridge, UK; 2AstraZeneca, Cambridge, UK; 3GSK R&D, Discovery Medicine, Collegeville, PA, USA; 4GSK R&D Patient-Centred Outcomes: Value, Evidence and Outcomes, Uxbridge, UK; 5CEBSTAT Consultancy Ltd, London, UK; 6Cornell Medical College, New York, NY, USA; 7Division of Pulmonary and Critical Care at the University of Michigan, Ann Arbor, MI, USA; 8Children’s of Alabama, Children’s Health Research Unit/University of Alabama, Birmingham, AB, USA; 9Pulmonary and Critical Care Medicine, Johns Hopkins University, Baltimore, MD, USA; 10David Geffen School of Medicine at UCLA, Los Angeles, CA, USA; 11UCSF Department of Medicine, San Francisco, CA, USA; 12Wake Forest School of Medicine, Winston Salem, NC, USA; 13Carver College of Medicine, University of Iowa, Iowa City, IA, USA; 14Division of Pulmonary and Critical Care Medicine, University of Utah, Salt Lake City, UT, USA; 15Division of Pulmonary Diseases and Critical Care Medicine, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA; 16Department of Thoracic Medicine and Surgery, Lewis Katz School of Medicine at Temple University, Philadelphia, PA, USA; 17COPD Foundation, Washington, DC, USA
Correspondence: Aili L Lazaar GSK, 1250 S. Collegeville Road, Collegeville, PA 19426-0989, USA
Tel +1 484-923-3730
Email [email protected]
Background: Chronic cough and phlegm are frequently reported chronic obstructive pulmonary disease (COPD) symptoms. Prior research classified chronic mucus hypersecretion (CMH) based on the presence of these symptoms for ≥ 3 months, called chronic bronchitis (CB) if respiratory infection symptoms were present for 1– 2 years (Medical Research Council [MRC] definition). We explored whether the COPD Assessment Test (CAT), a simple measure developed for routine clinical use, captures CMH populations and outcomes similarly to MRC and St. George’s Respiratory Questionnaire (SGRQ) definitions.
Methods: We identified CMH in the SPIROMICS COPD cohort using (a) MRC definitions, (b) SGRQ questions for cough and phlegm (both as most/several days a week), and (c) CAT cough and phlegm questions. We determined optimal cut-points for CAT items and described exacerbation frequencies for different CMH definitions. Moderate exacerbations required a new prescription for antibiotics/oral corticosteroids or emergency department visit; severe exacerbations required hospitalization. Results were stratified by smoking status.
Results: In a population of 1431 participants (57% male; mean FEV1% predicted 61%), 47% and 49% of evaluable participants had SGRQ- or CAT-defined CMH, respectively. A cut-point of ≥ 2 for cough and phlegm items defined CMH in CAT. Among SGRQ-CMH+ participants, 80% were also defined as CMH+ by the CAT. CMH+ participants were more likely to be current smokers. A higher exacerbation frequency was observed for presence of CMH+ versus CMH− in the year prior to baseline for all CMH definitions; this trend continued across 3 years of follow-up, regardless of smoking status.
Conclusion: Items from the CAT identified SGRQ-defined CMH, a frequent COPD trait that correlated with exacerbation frequency. The CAT is a short, simple questionnaire and a potentially valuable tool for telemedicine or real-world trials. CAT-based CMH is a novel approach for identifying clinically important characteristics in COPD that can be ascertained in these settings.
Keywords: COPD, SGRQ, exacerbation, CAT, cough, phlegm
Introduction
Chronic cough and phlegm production are frequently reported symptoms of chronic obstructive pulmonary disease (COPD). Historically, these symptoms have been reported as “chronic mucus hypersecretion” (CMH), “chronic sputum production,” “mucoid cough,” or “chronic bronchitis” (CB) (with the latter term used in the presence of acute bronchitis or COPD exacerbation history). CMH or CB have been associated with declines in lung function,1–3 increased frequency of exacerbations and hospitalizations,1,3–5 and mortality.6,7 Even in the absence of fixed airflow obstruction, the presence of CMH symptoms has been associated with a variety of poor outcomes, including respiratory complications and an increased risk of death.3,8
At the Ciba Foundation Guest Symposium in 1959, CB was defined as cough with expectoration not attributable to other lung diseases.9 The British Medical Research Council (MRC) definition of CMH has its basis in the CB definition emanating out of the CIBA Symposium, with the only distinction being that it relaxes the requirement of 2 years of symptoms. CMH has been characterized using cough and phlegm items from the St. George’s Respiratory Questionnaire (SGRQ), which comprises items that evaluate: (1) frequency and severity of respiratory symptoms; (2) impact of symptoms on social and psychological functioning, as well as activities that induce/are limited by breathlessness.10 In a comparison of the SGRQ definition of CMH/CB versus the classic definition of CB in the COPDGene study, a greater proportion of subjects were identified with CB with the SGRQ definition compared with the classic CB definition although the groups were substantially similar, with characteristic respiratory symptoms and exacerbations, as well as worse lung function and greater airway wall thickness compared to those without CMH by either definition.10 In addition, the SGRQ definition identified more subjects at risk for future exacerbations than the classic CB definition.11
The COPD Assessment Test (CAT), another tool with increasingly widespread clinical use, is a self-completion questionnaire that contains questions relating to the impact of COPD symptoms. It is short and easy to administer, while retaining the ability to reflect disease severity, risk of future adverse outcomes, and responsiveness to intervention.12,13 The simplicity and ease of administration of the CAT make it an ideal tool for use in routine clinical practice, as well as in real-world trials and digitally enabled telemedicine. Given the frequency of CMH among COPD patients and given the association with poorer outcomes, the ability to identify the trait of CMH using the CAT would be of great pragmatic benefit. We thus sought to explore various cut-points of the cough and phlegm parameters in the CAT to find an optimal definition for CMH.
We aimed to identify and compare CMH populations using the classic MRC-CB definition, the SGRQ definition based on cough and sputum items, and to determine optimal cut-points for a novel CAT-based definition based on its cough and phlegm production items. The objective was to better understand the similarities and differences in populations defined using different instruments, given that there are well-known differences in wording and time frames related to cough and phlegm production items reflected in derived CMH algorithms. In the comparisons, participants with CMH were characterized in terms of demographic and clinical/lifestyle factors and comorbidities. We additionally characterized exacerbation frequency prior to start of study follow-up and evaluated the relationship between CMH and exacerbations occurring during study follow-up.
Materials and Methods
Study Population
Data on COPD participants from the Subpopulations and Intermediate Outcomes Measures in COPD Study (SPIROMICS) were used. SPIROMICS is a multicenter longitudinal study funded by the National Health Lung and Blood Institute (ClinicalTrials.gov identifier: NCT01969344) that was designed to identify different COPD subpopulations and to validate intermediate outcome measures.14 SPIROMICS recruited participants aged 40–80 years between November 2010 and July 2015 who were either healthy never-smokers or current/former smokers with at least a 20 pack-year smoking history, with and without airflow obstruction (defined as post-bronchodilator forced expiratory volume in 1 second [FEV1]/forced vital capacity [FVC] <0.7). The SPIROMICS protocol was approved by the institutional review boards (IRBs) of all participating institutions and all participants gave written informed consent, including how their data and samples were to be stored, used, and shared. Details on the study design and baseline characteristics of the SPIROMICS cohort have been described previously.14 The focus of the present study was only on participants with COPD (strata III and IV in the SPIROMICS cohort) with available data and who provided informed consent for their data to be shared with outside investigators. IRB approval was not required for the present study. Data were provided from the SPIROMICS Genomics and Informatics Center as well as the Publications and Steering Committees thorough formalized processes and policies.
Definitions to Identify CB and CMH
CMH was identified using four separate definitions in the SPIROMICS baseline COPD cohort (Table 1). We assessed CMH in all participants with available data; however, not all participants had complete questionnaire data and therefore could not be characterized for one or more of the definitions.
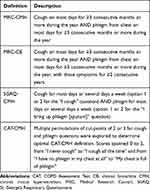 |
Table 1 Questions for Determination of Various Definitions of CMH |
MRC-CB was defined at baseline as per the MRC convention (answering “yes” to questions on whether cough and phlegm production symptoms were experienced over ≥3 months lasting for ≥2 years) using a modified American Thoracic Society – Division of Lung Diseases (ATS-DLD) questionnaire.15 MRC-CMH was defined as symptoms of cough and phlegm over ≥3 months using responses of “yes” to both “Do you usually cough on most days for 3 consecutive months or more during the year?” and “Do you usually bring up phlegm on most days for 3 consecutive months or more during the year?”
SGRQ-CMH was defined from the SGRQ-C, a 40-item questionnaire that is a shorter, disease-specific version of the SGRQ questionnaire and that does not have a defined recall period.16 SGRQ-CMH was present if participants selected the options of “cough for most days a week” or “several days a week” and also selected the options of phlegm/sputum production “most days a week” or “several days a week”.10 These options correspond to the most severe ratings of frequency of symptoms.
CAT-CMH was defined using the CAT, a short 8-item questionnaire developed to assess the impact of COPD on health status.12 Patients score their symptoms on a 0–5 scale, with anchor statements for each item. For cough and phlegm, these span the range from “I never cough” to “I cough all the time” and “I have no phlegm in my chest at all” to “My chest is full of phlegm.” There is no time frame for symptom recall period. We explored definitions using cut-points of 2 or 3 on one of the parameters, as well as all possible combinations of 2 and 3 scores on combined parameters collected at baseline. The final definition of CAT-CMH based on CAT cut-points for cough and phlegm was then selected based on measures of sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) using SGRQ-CMH and MRC-CMH as “gold standards.”
Data Analysis
Data analysis was performed using SAS 9.4 software (SAS Institute, Cary, NC). To understand the distributions of the CAT and SGRQ items used to derive the CMH definitions, histograms and descriptive statistics for CAT cough and sputum questions and SGRQ cough and sputum questions were generated. To better understand the concordance between the various definitions of CMH, cross-tabulations of participants meeting the definitions were generated. We calculated sensitivity, specificity, PPV, NPV, and Cohen’s kappa for multiple potential cut-points for the CAT-based definitions versus SGRQ items and the CAT-based definitions versus the MRC-CMH definition.
We assessed the distribution of the following variables by the CMH definitions: age, sex, body mass index (BMI), self-reported smoking status at baseline, number of pack-years, post-bronchodilator FEV1% predicted, global initiative for chronic obstructive lung disease (GOLD) grade of airflow limitation (I–IV), GOLD A–D classification based on the 2017 GOLD guidelines,17 SGRQ total score, CAT total score, history of previous exacerbations, and exacerbations during follow-up (Years 1, 2, and 3). Exacerbations were defined as: (1) moderate exacerbations requiring a new prescription for antibiotics or oral corticosteroids, or an emergency department visit, or (2) severe exacerbations requiring hospitalization.
Baseline characteristic tables were generated to describe demographics, medical history, pulmonary function, respiratory and disease-related symptoms for all definitions of CMH. For each of the three definitions of CMH and MRC-CB, exacerbation history during the 12 months prior to baseline and exacerbations during the first, second and third year of follow up were quantified. Exacerbations were examined separately for (1) ≥1 moderate exacerbation, (2) ≥1 severe exacerbation, and (3) ≥1 severe and ≥2 moderate exacerbations. Analyses were further stratified by smoking status (current vs former). Although the purpose of the present study was to present descriptive data and visual trends only and is in line with recent trends that move away from reliance on p-values,18,19 simple chi-square tests were performed to compare CMH+ and CMH− history of exacerbations at baseline.
No imputation was performed for patients with missing data. To allow comparisons between the populations of patients with missing SGRQ or CAT scores, the assumption that SGRQ and/or CAT score were missing at random was assessed. This was achieved by ensuring that similar results were obtained when calculating descriptive statistics using the overall population and the populations of patients with missing SGRQ or CAT scores.
Results
There were 1431 participants in the initial pool of eligible participants. Some participants were missing assessment data needed to characterize CMH from one or more of the MRC questions, SGRQ items or CAT items; thus, the number of eligible participants with available data differed across definitions. The number of eligible participants with available questionnaire data was slightly higher for CAT (n=1407) compared with SGRQ (n=1361) and substantially higher compared with MRC-CMH (n=1044) (Table 2). The proportion of CMH+ identified was similar for SGRQ and CAT (using a threshold of ≥2 for both cough and phlegm items), with proportions of 47% and 49%, respectively. A substantially smaller proportion of CMH+ was observed using the MRC-CB definitions or a CAT-based definition that used a threshold of ≥3 for both cough and phlegm items. The MRC-CMH definition identified an intermediate number of participants with CMH.
 |
Table 2 Frequency of CMH by Multiple Definitions |
CAT-Based Definition of CMH
In the evaluation of multiple different CAT thresholds for the CMH definition using SGRQ and MRC as the “gold standards,” a combination of cut-points of ≥2 for the cough question and ≥2 for the phlegm production question in the CAT appeared to provide the best balance across measures of sensitivity, specificity, PPV, and NPV (Table 3). This threshold in the CAT was also the optimal selection based on Cohen’s kappa, which attempts to account for trade-offs between sensitivity, specificity, PPV and NPV. Cohen’s kappa was 0.56 and 0.58 against MRC-CMH and SGRQ-CMH, respectively, suggesting moderate to good agreement.20 Thus, a threshold of ≥2 for both questions in the identification of CMH was chosen as the most pragmatic cut-off for the CAT-based definition in further descriptions.
 |
Table 3 Evaluation of Different CAT Cut-Points Using SGRQ-CMH and MRC-CMH as Gold Standard |
Demographics and Clinical Characteristics
In the overall study population, 57.4% were male with a mean FEV1% predicted of 60.8% (Table 4). There were no appreciable differences in distribution of age, sex, and GOLD grade of airflow limitation in CMH+ versus CMH− participants identified by the CAT or SGRQ (Table 4). Smokers were more likely to be CMH+, while most of the CMH− participants were ex-smokers, regardless of the instrument used.
 |
Table 4 Demographics and Clinical Characteristics of CMH+ and CMH− Participants, Based on SGRQ and CAT Definitions at Baseline |
History of COPD Exacerbations at Baseline and Exacerbations During Follow-Up
A higher percentage of CMH+ participants experienced COPD exacerbations compared with CMH− participants during the 12 months prior to the baseline visit, as well as across all follow-up time points (Years 1, 2, and 3), whether using the SGRQ- and CAT-based definitions (Table 5) or the MRC-CMH and MRC-CB definitions (Figure S1). However, in general, the proportion of participants experiencing exacerbations decreased as follow-up continued. Similar patterns of increased exacerbation frequency among CMH+ participants were observed regardless of smoking status (Figure 1, Figure 2). Given the relatively low frequency of exacerbations in SPIROMICS and the variation in exacerbation status year to year, we also examined exacerbation frequency across all 3 years of follow-up combined, and observed similar trends (Table 5). The trend for greater frequency of exacerbations in CMH+ versus CMH− participants was observed not only across the different definitions of CMH, but also across the different categories of exacerbations (≥1 moderate exacerbation, ≥1 severe exacerbation, and ≥1 moderate and ≥2 severe exacerbations). Chi-square tests to compare history of CMH+ and CMH− exacerbations at baseline indicated significant differences (Table S1).
 |
Table 5 COPD Exacerbations for CMH+ and CMH− Participants Based on SGRQ and CAT Definitions |
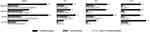 |
Figure 1 COPD exacerbations (percentage) among current smokers by SGRQ and CAT definitions of CMH 12 months prior to baseline and during first, second and third year of follow-up. |
 |
Figure 2 COPD Exacerbations (percentage) among ex-smokers by SGRQ and CAT definitions of CMH 12 months prior to baseline and during first,second and third year of follow-up. |
Similar patterns of increased exacerbation frequency among CMH+ participants were observed regardless of smoking status (Figure 1, Figure 2). In general, the proportion of participants experiencing exacerbations decreased as follow-up continued.
Discussion
The CAT is a short, simple questionnaire for assessing and monitoring health status in patients with COPD.12 The reliability and validity of the CAT, along with its reduced patient burden, make it a potentially useful tool for telemedicine or remote clinical trials. The aim of the present study was to derive a novel definition of CMH in patients with COPD based on the cough and sputum items within the CAT questionnaire.
Previous studies have demonstrated that the SGRQ identifies subjects with chronic cough and sputum who share a similar phenotype as the classic CB definition.10 In this analysis, the prevalence of CMH varied by each definition used, with 38% identified as MRC-CMH and 47% as SGRQ-CMH. Using a CAT cut-point of ≥2 for both cough and phlegm items identified a similar proportion of CMH+ participants as the SGRQ. Using a cut-point of ≥3 identified a smaller population, more analogous to the most stringent definition of CB. The ultimate choice of cut-point may depend on the relative importance of sensitivity versus specificity in relation to the purpose of the analyses. We opted for the cut-point that achieved a balance across all measures: ≥2 for both cough and phlegm items. Our recommended definition of CMH utilizing CAT items yielded very good sensitivity (78%) and specificity (80%) and PPV (77%), indicating very good agreement between CAT-CMH and SGRQ-CMH, suggesting these two questionnaires measure similar aspects of the disease.21 Both populations were similar with regard to demographics and smoking status. The moderate to good agreement observed in Cohen’s kappa score suggests that we identified largely the same participants.
As has been observed in this and other large cohorts,4,22 history of exacerbations was higher in CMH+ participants than CMH− participants. We additionally examined exacerbation frequency prospectively and observed that a higher percentage of CMH+ participants experienced COPD exacerbations compared with CMH− participants. When followed prospectively, we observed that the relative proportion of exacerbators was similar whether using SGRQ or CAT.
Regardless of smoking status or presence of CMH, the number of exacerbations identified in Years 1–3 was lower than the reporting of historical exacerbations at baseline, as has been previously reported.22,23 This is consistent with data demonstrating only modest repeatability of exacerbation recall in COPD patients and the variability of exacerbations in any individual.21,22 In general, the proportion of participants experiencing exacerbations decreased with follow up. Participants who experienced exacerbations earliest may also possibly have had the poorest health and were thus less likely to return for follow-up visits or calls.
Our study has several limitations. There is no specific patient-reported outcome (PRO) to collect information on CMH that can serve as a “gold standard.” We are using proxy measures of a traditional chronic bronchitis definition and individual items from questionnaires developed to measure health status in COPD. Further, we expect there can be patient selection bias impacting the SPIROMICS COPD patient population; participants were either selected based on their attendance of the investigator’s clinic or volunteered based on the study-related advertisement at clinics. Additionally, this prospective study used a questionnaire to collect data on participant medical history. Some of its modules were largely self-reported including healthcare utilization, exacerbations of COPD, and current treatment for COPD. We may expect recall bias to impact these variables.
Conclusions
CMH is a common COPD trait, occurring in nearly half of the SPIROMICS cohort. The CAT-based definition, using the selected threshold of 2 for both cough and phlegm items, identified a very similar proportion of CMH+ participants as the SGRQ definition. In addition to its use in health status assessment and clinical practice, the CAT has frequently been used as an entry criterion and efficacy outcome in clinical development programs and has thus been proposed for use as a clinical outcomes assessment drug development tool.24 The time to complete the CAT is significantly shorter than that for the SGRQ and it requires less assistance;25 it has been used in telehealth cohorts to monitor exacerbation risk or the impact of pulmonary rehabilitation.26,27 The use of the CAT-based definition would thus be of particular benefit in real-world trials where CMH is a trait to be identified. Further work and validation in other cohorts will be necessary to understand whether subtle differences exist between the two, with potential impact on patient selection or risk assessment.
Data Sharing Statement
More information about the study and how to access SPIROMICS data is available at https://www.spiromics.org/spiromics/obtaining-data.
Acknowledgments
The authors thank the SPIROMICS participants and participating physicians, investigators and staff for making this research possible. We would like to acknowledge all current and former investigators of the SPIROMICS sites and reading centers. Editorial support (in the form of copyediting and referencing) was provided by Jenni Lawton, PhD, of Gardiner-Caldwell Communications, Macclesfield, United Kingdom, and was funded by GlaxoSmithKline plc. The authors also thank Wilhelmine H Meeraus, PhD, for review and input into the manuscript. Trademarks are owned by or licensed to the GlaxoSmithKline group of companies.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
SPIROMICS was supported by contracts from the NIH/NHLBI (HHSN268200900013C, HHSN268200900014C, HHSN268200900015C, HHSN268200900016C, HHSN268200900017C, HHSN268200900018C, HHSN268200900019C, HHSN268200900020C), grants from the NIH/NHLBI (U01 HL137880 and U24 HL141762), and supplemented by contributions made through the Foundation for the NIH and the COPD Foundation from AstraZeneca/MedImmune; Bayer; Bellerophon Therapeutics; Boehringer-Ingelheim Pharmaceuticals, Inc.; Chiesi Farmaceutici S.p.A.; Forest Research Institute, Inc.; GlaxoSmithKline; Grifols Therapeutics, Inc.; Ikaria, Inc.; Novartis Pharmaceuticals Corporation; Nycomed GmbH; ProterixBio; Regeneron Pharmaceuticals, Inc.; Sanofi; Sunovion; Takeda Pharmaceutical Company; and Theravance Biopharma and Mylan.
Disclosure
MS-M, BM, ALL, MT, and TK are employees and shareholders of GlaxoSmithKline plc. HM, CEB and RT-S are former employees and shareholders of GlaxoSmithKline plc. HM is a current employee of AstraZeneca. CEB has also provided contracting for Eli Lilly, outside of the submitted work. RT-S also reports receipt of consulting fees from ImmunoMet outside of the submitted work. FJM reports a grant from NHLBI during the conduct of the study; serving on steering committees for GlaxoSmithKline plc., Afferent/Merck, AstraZeneca, Bayer, Boehringer Ingelheim, Gilead, Nitto, Patara/Respivant, Pearl Pharmaceuticals, ProMedior/Roche, ProMetic, Stromedix/Biogen and Veracyte; being a member of advisory boards for GlaxoSmithKline plc., AstraZeneca, Boehringer Ingelheim, Bioscale/Proterrix Bio, Chiesi, Gala, Genentech, Novartis, Pearl Pharmaceuticals, Physicians Education Resource, CSL Behring, Sunovion, Teva and Zambon; consulting for BristolMyersSquibb, Bridge Biotherapeutics and two XR; has received continuing medical education presentation support from the Canadian Respiratory Network, Chiesi, CME outfitters, Dartmouth University, France Foundation, Inova Fairfax, MD Magazine, Methodist Hospital, Miller Communications, National Association for Continuing Education/Haymarket, New York University, PeerView, Prime Education, Rare Diseases Healthcare Communication, Rockpointe, University of Alabama Birmingham, UpToDate, Vindico, WebMD/MedScape, Zambon; also DSMB for Boehringer Ingelheim and GlaxoSmithKline plc. MH reports a grant from NHLBI during the conduct of the study; personal fees from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline plc., Merck and Mylan; research support from Novartis and Sunovion outside of the submitted work. MD reports grants from NIH during the conduct of the study and from NIH, American Lung Association, Department of Veterans Affairs, and Department of Defense outside of the submitted work. He reports personal fees from Boehringer Ingelheim, GlaxoSmithKline plc., PneumRx/BTG, AstraZeneca, Quark Pharmaceuticals, and Mereo; has contracted clinical trials for Boehringer Ingelheim, GlaxoSmithKline plc., Novartis, AstraZeneca, Yungjin, PneumRx/BTG, Pulmonx, Boston Scientific, Gala and Nuveira; and has received non-financial support from Pulmonx, outside the submitted work. NNH reports research grants from AstraZeneca, Boehringer Ingelheim, COPD Foundation, GlaxoSmithKline plc., NIH; advisory board fees for AstraZeneca, GlaxoSmithKline plc., Mylan. CBC reports grants from NIH/NHLBI and the NIH Foundation during the conduct of the study; personal fees from MGC Diagnostics, NUVEIRA and PulmonX; and has acted as a global medical expert for GlaxoSmithKline plc., outside of the submitted work. VK has received personal fees from Gala Therapeutics, AstraZeneca, Boehringer Ingelheim, and the American Board of Internal Medicine over the last 3 years, outside of the submitted work. APC reports a grant from NIH and receipt of consulting fees from GlaxoSmithKline plc., and non-financial support from VIDA outside of the submitted work. RP III reports research grants from COPD Foundation and NHLBI, and from the Department of Veterans Affairs outside of the submitted work. MBD reports a grant from NIH-NHLBI during the conduct of the study and receipt of consulting fees from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline plc., Mylan Theravance, and Parion, outside of the submitted work. REK, PW, VEO and WA have no conflicts of interest to report. The authors report no other conflicts of interest in this work.
References
1. Vestbo J, Prescott E, Lange P. Association of chronic mucus hypersecretion with FEV1 decline and chronic obstructive pulmonary disease morbidity. Copenhagen City Heart Study Group. Am J Respir Crit Care Med. 1996;153(5):1530–1535. doi:10.1164/ajrccm.153.5.8630597
2. Guerra S, Sherrill DL, Venker C, Ceccato CM, Halonen M, Martinez FD. Chronic bronchitis before age 50 years predicts incident airflow limitation and mortality risk. Thorax. 2009;64(10):894–900. doi:10.1136/thx.2008.110619
3. Dotan Y, So JY, Chronic Bronchitis KV. Where Are We Now? Chronic Obstr Pulm Dis. 2019;6:178–192.
4. Kim V, Han MK, Vance GB, et al. The chronic bronchitic phenotype of COPD: an analysis of the COPDGene Study. Chest. 2011;140(3):626–633. doi:10.1378/chest.10-2948
5. Agusti A, Calverley PM, Celli B, et al. Characterisation of COPD heterogeneity in the ECLIPSE cohort. Respir Res. 2010;11(1):122. doi:10.1186/1465-9921-11-122
6. Mannino DM, Buist AS, Petty TL, Enright PL, Redd SC. Lung function and mortality in the United States: data from the First National Health and Nutrition Examination Survey follow up study. Thorax. 2003;58(5):388–393. doi:10.1136/thorax.58.5.388
7. Prescott E, Lange P, Vestbo J. Chronic mucus hypersecretion in COPD and death from pulmonary infection. Eur Respir J. 1995;8(8):1333–1338. doi:10.1183/09031936.95.08081333
8. Mannino DM, Doherty DE, Buist AS. Global Initiative on Obstructive Lung Disease (GOLD) classification of lung disease and mortality: findings from the Atherosclerosis Risk in Communities (ARIC) study. Respir Med. 2006;100:115–122.
9. Fletcher CM, Pride NB. Definitions of emphysema, chronic bronchitis, asthma, and airflow obstruction: 25 years on from the Ciba symposium. Thorax. 1984;39(2):81–85. doi:10.1136/thx.39.2.81
10. Kim V, Crapo J, Zhao H, et al. Comparison between an alternative and the classic definition of chronic bronchitis in COPDGene. Ann Am Thorac Soc. 2015;12(3):332–339. doi:10.1513/AnnalsATS.201411-518OC
11. Kim V, Zhao H, Regan E, et al. The St. George’s Respiratory Questionnaire Definition of Chronic Bronchitis May Be a Better Predictor of COPD Exacerbations Compared With the Classic Definition. Chest. 2019;156(4):685–695. doi:10.1016/j.chest.2019.03.041
12. Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD Assessment Test. Eur Respir J. 2009;34(3):648–654. doi:10.1183/09031936.00102509
13. Jones PW, Brusselle G, Dal Negro RW, et al. Properties of the COPD assessment test in a cross-sectional European study. Eur Respir J. 2011;38(1):29–35. doi:10.1183/09031936.00177210
14. Couper D, Lavange LM, Han M, et al. Design of the Subpopulations and Intermediate Outcomes in COPD Study (SPIROMICS). Thorax. 2014;69(5):491–494. doi:10.1136/thoraxjnl-2013-203897
15. Ferris BG. Epidemiology Standardization Project (American Thoracic Society). Am Rev Respir Dis. 1978;118::1–120.
16. Meguro M, Barley EA, Spencer S, Jones PW. Development and Validation of an Improved, COPD-Specific Version of the St. George Respiratory Questionnaire. Chest. 2007;132(2):456–463. doi:10.1378/chest.06-0702
17. Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report. GOLD Executive Summary. Am J Respir Crit Care Med. 2017;195(5):557–582. doi:10.1164/rccm.201701-0218PP
18. Wasserstein RLN, Lazar NA. The ASA Statement on p -values: context, Process, and Purpose. Am Stat. 2016;70(2):129–133. doi:10.1080/00031305.2016.1154108
19. Harrington D, D’Agostino RB, Gatsonis C. New Guidelines for Statistical Reporting in the Journal. N Engl J Med. 2019;381(3):285–286. doi:10.1056/NEJMe1906559
20. Mchugh ML. Interrater reliability: the kappa statistic. Biochem Med (Zagreb). 2012;22:276–282.
21. Anderson WH, Ha JW, Couper DJ, et al. Variability in objective and subjective measures affects baseline values in studies of patients with COPD. PLoS One. 2017;12(9):e0184606. doi:10.1371/journal.pone.0184606
22. Han MK, Quibrera PM, Carretta EE, et al. Frequency of exacerbations in patients with chronic obstructive pulmonary disease: an analysis of the SPIROMICS cohort. Lancet Respir Med. 2017;5(8):619–626. doi:10.1016/S2213-2600(17)30207-2
23. Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363(12):1128–1138. doi:10.1056/NEJMoa0909883
24. Mullerova H, Dransfield MT, Thomashow B, et al. Clinical Development and Research Applications of the COPD Assessment Test (CAT). Am J Respir Crit Care Med. 2019. doi:10.1164/rccm.201907-1369PP.
25. Ringbaek T, Martinez G, Lange P. A comparison of the assessment of quality of life with CAT, CCQ, and SGRQ in COPD patients participating in pulmonary rehabilitation. COPD. 2012;9(1):12–15. doi:10.3109/15412555.2011.630248
26. Rassouli F, Baty F, Stolz D, et al. Longitudinal change of COPD assessment test (CAT) in a telehealthcare cohort is associated with exacerbation risk. Int J Chron Obstruct Pulmon Dis. 2017;12:3103–3109. doi:10.2147/COPD.S141646
27. Bhatt SP, Patel SB, Anderson EM, et al. Video Telehealth Pulmonary Rehabilitation Intervention in Chronic Obstructive Pulmonary Disease Reduces 30-Day Readmissions. Am J Respir Crit Care Med. 2019;200(4):511–513. doi:10.1164/rccm.201902-0314LE
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
