Back to Journals » Research and Reviews in Parkinsonism » Volume 8
Deep-brain stimulation for Parkinson's disease: current perspectives on patient selection with an emphasis on neuropsychology
Authors Tröster AI , Ponce FA, Moguel-Cobos G
Received 26 February 2018
Accepted for publication 15 June 2018
Published 17 September 2018 Volume 2018:8 Pages 33—48
DOI https://doi.org/10.2147/JPRLS.S125332
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Peter Hedera
Alexander I Tröster,1,2 Francisco A Ponce,2,3 Guillermo Moguel-Cobos4,5
1Department of Clinical Neuropsychology, Barrow Neurological Institute, 2Center for Neuromodulation, Barrow Neurological Institute, 3Department of Neurology, Barrow Neurological Institute, 4Department of Neurosurgery, Barrow Neurological Institute, 5Muhammad Ali Movement Disorders Center, Barrow Neurological Institute, Phoenix, AZ, USA
Abstract: For some persons with Parkinson’s disease, deep-brain stimulation (DBS) is an effective treatment that enhances function and quality of life. It is critical that the preoperative evaluation process yields information that allows the treatment team to determine the likelihood that DBS (directed at a specific target) will be an effective and safe treatment for a given person and that the treatment will meet that person’s goals and expectations. Such information allows the team and the patient to perform a cost–benefit analysis and the patient and family to make an informed decision about the potential appropriateness of DBS, and ultimately whether or not to undergo DBS or alternative treatments. We review the multidisciplinary DBS evaluation and education process in general (and by exemplar at Barrow Neurological Institute) engaging the patient with neurology, neuropsychology, neurosurgery, neuroscience nursing, and when needed, psychiatry, social work, and additional medical subspecialties. The review first covers screening, including two standardized instruments, and then the more detailed preoperative evaluation that ensues after screening. Neuropsychological issues in patient selection, and especially cognition, are emphasized, because they remain the most controversial and yet often underlie the judgment that DBS is not an appropriate treatment for a given patient. Outcome studies, perhaps via large, multisite patient registries, are needed to identify neuropsychological risks for unsatisfactory outcome and to define better which surgery (e.g., target, side, timing) is best for a given patient. Such studies would ultimately allow one to judge whether current selection criteria are adequate, need to be stricter, or can be relaxed, and, consequently ensure that the therapy is accessible to the maximum number of persons who will benefit from it without significant adverse effects.
Keywords: neurosurgery, neuropsychology, cognition, quality of life, emotion, patient expectations
Introduction
Deep brain stimulation (DBS) for Parkinson’s disease (PD) entered widespread clinical use in the early 2000s. DBS is an effective symptomatic treatment that improves function and quality of life in PD, regardless of whether the therapy uses constant-voltage or constant-current devices.1–5 Furthermore, DBS may even benefit patients relatively early in the disease course, especially if they have motor complications, such as dyskinesias,6,7 and do so in a cost-effective manner.8 For PD, thalamic DBS9,10 is rarely used anymore, given that internal globus pallidus (GPi) and subthalamic nucleus (STN) DBS provide more potent and broader symptomatic improvement. Since Pollak et al published their case report of STN DBS,11 many more studies have appeared. A PubMed search using the terms “Parkinson’s disease and DBS” yields more than 2,400 articles, although the annual publication rate on the topic has recently plateaued.12 Early on, the preference for the STN vs GPi target seemed to be based on personal experience and opinion, the strength of which at times appeared inversely proportional to the empirical evidence available to support that opinion. Increases in the past decade in the availability of methodologically sound, clinical randomized trials comparing DBS outcomes of the two targets have led some to propose a more rational, patient-centered approach to DBS based on the emerging STN and GPi symptomatic benefit and safety profiles.13–15
It is obvious that presurgical evaluation, if it is to determine appropriateness of DBS for a given individual, must address at least two elements of the treatment: its effectiveness and safety. It is increasingly clear that a third element, namely patient goals and expectations16,17 also needs to be considered if DBS outcome is to be optimized. Consideration must specifically be given to which symptoms are most important to control from the patient’s perspective and a functional standpoint, and to the extent of symptomatic, functional, and quality-of-life (QoL) improvement (in the absence of significant adverse events) expected by the patient. Certainly, the importance of patient expectations in surgical outcome has been known for other functional neurosurgical interventions (eg, lobectomy for epilepsy) for some time.18–20 Preoperative evaluation provides a valuable opportunity for patient and care-partner education that can be especially helpful in shaping realistic expectations, allow the patient to feel prepared for surgery, and facilitate postoperative adjustment.21,22
Given the complexity of presurgical evaluation and the time and cost involved, some proposed a screening process (that can especially be used by primary-care physicians and general neurologists) to ensure adequate and efficient access of patients to a limited number of specialty centers.23 Additionally, structured, standardized prescreening questionnaires have been developed to aid in efficient patient evaluation and determination of the potential appropriateness of DBS.
This review is not a systematic one, but like an umbrella review began by considering recent reviews and meta-analyses identified by a PubMed search. Articles were then supplemented by others found in literature citations of the chosen articles. The PubMed search included the terms Parkinson* AND (deep brain stimulation OR DBS) AND neuropsych* AND selection. This search yielded 64 articles. The review first examines standardized screening tools and initial screening considerations. Thereafter, it describes in more detail the fuller presurgical evaluation process and especially the neuropsychological evaluation that remains a debated but almost universally accepted aspect of the presurgical evaluation.24–27 The appropriateness of subthalamic vs pallidal, unilateral vs bilateral, and asleep vs awake surgery is also briefly discussed.
Screening
Both the referral of persons for whom DBS is inappropriate and nonreferral of those for whom DBS is appropriate diminish the efficiency of the presurgical patient-selection process.23,28 At least two standardized questionnaires have been developed for use by referring primary-care physicians and non-subspecialists in movement disorders to enhance referral of those persons for whom DBS has a high likelihood of being an appropriate treatment. The Florida Surgical Questionnaire for Parkinson Disease (FLASQ-PD) was developed at one US movement-disorder surgery center (University of Florida) to improve surgical referral patterns.29 The 5-section questionnaire addresses the criteria-based diagnosis of probable PD, absolute contraindications to surgery, or “red flags” (eg, supranuclear gaze palsy, apraxia, greater than mild dementia), general patient characteristics (eg, age, disease duration), characteristics that are (un)favorable toward good outcomes (eg, levodopa responsiveness of motor symptoms, use of anticoagulants, incontinence), and prior medication-trial information. The best score is high (maximum 34, with no red flags) while the worst score is 0, with eight red flags.
A second screening instrument (available as an electronic decision tool) was developed by a group of movement-disorder surgery experts from Europe and Canada.28 The instrument (Stimulus-DBS) was developed using the Research and Development Corporation/University of California at Los Angeles appropriateness method that combines the best available empirical evidence with expert consensus to formulate a statement regarding the appropriateness of performing a procedure at the level of patient-specific symptoms, medical history, and test results. The first step in using the tool is to determine whether a person meets all five inclusion criteria. These criteria cover diagnosis of PD, existence of troublesome symptoms despite optimal pharmacotherapy, levodopa responsiveness, medical conditions contraindicating surgery, and “absence of significant medically resistant mental diseases”. If the inclusion criteria are met, seven other factors are assessed via two- or three-alternative forced-choice responses (eg, age, disease duration). The domains covered overlap almost completely with the FLASQ-PD, but the Stimulus-DBS is briefer. One center (University of Michigan) compared the use of the two instruments or algorithms. It was found that when compared to the center’s questionnaire-independent, multidisciplinary decision to offer DBS to 50 of 130 consecutive persons referred and presenting for evaluation (82 were placed on the DBS-evaluation pathway), Stimulus-DBS was superior (positive predictive value [PPV] about 60%, negative predictive value [NPV] 93%) to FLASQ-PD (PPV 50%, NPV 69%) in predicting from suggested cutoff scores (Stimulus-DBS ≥7, FLASQ-PD ≥25) which patients were ultimately offered DBS.23 A subsequent multicenter study in Europe found the Stimulus-DBS tool to have PPV of 79% and NPV of 75%,30 and that 77% of those screened (and in whom DBS was appropriate according to the tool) were accepted for DBS, as opposed to 48% of an unscreened group.
Notwithstanding their utility, it should be noted that both screening tools well define the neurological and demographic parameters to be evaluated, but are quite vague about cognitive and neuropsychiatric issues. As noted by Okun et al, perhaps the most controversial aspect of patient selection is the definition of what is an acceptable level of cognitive dysfunction.31 The FLASQ-PD considers “more than mild dementia” a red flag, and a general rule is that persons with multiple memory or cognitive problems and a tendency toward frequent disorientation are poor surgical candidates. That rule is consonant with the definition of severe intellectual impairment offered by the Stimulus-DBS tool: severe memory loss with disorientation for time and often to place; severe impairment in handling problems. These impairment characteristics probably remove from the continued DBS-evaluation process those with obvious dementia, but leave for consideration a large number of patients with still clinically evident cognitive disturbance (eg, with mild cognitive impairment associated with PD [PD-MCI]). Neither tool, however, offers explicit diagnostic criteria or assessment guidelines for cognition and behavior.
As suggested by Tröster,32 cognitive screening instruments might most appropriately be used to terminate the presurgical evaluation process and avoid unnecessary, taxing, and lengthy procedures when it is already evident that DBS is not indicated due to dementia. However, full neuropsychological evaluation is probably the best course of action among those passing screening, given the considerable prevalence of PD-MCI, the generally poor sensitivity of screening instruments to PD-MCI, and currently inadequate knowledge about the impact of various degrees and phenotypes of PD-MCI on DBS outcomes. Additionally, it is our experience that occasionally, cognitively intact patients (or their family members) will report during neuropsychological evaluation (perhaps given the appropriateness of the setting) previously undisclosed psychiatric issues, especially suicidal ideation, substance abuse, and possibly embarrassing behaviors, such as hypersexuality. The need for detailed neuropsychological evaluation is also apparent when one considers that when DBS is considered inappropriate for a patient: it is considered inappropriate for neuropsychological reasons in as many as about 50% of cases.
Reasons for screening/evaluation failures
Several studies have examined why DBS was not deemed to be an appropriate treatment for some patients during screening or full multidisciplinary evaluation. Although an early study by a specialty movement-disorder surgery center (University of Florida) in 2004 reported that DBS was deemed inappropriate for about 63% of referred patients,29 it is likely that screening and referral appropriateness has improved since then. More recent reports by several centers indicate that they did not deem DBS appropriate in about a quarter of patients who had undergone screening.30 Of those patients excluded, about a third were excluded for cognitive reasons (eg, dementia, MCI “suggestive of cortical dysfunction”) and about a fifth for behavioral reasons (eg, uncontrolled anxiety or depression), either alone or in combination with other factors.33 These numbers are close to ones suggesting that of those excluded, about 48% were not considered further, due to cognitive or psychiatric reasons.34 Of all those already screened and considered further (ie, initially included), about 10% were later excluded for cognitive reasons in one center.33 Another center reported that despite passing screening, about 9% of those on the DBS-evaluation pathway were excluded for dementia and about 4% for depression on the basis of neuropsychological evaluation.35 These findings again highlight the importance of neuropsychological evaluation. It appears rare that persons identified as having normal or only very mildly impaired cognition by full neuropsychological evaluation go on to develop disabling cognitive deficits soon after DBS, although such cases have been reported.36
Communicating screening and DBS-evaluation failures
Uniform guidelines for communicating screening results to potential surgical candidates are neither available, nor to our knowledge are there studies available that address patient satisfaction with how the outcomes of screening or DBS evaluation were communicated to them. In patient-centered care and shared decision making, it is important for the treatment team to communicate information about treatment benefits, costs, and options, and to help patients understand the importance of their values and preferences in making treatment decisions.37 Patients undergoing DBS evaluation differ in their decision-making style, with some relying more on their own initiative, while others rely more on recommendations made by the treatment team.38 Nonetheless, in our clinical experience, in the case of screening/evaluation failure, persons are less stigmatized and more engaged in treatment decision making when treatment information and recommendations are couched in terms of the appropriateness of treatment for the patient, rather than adequacy of the patient for DBS, ie, an explanation focusing on likely lack of treatment benefit, high risk of adverse events, or lack of fit between anticipated treatment effects and patient expectations and goals is more likely to be heard by the patient than one describing the patient (due to certain characteristics) as a poor candidate for DBS. The onus of benefit and acceptable cost is thereby placed on the treatment, and this conceptualization lifts responsibility from the patient, thereby avoiding potential blame, guilt, and anger about “not being a good candidate” for DBS due to personal characteristics. Care should be taken, however, not to absolve the patient from responsibility for treatment/education participation in the event of a potentially “temporary” or “remediable” screening failure, eg, due to treatable depression or the patient holding misconceptions about DBS during screening or evaluation. It is also helpful to discuss with persons alternative treatment options, including existing pharmacotherapy, allied health treatments (eg, physical therapy), and other experimental treatments.
Evaluation process for DBS candidates
The process followed in evaluating persons for DBS candidacy after screening includes required steps (neurological, neurosurgical, and neuropsychological evaluation, neuroradiological and other laboratory investigations, preoperative education, and multidisciplinary-team discussion) but at our center, there is some fluidity to the process, depending upon where the person enters the evaluation process and whether certain steps in the process need to be repeated (eg, when a patient is initially markedly depressed and inadequately treated and reconsidered after further treatment of depression). Although the vast majority of persons at our center enter or start the presurgical evaluation process via the neurology clinic, some may enter via the neurosurgery clinic, or rarely through the neuropsychology clinic. The flexibility and fluidity of our center’s screening and evaluation process are highlighted in Figure 1, which shows the relationship between neuropsychological screening and evaluation outcomes and progress through subsequent outcome-dependent evaluation and treatment steps.
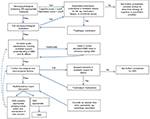  | Figure 1 Flowchart of deep-brain stimulation (DBS) candidacy-evaluation process for persons with Parkinson’s disease (PD). |
Some factors that potentially influence DBS outcomes are presented in Table 1, and are considered within neurological, neurosurgical, and neuropsychological evaluations. The remaining sections of this paper address evaluation considerations from neuropsychological, neurological, and neurosurgical perspectives in addressing three sequential questions: Is DBS an appropriate treatment for this individual?; If yes, which anatomical target is most appropriate, given the individual’s disease, demographic characteristics, and therapy goals?; Is unilateral or bilateral DBS most appropriate, given the individual’s goals, symptoms, and safety considerations?
  | Table 1 Neuropsychological, patient-demographic, disease, and anatomical factors that may impact DBS outcome and are considered during patient selection Abbreviation: DBS, deep-brain stimulation. |
Neuropsychological evaluation basics
Purposes of the neuropsychological evaluation
Neuropsychological evaluations can be broadly conceived as serving four general goals:
- ascertaining that the pattern of cognitive and emotional assets and liabilities is generally consistent with PD, rather than atypical parkinsonism or other disorder, such as bipolar disorder or Alzheimer’s disease;
- to determine whether the individual’s cognitive and emotional functioning and coping resources allow them to:
- understand the DBS process and anticipated effects (including potential adverse events);
- express their therapeutic goals and preferences;
- provide informed consent (including suggested reading level of educational and consent documents);
- cooperate and comply with the evaluation and peri- and post-operative demands.
- to determine whether the nature and severity of cognitive and emotional liabilities constitute significant concerns or relative contraindications to DBS and to recommend potential treatment and reevaluation of liabilities (eg, medication-related cognitive deficits, marked depression, misconceptions about DBS effects and success rates);
- to provide information relevant to the individual’s and treatment team’s decision making regarding surgical target.
Content of neuropsychological evaluation
The exact content of the neuropsychological evaluations is variable among US centers.39 Variability is likely to be even greater across international boundaries, due to practitioner preferences, test availability, and relevance of tests to the cultural, socioeconomic, educational, and linguistic background of the person being evaluated. Additionally, some centers may rely purely on computerized tests, and the potential advantages and significant limitations of this approach have been discussed in detail elsewhere.32,40 Most large movement-disorder centers in the US evaluate similar domains of function, even if the exact neuropsychological tests used differ. Table 2 presents the current domains evaluated (and specific tests used) at the Barrow Neurological Institute. In addition to the tests, which are typically completed in 2.5 hours or less, persons being evaluated for DBS undergo a neuropsychological interview lasting 30–60 minutes, depending upon the complexity of the person’s medical, surgical, psychiatric, and psychosocial history. Medical records are reviewed prior to appointment and potential motor and sensory limitations (and patient’s motor fluctuations and dyskinesias) are addressed with the patient and caregiver (and physician when needed) when the appointment is made. This allows for planning of an efficient examination and necessary test modifications. In addition to covering the traditional areas of inquiry, the interview specifically addresses the individual’s expectations and goals for DBS, their understanding of the procedure and its potential effects, and their insight into current motor, cognitive, and emotional deficits. Also addressed are social-support availability, current family dynamics, and caregiver expectations. Behavioral observations regarding the individual’s interaction with health care providers and family are also used to infer whether there are potential barriers that might complicate care provision, especially during hospitalization and postoperative follow-up. The individual’s ability to cope with the stress of evaluation is considered an index of their ability to tolerate lengthy investigations, surgery, and demanding postoperative visits.
  | Table 2 Neuropsychological domains evaluated and tests and scales used to evaluate these domains at the Barrow Neurological Institute |
Potential predictors of outcome considered in neuropsychological evaluation
Centers very rarely perform DBS on persons with PD who have dementia. Systematic studies of DBS outcome in PD with dementia (PDD) are lacking for obvious practical and ethical reasons, but case studies raise concern that persons with marked cognitive impairment may become more impaired and lose functional independence after DBS.41 Furthermore, there is concern whether a person with dementia or significant cognitive impairment can give truly informed consent and cooperate with a complex and arduous pre-, peri-, and postoperative evaluation and treatment protocol.42 Dementia in our experience also complicates an individual’s ability to engage adequately in the evaluation process, learn material in the DBS-education class, and reliably voice expectations and goals for treatment. If dementia is thought to be associated with medications or a reversible cause, further evaluation and treatment of that condition are initiated. The individual can then be reevaluated for DBS if cognition improves. There may, however, be circumstances where centers consider palliative DBS appropriate for a person with PDD after bioethical consultation.43 Additionally, pilot studies have evaluated the ability of DBS, eg, of the nucleus basalis of Meynert, to enhance cognition in PDD.44,45
In regard to psychiatric conditions, our center does not consider DBS appropriate for persons with PD who experience suicidality or currently inadequately controlled depression, anxiety disorder, psychosis, addiction, or impulsive/compulsive behavior. Typically, persons with such conditions are referred for further treatment and then reevaluated if they continue to be interested in DBS.
One reason for reconsidering persons for DBS only after adequate control of mental health conditions is that DBS has the potential to exacerbate prior illness or trigger de novo illness in patients with PD.46–48 Some authors have suggested that DBS should not be done in patients with active depression49 or unstable depression or psychosis.50 The most common psychiatric adverse event after DBS is depression, which is thought to occur in about 10% of persons within 6 months of surgery, according to well-designed clinical trials.32 Although moderate–severe depression has been reported to occur in as many as 36% of patients within 24 months after surgery, the difference in depression incidence at shorter and longer postoperative intervals leads one to suspect, given the large problems of depression in PD, the higher incidence of depression at longer follow-up intervals is associated with the natural course of PD. Nonetheless, it seems prudent to exclude patients with depression, because depression is an independent risk factor for postoperative suicide.51 Suicidality occurs in probably <1%–2% of patients, but it has been noted that the suicide rate after DBS is 12- to 15-fold higher than the global suicide rate reported by the World Health Organization.51 Other acute behavioral changes after DBS may be related to dopaminergic medication reduction (eg, apathy), whereas other changes, such as hypomania or impulsivity, may reflect the mimicking of hyperdopaminergic effects by DBS.47,48
Table 1 presents some of the cognitive, demographic, disease, and anatomic characteristics that have been – even if only inconsistently – associated with neuropsychological outcome after DBS. One of the first studies systematically to examine possible preoperative predictors of neuropsychological outcome after DBS in PD found that higher age, poorer motor-symptom response to levodopa, and attention impairment (when considered together) were associated with poorer cognitive outcome.52 Unfortunately, subsequent studies, perhaps because they used different neuropsychological tests and samples with different baseline disease and demographic characteristics, have not replicated this finding.53,54 It is also striking that demographic and disease variables occasionally identified as correlated with poorer outcomes, such as amount of baseline dopaminergic medication (levodopa equivalents), severity of motor deficit, disease duration, and age, were not related to outcome in any individual cognitive domain in a meta-analysis that sought retrospectively to identify potential predictors of neuropsychological outcome.55 A limitation of such a post hoc or retrospective analysis is that persons for whom DBS was considered inappropriate may already have been excluded from the meta-analyzed DBS studies, thereby not only restricting the range of values of predictors and outcome variables (and consequently the size of correlations) but perhaps also leading to exclusion of persons with other characteristics that might be associated with poor outcome. For ethical reasons, it is not possible to randomize to DBS vs medical treatment studies persons for whom DBS is deemed potentially unsafe or ineffective.
Even though reliable (and probably strong) predictors of poor emotional and cognitive outcomes have not been identified empirically, it is likely that baseline deficits in attention, executive functions, memory, and overall level of cognitive impairment, along with the discussed demographic and disease characteristics, are associated with poorer neuropsychological outcome. Consequently, the number of potentially unfavorable characteristics, as well as their extent or severity, should be considered when formulating DBS risk:benefit ratios. A greater presence of negative predictors might foretell a negative neuropsychological outcome.
The role of mild cognitive impairment
It is likely that the frequency of PD-MCI is higher after than before DBS surgery. A study not using MDS PD-MCI diagnostic criteria56 found that PD-MCI increased from 47% prior to surgery to 63% an average of 9 months after surgery among 30 STN DBS patients.57 Such an increase in MCI over just 9 months is noteworthy, given two observations: the rarity of large changes on neuropsychological tests over 18 months in PD (2%–8% of patients showed declines exceeding the reliable change index on various cognitive tests),58 and the 1-year MCI-development rate of about 10% in PD with normal cognition.59 Factors predisposing to postoperative PD-MCI remain to be defined.
DBS generally seems to be accepted as an appropriate treatment for persons with PD-MCI, although its role in neuropsychological and QoL outcomes after DBS is poorly understood. Few studies have examined the role of the pre-operative presence of PD-MCI in neuropsychological outcomes. None found that PD-MCI per se predicted cognitive outcome, but two found that dementia occurred sooner among those with PD-MCI at baseline than among those without PD-MCI. In one retrospective study, 60% of 130 patients had multiple-domain MCI and 21% single-domain MCI prior to surgery. Although PD-MCI was not predictive of length of hospital stay or confusion after bilateral STN DBS, patients with preoperative attention impairments were significantly more likely to have hospital stays ≥3 days (39% vs 12%) and tended to have postoperative confusion more frequently (11% vs 3%).60 Another retrospective study of 103 bilateral STN DBS patients found that 63% of the patients had MCI at baseline.61 Patients were followed for up to 7 years (mean 42 months). Although annual rate of decline on the Mini-Mental State Examination score was small (0.4±1.7), and not associated with MCI diagnosis, ten of the 103 patients developed dementia and the probability of developing dementia was significantly greater among those with than without baseline PD-MCI. Given similar rates of Mini-Mental State Examination score decline in those with than without PD-MCI at baseline, the higher probability of dementia in the PD-MCI-diagnosed group might reflect the natural history of PD and the fact that PD-MCI is a risk factor for PDD.62 Such a conclusion was drawn by the authors of another retrospective study63 of 184 patients, 23% of whom had PD-MCI prior to surgery. Although dementia affected those with PD-MCI sooner (median 6 years) than those without MCI (median 11 years) after surgery, no cases of dementia were observed early after DBS, suggesting that the more precocious development of dementia in the MCI group might reflect the natural history of the disease. PDD develops during 5-year follow-up in about 59.1% of incident PD cases with PD-MCI but only 7.2% of those with normal cognition during the first study year.59
Unfortunately, no studies have yet addressed cognitive outcome quantitatively among those with and without PD-MCI prior to DBS, so it remains possible that those with MCI have a poorer cognitive outcome, even if the decline does not warrant a diagnosis of dementia. Additionally, it remains unknown whether bilateral and unilateral DBS have a different impact in persons with PD-MCI and how this effect might differ across different PD-MCI phenotypes. Furthermore, because MCI represents a continuum of severity of functionally nonincapacitating cognitive impairment, it remains to be shown whether those with more “severe” MCI might be at greater risk of being propelled into the dementia diagnostic category after DBS than those with “milder” MCI.
Role of patient and caregiver expectations in outcome satisfaction
It is important to address patient expectations prior to surgery,16 specifically whether these are realistic in regard to outcomes that might reasonably be expected (eg, magnitude and duration of effect, symptoms impacted by treatment, and occurrence of adverse events). In an elegant study, patients were asked to rate their QoL covering several functional domains using the Parkinson’s Disease Questionnaire (PDQ-39) and to indicate on the same scale where they expect to see themselves after surgery. QoL improved significantly 6 months after surgery, but there was a marked discrepancy between expected and actual (much smaller) change. Nonetheless, most patients rated themselves as satisfied and having their expectations fulfilled on visual analog scales. Satisfaction was related to fulfillment of expectations, but not with actual PDQ39 changes, raising the possibility that at least some patients may be satisfied with surgical outcomes, despite lesser QoL gains. However, another study’s results question this possibility. In a study using semistructured interviews with 28 patients 12 months after STN DBS, dissatisfaction rate was similar to that in the previously described study (25% were disappointed by DBS outcome, 32% reported a “mixed” outcome, and 43% reported a satisfactory outcome).64 However, QoL improved only in the mixed and satisfied groups, and postoperative dissatisfaction was predicted by preoperative apathy and axial symptoms.
Caregiver expectations and support availability also need to be ascertained. Caregivers need to be advised that there is a possibility that even with good DBS motor outcomes, patients may not return to normal activities and that marital difficulties may ensue.65,66 Patient psychiatric disturbances after DBS may especially impact caregiver burden, and older caregivers with depression may be particularly prone to further disruption of their own QoL after their partner’s DBS.67
Neurological and neurosurgical considerations
From the neurological and neurosurgical point of view, DBS is indicated in PD for the alleviation of medication-refractory tremors and medication-refractory levodopa-induced motor complications, such as motor fluctuations and dyskinesia. One of the first case series of blinded STN DBS evaluation reported improvement with DBS in akinesia, rigidity, tremor, gait, and postural stability by 57%, 52%, 82%, and 49%, respectively.68 Subsequent larger and more sophisticated studies have confirmed significant improvement in PD motor symptoms with STN and GPi DBS.1,4,69 As noted earlier, however, careful and methodical patient selection procedures are essential if good therapeutic results are to be obtained. Several issues of particular importance confront the neurologist when determining whether DBS is an appropriate treatment option for a given person with PD.
Confirmation of diagnosis and PD characteristics
PD is characterized by resting tremors, bradykinesia (slowness of movement), rigidity, and gait disturbance/postural instability. Using only medication-refractory PD symptoms as the indication for DBS is not recommended, given that the range of such symptoms can be very wide and their identification prone to errors. Based on expert consensus, Bronstein et al25 recommended that for best DBS surgical outcomes, one select relatively younger persons with PD whose motor symptoms demonstrate adequate levodopa response, who have few or no axial, levodopa unresponsive motor symptoms, no or significant MCI, and no active psychiatric disease. These recommendations are consistent with those advocated by authors of the structured screening instruments already described. Although there is no absolute age limit for DBS in PD, studies suggest younger patients have a more robust motor-symptom response and greater QoL gains.26,70 A >25%–50% levodopa response is considered a positive treatment indicator, and the symptoms most likely to respond to GPi and STN DBS are “off”-period akinesia, rigidity, tremor, and dystonia and “on”-period dyskinesias.26
As noted in the discussion of standardized DBS-screening questionnaires, it is critical that a diagnosis of PD be confirmed. It is very important to exclude from DBS persons with atypical parkinsonian disorders. For example, DBS in multiple-system atrophy (MSA) will lead to at best brief and transient motor-symptom improvement, or worse to adverse effects or worsening of symptoms.71,72 Meissner et al72 summarized several red flags to avoid operating on persons with suspected MSA: rapid progression, early postural instability, orofacial dystonia as dyskinesia, severe dysarthria and dysphagia, early autonomic features, poor response to normal doses of levodopa, and the presence of MSA-related neuroimaging findings, such as the “hot cross bun” sign and hyperintensity in the posterolateral putamen or external capsule. During the first 5 years of parkinsonism, some MSA patients (particularly those with the parkinsonian variant of MSA) may be harder to distinguish from PD based on clinical grounds, because they can demonstrate peak-onset dyskinesia and some response to levodopa. Additionally, some patients with MSA have a slower or more benign symptom picture and progression, leading to the recommendation generally to avoid operating on patients with disease duration <5 years25 (although the US Food and Drug Administration in 2016 approved DBS therapy for use in people with PD of at least 4 years duration and with recent onset of motor complications, or motor complications of longer standing that are refractory to medication).
PD can be grouped on the basis of its main manifestations into tremor-dominant or -predominant, postural instability/gait difficulties or gait disturbance (PIGD), and mixed or indeterminate categories.73 Such grouping also impacts DBS success. In Katz et al,74 235 persons with PD were grouped by PD subtype (ie, tremor-dominant, intermediate, and PIGD) and DBS target (STN vs GPi). Primary outcomes were changed in Unified Parkinson’s Disease Rating Scale part III (UPDRS-III) or motor scores off medication from baseline to 24 months post-DBS. Tremor-dominant patients had greater overall motor improvement, and PIGD had the least improvement. Axial parkinsonian signs are very difficult to treat with standard STN or GPi DBS. A recent retrospective analysis revealed that nine of 13 patients with moderate postural abnormality and only two of five with severe postural abnormality improved on postural analysis. Because of this important limitation of STN and GPi DBS, research continues regarding the potential impact of pedunculopontine nucleus DBS on gait, balance, falls, and posture. Although Morita et al concluded pedunculopontine nucleus DBS to be feasible and promising in treating PD axial symptoms, further studies are needed,75–78 including more detailed analysis of the therapy’s neuropsychological impact.79
DBS: earlier or later in PD?
When in the course is the best time to operate on a PD patient? This is a question every neurologist asks when encountering a potential DBS patient. The mean duration of PD in most DBS studies is 12–15 years.26 As already noted, it is generally recommended to perform surgery at least 5 years after diagnosis, in order to avoid operating on misdiagnosed patients. More recently, there has been a trend to evaluate the effect of DBS earlier in PD.7 In the EARLYSTIM trial, Deuschl et al1 targeted patients who had had PD for at least 4 years and had recent onset of mild, levodopa-induced motor complications (≤3 years), but preserved social and occupational functioning. DBS improved all outcomes (motor disability, activities of daily living, levodopa-induced motor complications, and on time without dyskinesia) in comparison to best medical therapy. A small, open-label series of four cases reported by Mesnage et al supported the notion that early intervention can prevent motor handicap and adverse reactions to levodopa from interfering with socioprofessional integration and family life.80 Although early intervention appears to be associated with minimal neuropsychological morbidity,81 several cautionary points were raised by Mestre et al about DBS early in the disease course.82 Risks of early treatment include potentially operating on persons with atypical parkinsonism, less benefit to risk ratio for surgery in younger patients, doing DBS despite the fact that mild motor complications could remain stable for several years, potentially higher postsurgical suicide risk in younger patients, and that the long disease course in younger persons raises the risk of potential complications, such as hardware malfunction.
Target selection: GPi vs STN?
Will motor-symptom response differ depending on surgical target? Should neurologists be selecting surgical targets based on the most disabling symptoms of each patient? In 2010, Follett et al reported results from a randomized, blinded, controlled, prospective study of 299 patients comparing STN vs GPi DBS outcomes.83 Similar improvement in motor function (on UPDRS-III) was observed after GPi and STN DBS. The same VA Cooperative Study follow-up at 36 months again showed similar and stable motor improvements in both groups.84 Interestingly, Southwell et al85 found that after publication of the VA Cooperative Study, there was a sixfold increase in GPi- vs STN-target choice for patients with higher age, depression, and cognitive problems (perhaps reflecting higher neuropsychiatric adverse-event rates in the VA Cooperative Study STN DBS group). These authors concluded that these among other factors (such as brittle dyskinesia, having difficulties with follow-up) favor GPi, whereas in persons with higher preoperative levodopa-equivalent dose, the STN target is to be favored. It should be noted that although neuropsychiatric adverse-event rates might be higher after STN than GPi DBS (and some thus favor GPi over STN DBS in patients deemed at risk of neuropsychiatric adverse events),14,15 adverse-event reporting may not be highly reliable. Furthermore, the largest and highest-quality randomized trials reported minimal differences in cognitive and psychiatric outcomes, and differences in effect size were small.32,86
Odekerken et al, who randomized 128 patients to GPi or STN DBS, found no differences by surgical target on primary outcomes either (weighted Academic Medical Center Linear Disability Scale [ALDS] and composite score for cognitive, mood, and behavioral effects) 1 year after surgery, but they did find differences in secondary outcomes.2 Specifically, larger improvements were observed after STN than GPi DBS in the off-medication state (UPDRS-III, 20.3% vs 11.4% improvement; ALDS, 20.3% vs 11.8% improvement). In a 2014 meta-analysis of 563 patients in six trials by Liu et al,87 GPi and STN improved motor function (UPDRS-III medication off and on states) and activities of daily living (in the on state) similarly 1 year after surgery. However, STN DBS allowed greater medication reduction, whereas GPi DBS was associated with greater improvement in depressive symptoms (by Beck Depression Inventory). Only one case series has documented significant medication reduction with GPi DBS.88
Unilateral vs bilateral DBS
There have been no randomized controlled trials of unilateral vs bilateral pallidal or subthalamic DBS. Studies that have offered comparisons have typically examined patients after a first and then second surgery or compared patients after bilateral vs staged surgery. From a neuropsychological standpoint, such studies have suggested that whether one operates on the language-dominant or nondominant hemisphere89 or on the more or less diseased hemisphere90 may be more important than whether the surgery is unilateral or bilateral. From a motor standpoint, probably the most important consideration in deciding whether unilateral surgery might suffice is the patient’s degree of motor-symptom asymmetry. Patients with greater asymmetry are likely to obtain more satisfactory symptom control after unilateral surgery than those with minimal motor asymmetry, although bilateral surgery may be more frequently needed with STN than GPi DBS.91
DBS-education class and informed consent
At our institution, patients complete a DBS-education class prior to neurosurgical consultation, and the patient’s candidacy is discussed at the multidisciplinary DBS Consensus Conference, which is held twice a month. Our DBS team at the Barrow Neurological Institute involves six movement-disorder neurologists, two DBS nurses, one DBS coordinator, two neuropsychologists, three neurosurgeons, and one social worker. These multidisciplinary perspectives are maintained and represented in the education program, the contents of which are summarized in Table 3. The goal of education is to familiarize the patient as much as possible with the indications for DBS surgery, the anticipated benefits, the surgical procedure, and the associated risks. The patients should have a degree of insight into their condition, such that they know what it means to be “on” and “off”, recognize the concept of motor fluctuations, and are aware of what symptoms are medication-responsive vs unresponsive.
Informed consent consists of ensuring that the patient is aware of the risks, benefits, alternatives, and indications for surgery. Some of the significant risks of DBS include intracerebral hemorrhage, infection, hardware failure, electrode malposition, air embolism, seizure, hardware breakage, and erosion of hardware through the skin.83,92 These complications may require return of a patient to surgery. Intracerebral hemorrhage may result in paralysis and/or disability. Complications resulting from DBS surgery can also include death, coma, stroke, paralysis, or permanent total disability. While the risk of a catastrophic outcome is very small, addressing these risks preoperatively is a necessary part of informed consent. The patient may also have hardware-related discomfort. Postoperative confusion can occur, and a baseline neuropsychological evaluation can help to prepare patients and family for this possibility. Postoperative confusion may prevent discharge home and need for acute rehabilitation or a skilled nursing facility. Medical risks include deep-vein thrombosis, heart attack, allergic reactions, and pneumonia. It is also important to discuss stimulation-related side effects. These may include but are not limited to cognitive disturbances, impulsive behavior, and affective disturbances, such as mania or depression. Stimulation may also produce side effects affecting vision, sensation, speech, verbal fluency, and motor function. Stimulation-related side effects or lack of clinical benefit may result in return to surgery to reposition the DBS lead. Issues of awake vs asleep DBS are also discussed during education, and patients are advised that awake surgery with electrophysiological mapping remains the gold standard. Patients have a choice of which procedure they wish to undergo.
MRI
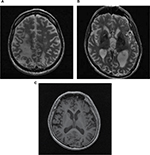
Typically, patients will also undergo MRI of the brain as part of screening to identify risk factors for surgery, such as cerebral atrophy or microvascular changes to white matter (Figure 2). Such changes may predispose the patient to postoperative cognitive decline. More refined MRI scans are obtained for surgical planning once patients have completed neurosurgical evaluation and informed consent. In addition to a high-resolution three-dimension sequence, specific MRI sequences can be used to visualize the DBS targets. At our institution, the preference is to use a 3 T magnet, and T2 fast-spin echo images are obtained for visualization of the STN and proton density for visualization of the GPi.
Asleep or awake surgery
The gold standard for surgery continues to be awake surgery using microelectrode recording to confirm the surgical target. However, several centers, including the Barrow Neurological Institute, are increasingly using direct anatomical targeting via intraoperative neuroimaging and conducting DBS operations for PD with patients under general endotracheal anesthesia. Such factors as claustrophobia, obstructive sleep apnea, or chronic pain may render awake surgery a challenge. Asleep surgery has the disadvantage that electrophysiological target confirmation cannot be obtained. Anatomical and functional heterogeneity among patients may thus be associated with adverse events in asleep surgery. Nonetheless, preliminary findings suggest that similar or better motor outcomes might be obtained with asleep surgery93,94 and that cognitive morbidity is minimal.95 The finding that asleep DBS may be associated with better QoL and speech outcomes than awake surgery93 requires replication. It is not yet known whether certain patient and disease characteristics might lead one to select one surgical method over another for a given patient.
Conclusion
Patient selection for DBS is a lengthy process. The process is best achieved by utilization of a multidisciplinary team that considers not only patient and disease characteristics in deciding whether DBS is an appropriate therapy for a given patient but also pays close attention to patient expectations, preferences, and QoL determinants. Further research is needed to allow more consistent electrode-location placements and perhaps work from connectomics or optogenetics will eventually translate into more individualized and circumscribed yet effective and safe stimulation. Research is also needed to compare the outcomes of awake and asleep surgery in a randomized study. Directional and segmental stimulation, which is thought to increase adverse-event thresholds,96,97 also remains to be more thoroughly investigated. Most importantly, large multicenter databases will probably be needed to more accurately predict for whom and when what type of DBS is appropriate. Indeed, it is likely that an intense search for preoperative biomarkers of DBS success and safety will take place. From a clinical standpoint, the beneficial effects of patient education require further empirical validation.
Acknowledgments
This review was supported in part by funding from the Barrow Neurological Foundation.
Disclosure
AIT has served as a consultant or on the scientific advisory boards of Medtronic, St Jude Medical (Abbott), Boston Scientific, Teva, Pfizer, Takeda, and Axovant. He receives royalties from Oxford University Press and has received financial support from the Michael J Fox Foundation and Barrow Neurological Foundation. The authors report no other conflicts of interest in this work.
References
Deuschl G, Schade-Brittinger C, Krack P, et al. A randomized trial of deep-brain stimulation for Parkinson’s disease. N Engl J Med. 2006;355(9):896–908. | ||
Odekerken VJ, van Laar T, Staal MJ, et al. Subthalamic nucleus versus globus pallidus bilateral deep brain stimulation for advanced Parkinson’s disease (NSTAPS study): a randomised controlled trial. Lancet Neurol. 2013;12(1):37–44. | ||
Okun MS, Gallo BV, Mandybur G, et al. Subthalamic deep brain stimulation with a constant-current device in Parkinson’s disease: an open-label randomised controlled trial. Lancet Neurol. 2012;11(2):140–149. | ||
Weaver FM, Follett K, Stern M, et al. Bilateral deep brain stimulation vs best medical therapy for patients with advanced Parkinson disease: a randomized controlled trial. JAMA. 2009;301(1):63–73. | ||
Williams A, Gill S, Varma T, et al. Deep brain stimulation plus best medical therapy versus best medical therapy alone for advanced Parkinson’s disease (PD SURG trial): a randomised, open-label trial. Lancet Neurol. 2010;9(6):581–591. | ||
Charles D, Konrad PE, Davis TL, Neimat JS, Hacker ML, Finder SG. Deep brain stimulation in early stage Parkinson’s disease. Parkinsonism Relat Disord. 2015;21(3):347–348. | ||
Schuepbach WM, Rau J, Knudsen K, et al. Neurostimulation for Parkinson’s disease with early motor complications. N Engl J Med. 2013;368(7):610–622. | ||
Fundament T, Eldridge PR, Green AL, et al. Deep brain stimulation for Parkinson’s disease with early motor complications: a UK cost-effectiveness analysis. PLoS One. 2016;11(7):e0159340. | ||
Benabid AL, Pollak P, Hoffmann D, Gervason C, et al. Long-term suppression of tremor by chronic stimulation of the ventral intermediate thalamic nucleus. Lancet. 1991;337(8738):403–406. | ||
Koller W, Pahwa R, Busenbark K, et al. High-frequency unilateral thalamic stimulation in the treatment of essential and parkinsonian tremor. Ann Neurol. 1997;42(3):292–299. | ||
Pollak P, Benabid AL, Gross C, et al. Effects of the stimulation of the subthalamic nucleus in Parkinson disease. Rev Neurol. 1993;149(3):175–176. | ||
Lozano CS, Tam J, Lozano AM. The changing landscape of surgery for Parkinson’s disease. Mov Disord. 2018;33(1):36–47. | ||
Williams NR, Foote KD, Okun MS. STN vs. GPi deep brain stimulation: translating the rematch into clinical practice. Mov Disord Clin Pract. 2014;1(1):24–35. | ||
Ramirez-Zamora A, Ostrem JL. Globus pallidus interna or subthalamic nucleus deep brain stimulation for Parkinson disease. JAMA Neurol. 2018;75(3):367–372. | ||
Rughani A, Schwalb JM, Sidiropoulos C, et al. Congress of Neurological Surgeons Systematic review and evidence-based guideline on subthalamic nucleus and globus pallidus internus deep brain stimulation for the treatment of patients with Parkinson’s disease: executive summary. Neurosurgery. 2018;82(6):753–756. | ||
Maier F, Lewis CJ, Horstkoetter N, et al. Patients’ expectations of deep brain stimulation, and subjective perceived outcome related to clinical measures in Parkinson’s disease: a mixed-method approach. J Neurol Neurosurg Psychiatry. 2013;84(11):1273–1281. | ||
Hogg E, Wertheimer J, Graner S, Tagliati M. Deep brain stimulation and nonmotor symptoms. Int Rev Neurobiol. 2017;134:1045–1089. | ||
Rose KJ, Derry PA, Mclachlan RS. Patient expectations and postoperative depression, anxiety, and psychosocial adjustment after temporal lobectomy: A prospective study. Int J Behav Med. 1995;2(1):27–40. | ||
Wheelock I, Peterson C, Buchtel HA. Presurgery expectations, postsurgery satisfaction, and psychosocial adjustment after epilepsy surgery. Epilepsia. 1998;39(5):487–494. | ||
Wilson SJ, Saling MM, Kincade P, Bladin PF. Patient expectations of temporal lobe surgery. Epilepsia. 1998;39(2):167–174. | ||
dos Santos JF, du Montcel ST, Gargiulo M, et al. Tackling psychosocial maladjustment in Parkinson’s disease patients following subthalamic deep-brain stimulation: a randomised clinical trial. PLoS One. 2017;12(4): e0174512. | ||
Lanier-Bohan EM, Heath SL. Patient and caregiver perspectives of preoperative teaching for deep brain stimulation surgery. J Neurosci Nurs. 2016;48(5):247–255. | ||
Coleman RR, Kotagal V, Patil PG, Chou KL. Validity and efficacy of screening algorithms for assessing deep brain stimulation candidacy in Parkinson’s disease. Mov Disord Clin Pract. 2014;1(4):342–347. | ||
Anderson DG, van Coller R, Carr J. South African guideline on deep brain stimulation for Parkinson’s disease. S Afr Med J. 2017;107(10):1027–1032. | ||
Bronstein JM, Tagliati M, Alterman RL, et al. Deep brain stimulation for Parkinson disease: an expert consensus and review of key issues. Arch Neurol. 2011;68(2):165. | ||
Lang AE, Houeto JL, Krack P, et al. Deep brain stimulation: preoperative issues. Mov Disord. 2006;21(S14):S171–S196. | ||
Panisset M, Picillo M, Jodoin N, et al. Establishing a Standard of Care for Deep Brain Stimulation Centers in Canada. Can J Neurol Sci. 2017;44(2):132–138. | ||
Moro E, Allert N, Eleopra R, et al. A decision tool to support appropriate referral for deep brain stimulation in Parkinson’s disease. J Neurol. 2009;256(1):83–88. | ||
Okun MS, Fernandez HH, Pedraza O, et al. Development and initial validation of a screening tool for Parkinson disease surgical candidates. Neurology. 2004;63(1):161–163. | ||
Wächter T, Mínguez-Castellanos A, Valldeoriola F, Herzog J, Stoevelaar H. A tool to improve pre-selection for deep brain stimulation in patients with Parkinson’s disease. J Neurol. 2011;258(4):641–646. | ||
Okun MS, Fernandez HH, Rodriguez RL, Foote KD. Identifying candidates for deep brain stimulation in Parkinson’s disease: the role of the primary care physician. Geriatrics. 2007;62(5):18–24. | ||
Tröster AI. Some clinically useful information that neuropsychology provides patients, carepartners, neurologists, and neurosurgeons about deep brain stimulation for Parkinson’s disease. Arch Clin Neuropsychol. 2017;32(7):810–828. | ||
Abboud H, Mehanna R, Machado A. Comprehensive, multidisciplinary deep brain stimulation screening for Parkinson patients: no room for “short cuts”. Mov Disord Clin Pract. 2014;1(4):336–341. | ||
Lopiano L, Rizzone M, Bergamasco B, et al. Deep brain stimulation of the subthalamic nucleus in PD: an analysis of the exclusion causes. J Neurol Sci. 2002;195(2):167–170. | ||
Coleman RR, Kotagal V, Patil PG, Chou KL. Validity and efficacy of screening algorithms for assessing deep brain stimulation candidacy in Parkinson’s disease. Mov Disord Clin Pract. 2014;1(4):342–347. | ||
Mirza S, Yazdani U, Dewey R, et al. Comparison of globus pallidus interna and subthalamic nucleus in deep brain stimulation for Parkinson disease: an institutional experience and review. Parkinsons Dis. 2017;2017(3):3410820. | ||
Barry MJ, Edgman-Levitan S. Shared decision making: the pinnacle of patient-centered care. N Engl J Med. 2012;366(9):780–781. | ||
Hamberg K, Hariz GM. The decision-making process leading to deep brain stimulation in men and women with Parkinson’s disease: an interview study. BMC Neurol. 2014;14:89. | ||
Burandt CA, Lebowitz BK, Tröster AI, O’Connor MG. The role of neuropsychology in the evaluation of surgical candidates for deep brain stimulation in Parkinson’s disease: a survey study. Clin Neuropsychol. 2008;22(3):390. | ||
Marras C, Tröster AI, Kulisevsky J, Stebbins GT. The tools of the trade: a state of the art “how to assess cognition” in the patient with Parkinson’s disease. Mov Disord. 2014;29(5):584–596. | ||
Hariz MI, Johansson F, Shamsgovara P, Johansson E, Hariz GM, Fagerlund M. Bilateral subthalamic nucleus stimulation in a parkinsonian patient with preoperative deficits in speech and cognition: persistent improvement in mobility but increased dependency – a case study. Mov Disord. 2000;15(1):136–139. | ||
Massano J, Garrett C. Deep brain stimulation and cognitive decline in Parkinson’s disease: a clinical review. Front Neurol. 2012;3:66. | ||
Kubu CS, Ford PJ. Clinical ethics in the context of deep brain stimulation for movement disorders. Arch Clin Neuropsychol. 2017;32(7):829–839. | ||
Freund HJ, Kuhn J, Lenartz D, et al. Cognitive functions in a patient with Parkinson-dementia syndrome undergoing deep brain stimulation. Arch Neurol. 2009;66(6):781–785. | ||
Gratwicke J, Zrinzo L, Kahan J, et al. Bilateral deep brain stimulation of the nucleus basalis of Meynert for Parkinson disease dementia: a randomized clinical trial. JAMA Neurol. 2018;75(2):169–178. | ||
Castrioto A, Lhommée E, Moro E, Krack P. Mood and behavioural effects of subthalamic stimulation in Parkinson’s disease. Lancet Neurol. 2014;13(3):287–305. | ||
Volkmann J, Daniels C, Witt K. Neuropsychiatric effects of subthalamic neurostimulation in Parkinson disease. Nat Rev Neurol. 2010;6(9):487–498. | ||
Witt K, Daniels C, Volkmann J. Factors associated with neuropsychiatric side effects after STN-DBS in Parkinson’s disease. Parkinsonism Relat Disord. 2012;18 Suppl 1:S168–S170. | ||
Erasmi R, Deuschl G, Witt K. Tiefe Hirnstimulation bei Morbus Parkinson: wann und fuer wen? [Deep brain stimulation for Parkinson’s disease: timing and patient selection]. Nervenarzt. 2014;85(2):137–146. German. | ||
Munhoz RP, Picillo M, Fox SH, et al. Eligibility criteria for deep brain stimulation in Parkinson’s disease, tremor, and dystonia. Can J Neurol Sci. 2016;43(4):462–471. | ||
Voon V, Krack P, Lang AE, et al. A multicentre study on suicide outcomes following subthalamic stimulation for Parkinson’s disease. Brain. 2008;131(10):2720–2728. | ||
Smeding HM, Speelman JD, Huizenga HM, Schuurman PR, Schmand B. Predictors of cognitive and psychosocial outcome after STN DBS in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2011;82(7):754–760. | ||
Odekerken VJ, Boel JA, Geurtsen GJ, et al. Neuropsychological outcome after deep brain stimulation for Parkinson disease. Neurology. 2015;84(13):1355–1361. | ||
Tröster AI, Jankovic J, Tagliati M, Peichel D, Okun MS. Neuropsychological outcomes from constant current deep brain stimulation for Parkinson’s disease. Mov Disord. 2017;32(3):433–440. | ||
Combs HL, Folley BS, Berry DT, et al. Cognition and depression following deep brain stimulation of the subthalamic nucleus and globus pallidus pars internus in Parkinson’s disease: a meta-analysis. Neuropsychol Rev. 2015;25(4):439–454. | ||
Litvan I, Goldman JG, Tröster AI, et al. Diagnostic criteria for mild cognitive impairment in Parkinson’s disease: Movement Disorder Society task force guidelines. Mov Disord. 2012;27(3):349–356. | ||
Yágüez L, Costello A, Moriarty J, et al. Cognitive predictors of cognitive change following bilateral subthalamic nucleus deep brain stimulation in Parkinson’s disease. J Clin Neurosci. 2014;21(3):445–450. | ||
Tröster A, Woods S, Morgan E. Assessing cognitive change in Parkinson’s disease: development of practice effect-corrected reliable change indices. Arch Clin Neuropsychol. 2007;22(6):711–718. | ||
Pedersen KF, Larsen JP, Tysnes OB, Alves G. Natural course of mild cognitive impairment in Parkinson disease: a 5-year population-based study. Neurology. 2017;88(8):767–774. | ||
Abboud H, Floden D, Thompson NR, et al. Impact of mild cognitive impairment on outcome following deep brain stimulation surgery for Parkinson’s disease. Parkinsonism Relat Disord. 2015;21(3):249–253. | ||
Kim H-J, Jeon BS, Paek SH, et al. Long-term cognitive outcome of bilateral subthalamic deep brain stimulation in Parkinson’s disease. J Neurol. 2014;261(6):1090–1096. | ||
Hoogland J, Boel JA, de Bie RM, et al. Mild cognitive impairment as a risk factor for Parkinson’s disease dementia. Mov Disord. 2017;32(7):1056–1065. | ||
Merola A, Rizzi L, Artusi CA, et al. Subthalamic deep brain stimulation: clinical and neuropsychological outcomes in mild cognitive impaired parkinsonian patients. J Neurol. 2014;261(9):1745–1751. | ||
Maier F, Lewis CJ, Horstkoetter N, et al. Subjective perceived outcome of subthalamic deep brain stimulation in Parkinson’s disease one year after surgery. Parkinsonism Relat Disord. 2016;24:41–47. | ||
Schüpbach M, Gargiulo M, Welter ML, et al. Neurosurgery in Parkinson disease: a distressed mind in a repaired body? Neurology. 2006;66(12):1811–1816. | ||
Houeto JL, Mesnage V, Mallet L, et al. Behavioural disorders, Parkinson’s disease and subthalamic stimulation. J Neurol Neurosurg Psychiatry. 2002;72(6):701–707. | ||
Lewis CJ, Maier F, Horstkötter N, et al. The impact of subthalamic deep brain stimulation on caregivers of Parkinson’s disease patients: an exploratory study. J Neurol. 2015;262(2):337–345. | ||
Kumar R, Lozano AM, Kim YJ, et al. Double-blind evaluation of subthalamic nucleus deep brain stimulation in advanced Parkinson’s disease. Neurology. 1998;51(3):850–855. | ||
Limousin P, Krack P, Pollak P, et al. Electrical stimulation of the subthalamic nucleus in advanced Parkinson’s disease. N Engl J Med. 1998;339(16):1105–1111. | ||
Dafsari HS, Reker P, Stalinski L, et al. Quality of life outcome after subthalamic stimulation in Parkinson’s disease depends on age. Mov Disord. 2018;33(1):99–107. | ||
Shih LC, Tarsy D. Deep brain stimulation for the treatment of atypical parkinsonism. Mov Disord. 2007;22(15):2149–2155. | ||
Meissner WG, Laurencin C, Tranchant C, et al. Outcome of deep brain stimulation in slowly progressive multiple system atrophy: a clinico-pathological series and review of the literature. Parkinsonism Relat Disord. 2016;24:69–75. | ||
Rodriguez-Violante M, Cervantes-Arriaga A, Fahn S, Tolosa E. Two-hundred years later: is Parkinson’s disease a single defined entity? Rev Invest Clin. 2017;69(6):308–313. | ||
Katz M, Luciano MS, Carlson K, et al. Differential effects of deep brain stimulation target on motor subtypes in Parkinson’s disease. Ann Neurol. 2015;77(4):710–719. | ||
Thevathasan W, Debu B, Aziz T, et al. Pedunculopontine nucleus deep brain stimulation in Parkinson’s disease: a clinical review. Mov Disord. 2018;33(1):10–20. | ||
Mazzone P, Scarnati E, Garcia-Rill E. The pedunculopontine nucleus: clinical experience, basic questions and future directions. J Neural Transm (Vienna). 2011;118(10):1391–1396. | ||
Morita H, Hass CJ, Moro E, Sudhyadhom A, Kumar R, Okun MS. Pedunculopontine nucleus stimulation: where are we now and what needs to be done to move the field forward? Front Neurol. 2014; 5:243. | ||
Hamani C, Lozano AM, Mazzone PAM, et al. Pedunculopontine nucleus region deep brain stimulation in Parkinson disease: surgical techniques, side effects, and postoperative imaging. Stereotact Funct Neurosurg. 2016;94(5):307–319. | ||
Fischer J, Schwiecker K, Bittner V, et al. Modulation of attentional processing by deep brain stimulation of the pedunculopontine nucleus region in patients with parkinsonian disorders. Neuropsychology. 2015;29(4):632–637. | ||
Mesnage V, Houeto JL, Welter ML, et al. Parkinson’s disease: neurosurgery at an earlier stage? J Neurol Neurosurg Psychiatry. 2002;73(6):778–779. | ||
Tramontana MG, Molinari AL, Konrad PE, et al. Neuropsychological effects of deep brain stimulation in subjects with early stage Parkinson’s disease in a randomized clinical trial. J Parkinsons Dis. 2015;5(1):151–163. | ||
Mestre TA, Espay AJ, Marras C, Eckman MH, Pollak P, Lang AE. Subthalamic nucleus-deep brain stimulation for early motor complications in Parkinson’s disease – the EARLYSTIM trial: early is not always better. Mov Disord. 2014;29(14):1751–1756. | ||
Follett KA, Weaver FM, Stern M, et al. Pallidal versus subthalamic deep-brain stimulation for Parkinson’s disease. N Engl J Med. 2010;362(22):2077–2091. | ||
Weaver FM, Follett KA, Stern M, et al. Randomized trial of deep brain stimulation for Parkinson disease: thirty-six-month outcomes. Neurology. 2012;79(1):55–65. | ||
Southwell DG, Rutkowski MJ, San Luciano M, et al. Before and after the veterans affairs cooperative program 468 study: deep brain stimulator target selection for treatment of Parkinson’s disease. Parkinsonism Relat Disord. 2018;48:40–44. | ||
Boel JA, Odekerken VJ, Geurtsen GJ, et al. Psychiatric and social outcome after deep brain stimulation for advanced Parkinson’s disease. Mov Disord. 2016;31(3):409–413. | ||
Liu Y, Li W, Tan C, et al. Meta-analysis comparing deep brain stimulation of the globus pallidus and subthalamic nucleus to treat advanced Parkinson disease. J Neurosurg. 2014;121(3):709–718. | ||
Evidente VG, Premkumar AP, Adler CH, Caviness JN, Driver-Dunckley E, Lyons MK. Medication dose reductions after pallidal versus subthalamic stimulation in patients with Parkinson’s disease. Acta Neurol Scand. 2011;124(3):211–214. | ||
Rothlind JC, Cockshott RW, Starr PA, Marks WJ. Neuropsychological performance following staged bilateral pallidal or subthalamic nucleus deep brain stimulation for Parkinson’s disease. J Int Neuropsychol Soc. 2007;13(01):68–79. | ||
Hershey T, Wu J, Weaver PM, et al. Unilateral vs. bilateral STN DBS effects on working memory and motor function in Parkinson disease. Exp Neurol. 2008;210(2):402–408. | ||
Taba HA, Wu SS, Foote KD, et al. A closer look at unilateral versus bilateral deep brain stimulation: results of the National Institutes of Health COMPARE cohort. J Neurosurg. 2010;113(6):1224–1229. | ||
Larson PS. Deep brain stimulation for movement disorders. Neurotherapeutics. 2014;11(3):465–474. | ||
Brodsky MA, Anderson S, Murchison C, et al. Clinical outcomes of asleep vs awake deep brain stimulation for Parkinson disease. Neurology. 2017;89(19):1944–1950. | ||
Mirzadeh Z, Chapple K, Lambert M, et al. Parkinson’s disease outcomes after intraoperative CT-guided “asleep” deep brain stimulation in the globus pallidus internus. J Neurosurg. 2016;124(4):902–907. | ||
Sidiropoulos C, Rammo R, Merker B, et al. Intraoperative MRI for deep brain stimulation lead placement in Parkinson’s disease: 1-year motor and neuropsychological outcomes. J Neurol. 2016;263(6):1226–1231. | ||
Dembek TA, Reker P, Visser-Vandewalle V, et al. Directional DBS increases side-effect thresholds: a prospective, double-blind trial. Mov Disorders. 2017;32(10):1380–1388. | ||
ten Brinke TR, Odekerken VJ, Dijk JM, van den Munckhof P, Schuurman PR, de Bie RM. Directional deep brain stimulation: first experiences in centers across the globe. Brain Stimul. 2018; 11(4):949–950. | ||
Witt K, Daniels C, Krack P, et al. Negative impact of borderline global cognitive scores on quality of life after subthalamic nucleus stimulation in Parkinson’s disease. J Neurol Sci. 2011;310(1–2):261–266. | ||
Hrabovsky D, Balaz M, Rab M, et al. Factors responsible for early postoperative mental alterations after bilateral implantation of subthalamic electrodes. Br J Neurosurg. 2017;31(2):212–216. | ||
Blume J, Lange M, Rothenfusser E, et al. The impact of white matter lesions on the cognitive outcome of subthalamic nucleus deep brain stimulation in Parkinson’s disease. Clin Neurol Neurosurg. 2017;159:87–92. | ||
Fukaya C, Watanabe M, Kobayashi K, Oshima H, Yoshino A, Yamamoto T. Predictive factors for long-term outcome of subthalamic nucleus deep brain stimulation for Parkinson’s disease. Neurol Med Chir (Tokyo). 2017;57(4):166–171. | ||
Daniels C, Krack P, Volkmann J, et al. Risk factors for executive dysfunction after subthalamic nucleus stimulation in Parkinson’s disease. Mov Disord. 2010;25(11):1583–1589. | ||
Geevarghese R, Lumsden DE, Costello A, et al. Verbal memory decline following DBS for Parkinson’s disease: structural volumetric MRI relationships. PLoS One. 2016;11(8):e0160583. | ||
Pilitsis JG, Rezai AR, Boulis NM, Henderson JM, Busch RM, Kubu CS. A preliminary study of transient confusional states following bilateral subthalamic stimulation for Parkinson’s disease. Stereotact Funct Neurosurg. 2005;83(2–3):67–70. | ||
Drapier S, Raoul S, Drapier D, et al. Only physical aspects of quality of life are significantly improved by bilateral subthalamic stimulation in Parkinson’s disease. J Neurol. 2005;252(5):583–588. | ||
Schoenberg MR, Mash KM, Bharucha KJ, Francel PC, Scott JG. Deep brain stimulation parameters associated with neuropsychological changes in subthalamic nucleus stimulation for refractory Parkinson’s disease. Stereotact Funct Neurosurg. 2008;86(6):337–344. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.